Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
A Plate-Based Assay for the Measurement of Endogenous Monoamine Release in Acute Brain Slices
* Wspomniani autorzy wnieśli do projektu równy wkład.
W tym Artykule
Podsumowanie
This method introduces a simple technique for the detection of endogenous monoamine release using acute brain slices. The setup uses a 48-well plate containing a tissue holder for monoamine release. Released monoamine is analyzed by HPLC coupled with electrochemical detection. Additionally, this technique provides a screening method for drug discovery.
Streszczenie
Monoamine neurotransmitters are associated with numerous neurologic and psychiatric ailments. Animal models of such conditions have shown alterations in monoamine neurotransmitter release and uptake dynamics. Technically complex methods such as electrophysiology, Fast Scan Cyclic Voltammetry (FSCV), imaging, in vivo microdialysis, optogenetics, or use of radioactivity are required to study monoamine function. The method presented here is an optimized two-step approach for detecting monoamine release in acute brain slices using a 48-well plate containing tissue holders for examining monoamine release, and high-performance liquid chromatography coupled with electrochemical detection (HPLC-ECD) for monoamine release measurement. Briefly, rat brain sections containing regions of interest, including prefrontal cortex, hippocampus, and dorsal striatum were obtained using a tissue slicer or vibratome. These regions of interest were dissected from the whole brain and incubated in an oxygenated physiological buffer. Viability was examined throughout the experimental time course, by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. The acutely dissected brain regions were incubated in varying drug conditions that are known to induce monoamine release through the transporter (amphetamine) or through the activation of exocytotic vesicular release (KCl). After incubation, the released products in the supernatant were collected and analyzed through an HPLC-ECD system. Here, basal monoamine release is detected by HPLC from acute brain slices. This data supports previous in vivo and in vitro results showing that AMPH and KCl induce monoamine release. This method is particularly useful for studying mechanisms associated with monoamine transporter-dependent release and provides an opportunity to screen compounds affecting monoamine release in a rapid and low-cost manner.
Wprowadzenie
A plethora of neurological and psychiatric diseases are associated with dysregulation or insufficient maintenance of monoamine neurotransmitter (dopamine [DA], serotonin [5-HT], norepinephrine [NE]) homeostasis1,2,3. These conditions include, but are not limited to, depression1,2, schizophrenia2, anxiety2, addiction4, menopause5,6,7, pain8, and Parkinson's disease3. For instance, several rat models of menopause have shown that the dysregulation or reduction of monoamines within the hippocampus, prefrontal cortex, and striatum may be associated with both depression and cognitive decline, which is seen in women experiencing menopause. The dysregulation of monoamines in these models have been extensively examined using HPLC-ECD, although the studies did not discriminate between measured neurotransmitter content versus neurotransmitter release5,6,7. Monoamines are classically released into the extracellular space through Ca2+-dependent vesicular release9, and are recycled back through their respective plasma membrane re-uptake system (dopamine transporter, DAT; serotonin transporter, SERT; norepinephrine transporter, NET)10,11. Conversely, data suggests that these transporters are able to release or efflux monoamines, since drugs of abuse such as amphetamine (AMPH) and 3,4-Methylenedioxymethamphetamine (MDMA) are known to release DA and 5-HT, respectively through their transporter systems12,13,14,15,16,17. Thus, a proper mechanistic understanding of monoamine release dynamics is crucial for developing specific and targeted pharmacotherapies.
A wide range of techniques have been employed to study monoamine release such as Fast Scan Cyclic Voltammetry (FSCV)18, in vivo microdialysis13, imaging19, preincubation with radiolabeled monoamines20, optogenetics, and more recently, genetically encoded fluorescent sensors and photometry21,22. FSCV and in vivo microdialysis are the primary techniques used for studying monoamine release. FSCV is used to study the stimulated exocytotic release of, primarily, DA in acute brain slices and in vivo23. Because FSCV uses electrodes to stimulate or evoke release, the primary source of neurotransmitter release is Ca2+-dependent vesicular release18,24,25,26,27,28,29,30,31. In vivo microdialysis coupled with HPLC measures changes in extracellular neurotransmitter levels using a probe placed in a brain area of interest13,32. Similar to FSCV, a major limitation to in vivo microdialysis is the difficulty in determining the source of neurotransmitter release: Ca2+ dependent vesicular release or transporter dependent. Noteworthy, both methods allow for the direct measurement of monoamine release. Through the recent advancement of optogenetics, research demonstrates detection of 5-HT and DA release in a short timespan with exquisite cell-type specificity21,22. However, these strategies require complex and costly techniques and equipment, and indirectly measure monoamine release, specifically through monoamine binding to receptors. Further, radiolabeled monoamines are also used for studying monoamine dynamics. Radiolabeled monoamines may be preloaded into various model systems such as heterologous cells overexpressing each monoamine transporter20,33,34,35,36,37,38,39,40, primary neurons20, synaptosomes33,39,41,42, and acute brain slices43,44. However, radioactivity poses potential harm to the experimenter, and the tritium-labeled analytes may not faithfully recapitulate endogenous monoamine dynamics45,46. Superfusion systems combined with off-line detection methods such as HPLC-ECD have allowed for the detection of monoamines from multiple tissue sources. Here, this protocol provides as an optimized and low-cost, simple, and precise method using acute brain slices to directly measure endogenous basal and stimulated monoamine release.
