Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Fluorescence Microscopy for ATP Internalization Mediated by Macropinocytosis in Human Tumor Cells and Tumor-xenografted Mice
* Wspomniani autorzy wnieśli do projektu równy wkład.
W tym Artykule
Podsumowanie
We developed a reproducible method to visualize the internalization of nonhydrolyzable fluorescent adenosine triphosphate (ATP), an ATP surrogate, with high cellular resolution. We validated our method using independent in vitro and in vivo assays-human tumor cell lines and immunodeficient mice xenografted with human tumor tissue.
Streszczenie
Adenosine triphosphate (ATP), including extracellular ATP (eATP), has been shown to play significant roles in various aspects of tumorigenesis, such as drug resistance, epithelial-mesenchymal transition (EMT), and metastasis. Intratumoral eATP is 103 to 104 times higher in concentration than in normal tissues. While eATP functions as a messenger to activate purinergic signaling for EMT induction, it is also internalized by cancer cells through upregulated macropinocytosis, a specific type of endocytosis, to perform a wide variety of biological functions. These functions include providing energy to ATP-requiring biochemical reactions, donating phosphate groups during signal transduction, and facilitating or accelerating gene expression as a transcriptional cofactor. ATP is readily available, and its study in cancer and other fields will undoubtedly increase. However, eATP study remains at an early stage, and unresolved questions remain unanswered before the important and versatile activities played by eATP and internalized intracellular ATP can be fully unraveled.
These authors' laboratories' contributions to these early eATP studies include microscopic imaging of non-hydrolysable fluorescent ATP, coupled with high- and low-molecular weight fluorescent dextrans, which serve as macropinocytosis and endocytosis tracers, as well as various endocytosis inhibitors, to monitor and characterize the eATP internalization process. This imaging modality was applied to tumor cell lines and to immunodeficient mice, xenografted with human cancer tumors, to study eATP internalization in vitro and in vivo. This paper describes these in vitro and in vivo protocols, with an emphasis on modifying and finetuning assay conditions so that the macropinocytosis-/endocytosis-mediated eATP internalization assays can be successfully performed in different systems.
Wprowadzenie
The opportunistic uptake of intratumoral extracellular (ie) nutrients has recently been named a key hallmark for cancer metabolism