Acute brain slices allow for testing mechanistic hypotheses, primarily as they preserve the in vivo anatomical microenvironment, and maintain intact synapses47,48,49,50,51,52. In a few studies, acute brain slices or chopped brain tissue have been used in conjunction with a superfusion technique using KCl to stimulate Ca2+ mediated release53,54,55,56. Superfusion systems have been critical to advance the field's understanding of neurotransmitter release mechanisms, including monoamines. However, these systems are relatively expensive, and the number of chambers available for tissue analysis ranges from 4-12. In comparison, the method presented here is inexpensive, allows the measurement of 48 tissue samples, and may be refined to use up to 96 tissue samples. Each well within the 48-well plate contains tissue holders that use filters to separate the released product from the tissue, and released monoamines are then collected and analyzed by HPLC-ECD. Importantly, this method allows for the simultaneous measurement of 5-HT, DA, and NE release from different brain areas such as the prefrontal cortex, the hippocampus, and the dorsal striatum after treatment with pharmacological agents that modulate monoamine release. Thus, the experimenter can answer multiple questions using an inexpensive multi-well system that increases the number of samples tested and thereby reducing the number of animals used.
Protokół
All experiments, including animal handling and tissue collection, were carried out in accordance with the University of Florida and the City College of New York Institutional Animal Care and Use Committee (IACUC), following the approved protocol 201508873 (UF) and 1071 (CCNY). For reagents and buffer please refer to the Supplementary File.
1. Prepare acute rat brain slices
NOTE: In this experiment adult male rats (250-350 g) were used. However, this set up is functional for different developmental points, female rats, and other species. If using a smaller animal, such as mice, the experimenter may adjust to optimize the protocol by using a different number of brain slices or punches per condition. Dissection buffer will be referred to as Buffer 1; efflux buffer will be referred to as Buffer 2.
- Prepare Buffer 1 as mentioned in the Supplementary File. Saturate Buffer 1 with oxygen by bubbling with 95%/5% (O2/CO2) for 20 min on ice. Remove 50 mL of Buffer 1, and chill on ice in a small beaker or a Petri dish. This buffer is used to hold the acutely harvested whole brain.
- Anesthetize one or two adult rats (250-350 g) with 1%-2% isoflurane, decapitate them using a guillotine, and rapidly remove their brains. Immediately place the brain in ice-cold oxygenated Buffer 1 in the container from step 1.1.
NOTE: Ensure isoflurane and guillotine are used safely. Open isoflurane under a fume hood. - Using a vibratome or compresstome, cut 300 µm coronal brain sections from each region of interest (Figure 1). Bubbling Buffer 1 must be present while sections are being made. Using a stainless-steel spatula, carefully and immediately transfer brain slices into a new Petri dish filled with ice-cold oxygenated Buffer 1 (Figure 2).
- Further dissect brain slices (e.g., punches, cut out) by carefully moving the slices to glass slides (Figure 1G) using rat brain atlas57. For instance, identify the dorsal striatum based off its dark, striated structure, and identify the hippocampus based off its proximity to the cortex and unique spiral structure. The right and left hemispheres may be separated to use as control and experimental slices (Figures 2G - H). Here, the dorsal striatum was further dissected into 2 mm punches (Figure 1G).
- Using a plastic transfer pipette with the tip cut off, transfer slices or brain punches into small containers immersed in oxygenated ice-cold Buffer 1 with oxygen bubbling. These containers may be stainless steel mesh, or small Petri dishes filled with buffer (Figure 1H).
2. Ex-vivo endogenous monoamine release from brain slices or punches
NOTE: The device used for this section consists of a 48-well plate and a tissue holder made of six microcentrifuge filter units without the inset-filters connected to a carbogen line (Figure 2). To make the holder, use a sturdy plastic rod (e.g., from a cell scrapper) and super glue the microcentrifuge filter units without the inset-filters to it. Let it dry for 1-2 days. Time required for the endogenous monoamine release experiment and concentrations of amphetamine, fluoxetine, and cocaine are based on the current literature and previous protocols13,20,58.
- Tissue activation
- Transfer the brain tissue from step 1.1.5 to each well of the efflux chamber and allow recovery for 30-50 min at 37 °C on a slide warmer in 0.5-1 mL of oxygenated Buffer 2, with constant, gentle bubbling (Figure 2B1).
- During this incubation, dilute the drugs to the desired concentration for the experiment. All the drugs must be dissolved in Buffer 2, and concentrations are based off the current literature.
- First incubation
- Move the tissue holder with brain tissue to wells containing 500 µL of oxygenated Buffer 2 and incubate for 20 min at 37 °C. Ensure that minimal to no buffer is transported over by tapping the holder on the edge of the well until no excess buffer is in the holder.
- In experiments with pharmacological agents such as monoamine transporter inhibitors, incubate the tissue samples with the drugs diluted in oxygenated Buffer 2 (e.g., 10 µM Fluoxetine, 40 µM cocaine; see Figure 2B2). The final volume in each well will be 500 µL.
- Second incubation
- Move the holder with the tissue to a new set of wells containing 500 µL total Buffer 2 with or without the desired concentration of each drug. Ensure there is no excess buffer leftover. Each well represents an n = 1 for experimental conditions. Each experimental condition is performed in triplicate.
- One well includes an oxygenated Buffer 2, the next 10-30 µM AMPH, and the final well includes 10-30 µM AMPH plus monoamine transporter inhibitors. Each drug is dissolved in oxygenated Buffer 2.
- Incubate the tissue for 20 min at 37 °C with 500 µL of the drug condition.
NOTE: Additional wells may include an oxygenated high K+ Buffer 2 with or without the monoamine transporter inhibitors. Dissolve each drug in the oxygenated Buffer 2 (500 µL). - During this second incubation of 20 min, collect the solution from the wells from the first incubation in step 2.2.1 and transfer to microcentrifuge tubes containing 50 µL of 1 N perchloric acid or phosphoric acid (dependent on the type of HPLC, final concentration 0.1 N). The final volume of the sample will be 550 µL. Maintain microcentrifuge tubes on ice, and label the tubes #1.
- After the second incubation of 20 min, move the tissue holder with brain sections or punches to an empty well and maintain the plate on ice. Transfer the supernatant to microcentrifuge tubes containing 50 µL of 1 N perchloric acid or phosphoric acid. The final volume of the sample will be 550 µL. Maintain microcentrifuge tubes on ice, and label the tubes #2.
- Add 1 mL of ice-cold Buffer 1 to each well containing tissue. Collect all of the tissue using small tweezers, and transfer to clean microcentrifuge tubes.
- Maintain tubes with brain tissue at -80 °C. Discard the 1 mL of Buffer 1 (Figure 2B4).
- Filter solutions obtained from each incubation using microcentrifuge filter tubes (0.22 µm) at 2,500 x g for 2min. Use the filtrate to determine monoamine content using HPLC with electrochemical detection (Figure 2B5).
3. Tissue viability
- MTT assay
NOTE: A significant concern regarding this experimental setup is tissue viability as the tissue may be used for up to several hours59. An MTT assay60,61 is used to determine tissue viability by the end of the experimentation. This assay is based on the conversion of the yellow tetrazolium salt MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) to purple formazan crystals by viable cells with adequate metabolism.- Post experiment maintain a separate group of tissue samples and separate them into two groups.
- Incubate one group for 20 min at 37 °C in Triton X-100 (1%) dissolved in Buffer 2 as a control. Triton X-100 treatment results in cell death. Maintain the second group in Buffer 2, and do not incubate in Triton X-100 (tissue viability control).
- Add MTT (stock solution 5 mg/mL in PBS, pH 7.4) to both groups in the oxygenated Buffer 2 to a final concentration of 0.5 mg/mL.
- Incubate the tissue samples for 20 min at 37 °C, wash with PBS, and transfer into microcentrifuge tubes containing 250 µL of a mixture of SDS (10%, w/v), DMF (25%, v/v), and water to dissolve the formazan crystals.
- Incubate the samples for 24 h.
- Centrifuge the tubes at 10,000 x g for 10 min and measure the absorbance of the supernatant (200 µL) at 562 nm and 690 nm using a microplate reader. Tissue viability is calculated as follows: (A562-A690)/tissue weight.
4. HPLC analysis of monoamines
- Quantify monoamine release from each experimental condition using HPLC-ECD according to previous protocols13,44, using a reverse phase column.
- Prepare the mobile phase required for detection. This consists of 100 mM phosphoric acid, 100 mM citric acid, 0.1 mM EDTA-Na2, 600 mg/L octanesulfonic acid, 8% v/v acetonitrile (final pH 6.0). The composition of the mobile phase is dependent on type of HPLC and column used.
- Set the potential of the electrochemical detector (2 mm glassy carbon electrode,) to 0.46 V, and set the flow rate to 0.05 mL/min.
- Load 5 µL of each sample, including neurotransmitter standards into the HPLC for autoinjection and detection. The amount of each sample added depends on the type of HPLC used.
- Once the HPLC has completed the run, use the given HPLC analysis software to acquire and analyze the chromatograph data.
- Analyze monoamine content using a standard curve composed of each monoamine (Dopamine: DA, Norepinephrine: NE, and Serotonin: 5-HT; Figure 2C). Use the resulting chromatograms to obtain the area under the curve (AUC) based on the manufacturer's guidelines.
5. Preparing tissue lysates for protein quantification
- Protein assay
- Add ice-cold lysis buffer plus protease inhibitors (0.1 g/1 mL) to each microcentrifuge tube containing brain sections/punches and homogenize using a pestle homogenizer. The microcentrifuge tubes must be maintained on ice while homogenizing to prevent protein degradation.
- Incubate tissue homogenates for 1 h at 4 °C with light rotation.
- Centrifuge tissue homogenates at 16,000 x g for 15 min at 4 °C and recover the supernatant.
- Determine protein concentration in the supernatants, with bovine serum albumin (BSA) as a standard.
- Normalize the monoamine content in each brain sample to the total content of protein (µg) measured in 250 µL of brain tissue lysed. Use the below formula to determine the nmol monoamine/g protein. df = dilution factor.
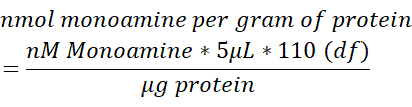
6. Statistical analysis
- Analyze monoamine release (nmol/g) using one-way ANOVA followed by Sidak's multiple comparisons test for post-hoc comparisons.
- Analyze tissue viability using an Unpaired Student's t-test for independent groups (Control vs. 1% Triton X-100).
- For all statistical analyses, set the alpha level to ≤ 0.05.
Wyniki
This technique describes the use of brain slices to measure the release of endogenous monoamines using HPLC with electrochemical detection based in a 48-well plate with an internal tissue holder. Experimental set up is depicted in Figure 1 and Figure 2. Initially, to ensure tissue viability by the end of the experimentation, an MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, a tetrazole) assay was performed. Af...
Dyskusje
Monoamine release measurements have been performed for years in a number of systems such as heterologous cells, neuronal cultures, brain synaptosomes, ex vivo acute brain slices, and whole animals13,20,41,42,58,64,65,66,67...
Ujawnienia
The authors have no disclosures.
Podziękowania
This work was supported by grants Fondecyt Initiation Fund N 11191049 to J.A.P. and NIH grant DA038598 to G.E.T.
Materiały
| Name | Company | Catalog Number | Comments |
| 48 Well plate | NA | NA | Assay |
| Acetonitrile | Fischer Scientific | A998-1 | Mobile Phase |
| Calcium Chloride Ahydrous | Sigma Aldrich | C1016 | Modified Artifical Cerebrospinal Fluid OR Efflux Buffer |
| Clarity Software | Anetc | ||
| Citric Acid | Sigma Aldrich | Mobile Phase | |
| D-(+)-Glucose | Sigma | 1002608421 | Dissection Buffer |
| DMF | Sigma Aldrich | D4551 | MTT Assay |
| EDTA-Na2 | Sigma Aldrich | Mobile Phase | |
| GraphPad Software | Graphpad Software, Inc | Statistical Analysis | |
| Glycerol | Sigma Aldrich | G5516 | Lysis buffer |
| HEPES | Sigma Aldrich | H3375 | Lysis buffer |
| HPLC, Decade Amperometric | Anetc | HPLC, LC-EC system | |
| HPLC | Amuza | HPLC HTEC-510. | |
| L-Asrobic Acid | Sigma Aldrich | A5960 | Dissection Buffer |
| Magnesium Sulfate | Sigma | 7487-88-9 | KH Buffer |
| Microcentrifuge Filter Units UltraFree | Millipore | C7554 | Assay - 6 to fit in 48 well plate |
| MTT | Thermo Fisher | M6494 | MTT Assay |
| Nanosep | VWR | 29300-606 | Assay; protein assay |
| Octanesulfonic acid | Sigma Aldrich | V800010 | Mobile Phase |
| Pargyline Clorohydrate | Sigma Aldrich | P8013 | Modified Artifical Cerebrospinal Fluid OR Efflux Buffer |
| Phosphoric Acid | Sigma Aldrich | Mobile Phase | |
| Potassium Chloride | Sigma | 12636 | KH Buffer |
| Potassium Phosphate Monobasic | Sigma | 1001655559 | KH Buffer |
| Precisonary VF-21-0Z | Precissonary | Compresstome | |
| Protease Inhibitor Cocktail | Sigma Aldrich | P2714 | Lysis buffer. |
| Sodium Bicarbonate | Sigma | S5761 | Dissection Buffer |
| Sodium Bicarbonate | Sigma Aldrich | S5761 | Dissection Buffer |
| Sodium Chloride | Sigma | S3014 | KH Buffer |
| Sodium Dodecyl Sulfate | Sigma Aldrich | L3771 | Lysis buffer |
| Triton X-100 | Sigma Aldrich | T8787 | MTT Assay / Lysis buffer |
Odniesienia
- Jesulola, E., Micalos, P., Baguley, I. J. Understanding the pathophysiology of depression: From monoamines to the neurogenesis hypothesis model - are we there yet. Behavioural Brain Research. 341, 79-90 (2018).
- Krystal, J. H., D'Souza, D. C., Sanacora, G., Goddard, A. W., Charney, D. S. Current perspectives on the pathophysiology of schizophrenia, depression, and anxiety disorders. Medical Clinics of North America. 85 (3), 559-577 (2001).
- Barone, P. Neurotransmission in Parkinson's disease: beyond dopamine. European Journal of Neurology. 17 (3), 364-376 (2010).
- Howell, L. L., Kimmel, H. L. Monoamine transporters and psychostimulant addiction. Biochemical Pharmacology. 75 (1), 196-217 (2008).
- Kirshner, Z. Z., et al. Impact of estrogen receptor agonists and model of menopause on enzymes involved in brain metabolism, acetyl-CoA production and cholinergic function. Life Sciences. 256, 117975 (2020).
- Long, T., et al. Comparison of transitional vs surgical menopause on monoamine and amino acid levels in the rat brain. Molecular and Cellular Endocrinology. 476, 139-147 (2018).
- Long, T., et al. Estradiol and selective estrogen receptor agonists differentially affect brain monoamines and amino acids levels in transitional and surgical menopausal rat models. Molecular and Cellular Endocrinology. 496, 110533 (2019).
- Burke, N. N., et al. Enhanced nociceptive responding in two rat models of depression is associated with alterations in monoamine levels in discrete brain regions. Neuroscience. 171 (4), 1300-1313 (2010).
- Lane, J. D., Aprison, M. H. Calciumm-dependent release of endogenous serotonin, dopamine and norepinephrine from nerve endings. Life Sciences. 20 (4), 665-671 (1977).
- Ramamoorthy, S., Shippenberg, T. S., Jayanthi, L. D. Regulation of monoamine transporters: Role of transporter phosphorylation. Pharmacology and Therapeutics. 129 (2), 220-238 (2011).
- Torres, G. E., Gainetdinov, R. R., Caron, M. G. Plasma membrane monoamine transporters: structure, regulation and function. Nature Reviews. Neuroscience. 4 (1), 13-25 (2003).
- Hilber, B., et al. Serotonin-transporter mediated efflux: A pharmacological analysis of amphetamines and non-amphetamines. Neuropharmacology. 49 (6), 811-819 (2005).
- Mauna, J. C., et al. G protein βγ subunits play a critical role in the actions of amphetamine. Translational Psychiatry. 9 (1), 81 (2019).
- Sitte, H. H., Freissmuth, M. Amphetamines, new psychoactive drugs and the monoamine transporter cycle. Trends in Pharmacological Sciences. 36 (1), 41-50 (2015).
- Johnson, L. A., Guptaroy, B., Lund, D., Shamban, S., Gnegy, M. E. Regulation of amphetamine-stimulated dopamine efflux by protein kinase C β. Journal of Biological Chemistry. 280 (12), 10914-10919 (2005).
- Kahlig, K. M., et al. Amphetamine induces dopamine efflux through a dopamine transporter channel. Proceedings of the National Academy of Sciences of the United States of America. 102 (9), 3495-3500 (2005).
- Kantor, L., Hewlett, G. H. K., Gnegy, M. E. Enhanced amphetamine- and K+ -mediated dopamine release in rat striatum after repeated amphetamine: differential requirements for Ca 2+ - and calmodulin-dependent phosphorylation and synaptic vesicles. The Journal of Neuroscience. 19 (10), 3801-3808 (2018).
- Brodnik, Z. D., et al. Susceptibility to traumatic stress sensitizes the dopaminergic response to cocaine and increases motivation for cocaine. Neuropharmacology. 125, 295-307 (2017).
- Henke, A., et al. Toward serotonin fluorescent false neurotransmitters: development of fluorescent dual serotonin and vesicular monoamine transporter substrates for visualizing serotonin neurons. ACS Chemical Neuroscience. 9 (5), 925-934 (2018).
- Garcia-Olivares, J., et al. Gβγ subunit activation promotes dopamine efflux through the dopamine transporter. Molecular Psychiatry. 22 (12), 1673-1679 (2017).
- Xiao, N., Privman, E., Venton, B. J. Optogenetic control of serotonin and dopamine release in Drosophila larvae. ACS Chemical Neuroscience. 5 (8), 666-673 (2014).
- Bass, C. E., et al. Optogenetic control of striatal dopamine release in rats. Journal of Neurochemistry. 114 (5), 1344-1352 (2010).
- Stamford, J. A. Fast cyclic voltammetry: measuring transmitter release in "real time". Journal of Neuroscience Methods. 34 (1-3), 67-72 (1990).
- Brodnik, Z. D., Ferris, M. J., Jones, S. R., España, R. A. Reinforcing doses of intravenous cocaine produce only modest dopamine uptake inhibition. ACS Chemical Neuroscience. 8 (2), 281-289 (2017).
- Brodnik, Z. D., España, R. A. Dopamine uptake dynamics are preserved under isoflurane anesthesia. Neuroscience Letters. 606, 129-134 (2015).
- Ferris, M. J., Calipari, E. S., Yorgason, J. T., Jones, S. R. Examining the complex regulation and drug-induced plasticity of dopamine release and uptake using voltammetry in brain slices. ACS Chemical Neuroscience. 4 (5), 693-703 (2013).
- Siciliano, C. A., Calipari, E. S., Ferris, M. J., Jones, S. R. Biphasic mechanisms of amphetamine action at the dopamine terminal. The Journal of Neuroscience The Official Journal of the Society for Neuroscience. 34 (16), 5575-5582 (2014).
- Rice, M. E., et al. Direct monitoring of dopamine and 5-HT release in substantia nigra and ventral tegmental area in vitro. Experimental Brain Research. 100 (3), 395-406 (1994).
- Bunin, M. A., Prioleau, C., Mailman, R. B., Wightman, R. M. Release and uptake rates of 5-hydroxytryptamine in the dorsal raphe and substantia nigra reticulata of the rat brain. Journal of Neurochemistry. 70 (3), 1077-1087 (1998).
- Park, J., Takmakov, P., Wightman, R. M. In vivo comparison of norepinephrine and dopamine release in rat brain by simultaneous measurements with fast-scan cyclic voltammetry. Journal of Neurochemistry. 119 (5), 932-944 (2011).
- Park, J., Bhimani, R. V., Bass, C. E. In vivo electrochemical measurements of norepinephrine in the brain: current status and remaining challenges. Journal of the Electrochemical Society. 165 (12), 3051-3056 (2018).
- Butcher, S. P., Fairbrother, I. S., Kelly, J. S., Arbuthnott, G. W. Amphetamine-induced dopamine release in the rat striatum: an in vivo microdialysis study. Journal of Neurochemistry. 50 (2), 346-355 (1988).
- Garcia-Olivares, J., et al. Inhibition of dopamine transporter activity by G protein βγ subunits. PLoS One. 8 (3), 1-9 (2013).
- Carneiro, A. M. D., Blakely, R. D. Serotonin-, protein kinase C-, and Hic-5-associated redistribution of the platelet serotonin transporter. Journal of Biological Chemistry. 281 (34), 24769-24780 (2006).
- Rajamanickam, J., et al. Akt-mediated regulation of antidepressant-sensitive serotonin transporter function, cell-surface expression and phosphorylation. The Biochemical Journal. 468 (1), 177-190 (2015).
- Egaña, L. A., et al. Physical and functional interaction between the dopamine transporter and the synaptic vesicle protein synaptogyrin-3. The Journal of Neuroscience The Official Journal of the Society for Neuroscience. 29 (14), 4592-4604 (2009).
- Guptaroy, B., Fraser, R., Desai, A., Zhang, M., Gnegy, M. E. Site-directed mutations near transmembrane domain 1 alter conformation and function of norepinephrine and dopamine transporters. Molecular Pharmacology. 79 (3), 520-532 (2011).
- Ordway, G. A., et al. Norepinephrine transporter function and desipramine: Residual drug effects versus short-term regulation. Journal of Neuroscience Methods. 143 (2), 217-225 (2005).
- Steinkellner, T., et al. Amphetamine action at the cocaine- and antidepressant-sensitive serotonin transporter is modulated by CaMKII. Journal of Neuroscience. 35 (21), 8258-8271 (2015).
- Guptaroy, B., et al. A juxtamembrane mutation in the N terminus of the dopamine transporter induces preference for an inward-facing conformation. Molecular Pharmacology. 75 (3), 514-524 (2009).
- Carpenter, C., et al. Direct and systemic administration of a CNS-permeant tamoxifen analog reduces amphetamine-induced dopamine release and reinforcing effects. Neuropsychopharmacology. 42 (10), 1940-1949 (2017).
- Aquino-Miranda, G., Escamilla-Sánchez, J., González-Pantoja, R., Bueno-Nava, A., Arias-Montaño, J. -. A. Histamine H3 receptor activation inhibits dopamine synthesis but not release or uptake in rat nucleus accumbens. Neuropharmacology. 106, 91-101 (2016).
- Reddy, I. A., et al. Glucagon-like peptide 1 receptor activation regulates cocaine actions and dopamine homeostasis in the lateral septum by decreasing arachidonic acid levels. Translational Psychiatry. 6 (5), 809 (2016).
- Koutzoumis, D. N., et al. Alterations of the gut microbiota with antibiotics protects dopamine neuron loss and improve motor deficits in a pharmacological rodent model of Parkinson's disease. Experimental Neurology. 325, 113159 (2020).
- Herdon, H., Strupish, J., Nahorski, S. R. Differences between the release of radiolabelled and endogenous dopamine from superfused rat brain slices: Effects of depolarizing stimuli, amphetamine and synthesis inhibition. Brain Research. 348 (2), 309-320 (1985).
- Thongsaard, W., Kendall, D. A., Bennett, G. W., Marsden, C. A. A simple method for measuring dopamine release from rat brain slices. Journal of Pharmacological and Toxicological Methods. 37 (3), 143-148 (1997).
- Dorris, D. M., Hauser, C. A., Minnehan, C. E., Meitzen, J. An aerator for brain slice experiments in individual cell culture plate wells. Journal of Neuroscience Methods. 238, 1-10 (2014).
- Humpel, C. Organotypic brain slice cultures: a review. Neuroscience. 305, 86-98 (2015).
- Papouin, T., Haydon, P. Obtaining acute brain slices. BIO-PROTOCOL. 8 (2), 477-491 (2018).
- Collingridge, G. L. The brain slice preparation: a tribute to the pioneer Henry McIlwain. Journal of Neuroscience Methods. 59 (1), 5-9 (1995).
- Yamamoto, C., McIlwain, H. Electrical activities in thin sections from the mammalian brain maintained in chemically-defined media in vitro. Journal of Neurochemistry. 13 (12), 1333-1343 (1966).
- Buskila, Y., et al. Extending the viability of acute brain slices. Scientific Reports. 4, 4-10 (2014).
- Kako, H., Fukumoto, S., Kobayashi, Y., Yokogoshi, H. Effects of direct exposure of green odour components on dopamine release from rat brain striatal slices and PC12 cells. Brain Research Bulletin. 75 (5), 706-712 (2008).
- McBride, W. J., Murphy, J. M., Lumeng, L., Li, T. -. K. Effects of ethanol on monoamine and amino acid release from cerebral cortical slices of the alcohol-preferring P line of rats. Alcoholism: Clinical and Experimental Research. 10 (2), 205-208 (1986).
- Chen, J. C., Turiak, G., Galler, J., Volicer, L. Effect of prenatal malnutrition on release of monoamines from hippocampal slices. Life Sciences. 57 (16), 1467-1475 (1995).
- Becker, J. B., Castañeda, E., Robinson, T. E., Beer, M. E. A simple in vitro technique to measure the release of endogenous dopamine and dihydroxyphenylacetic acid from striatal tissue using high performance liquid chromatography with electrochemical detection. Journal of Neuroscience Methods. 11 (1), 19-28 (1984).
- Paxinos, G., Watson, C. . The Rat Brain in Stereotaxic Coordinates. , (2007).
- Dailey, J. W., Reith, M. E. A., Steidley, K. R., Milbrandt, J. C., Jobe, P. C. Carbamazepine-induced release of serotonin from rat hippocampus in vitro. Epilepsia. 39 (10), 1054-1063 (1998).
- Buskila, Y., et al. Extending the viability of acute brain slices. Scientific Reports. 4, 5309 (2014).
- Mewes, A., Franke, H., Singer, D. Organotypic brain slice cultures of adult transgenic P301S mice-A model for tauopathy studies. PLoS One. 7 (9), (2012).
- Rönicke, R., et al. AB mediated diminution of MTT reduction - An artefact of single cell culture. PLoS One. 3 (9), (2008).
- Ihalainen, J. A., Riekkinen, P., Feenstra, M. G. P. Comparison of dopamine and noradrenaline release in mouse prefrontal cortex, striatum and hippocampus using microdialysis. Neuroscience Letters. 277 (2), 71-74 (1999).
- Richards, D. A., Obrenovitch, T. P., Symon, L., Curzon, G. Extracellular dopamine and serotonin in the rat striatum during transient ischaemia of different severities: a microdialysis study. Journal of Neurochemistry. 60 (1), 128-136 (1993).
- Fog, J. U., et al. Calmodulin kinase II interacts with the dopamine transporter C terminus to regulate amphetamine-induced reverse transport. Neuron. 51 (4), 417-429 (2006).
- Balázsa, T., Bíró, J., Gullai, N., Ledent, C., Sperlágh, B. CB1-cannabinoid receptors are involved in the modulation of non-synaptic [3H]serotonin release from the rat hippocampus. Neurochemistry International. 52 (1), 95-102 (2008).
- Schechter, L. E. The potassium channel blockers 4-aminopyridine and tetraethylammonium increase the spontaneous basal release of [3H]5-hydroxytryptamine in rat hippocampal slices. The Journal of Pharmacology and Experimental Therapeutics. 282 (1), 262-270 (1997).
- Boudanova, E., Navaroli, D. M., Stevens, Z., Melikian, H. E. Dopamine transporter endocytic determinants: carboxy terminal residues critical for basal and PKC-stimulated internalization. Molecular and Cellular Neuroscience. 39 (2), 211-217 (2008).
- Bowyer, J. F., et al. Interactions of MK-801 with glutamate-, glutamine- and methamphetamine-evoked release of [3H]dopamine from striatal slices. The Journal of Pharmacology and Experimental Therapeutics. 257 (1), 262-270 (1991).
- Perszyk, R. E., et al. GluN2D-containing N-methyl-D-aspartate receptors mediate synaptic transmission in hippocampal interneurons and regulate interneuron activity. Molecular Pharmacology. 90 (6), 689-702 (2016).
- Jones, S. R., et al. Profound neuronal plasticity in response to inactivation of the dopamine transporter. Proceedings of the National Academy of Sciences of the United States of America. 95 (7), 4029-4034 (1998).
- Jedema, H. P., Narendran, R., Bradberry, C. W. Amphetamine-induced release of dopamine in primate prefrontal cortex and striatum: striking differences in magnitude and timecourse. Journal of Neurochemistry. 130, 490-497 (2014).
- Buchmayer, F., et al. Amphetamine actions at the serotonin transporter rely on the availability of phosphatidylinositol-4,5-bisphosphate. Proceedings of the National Academy of Sciences. 110 (28), 11642-11647 (2013).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone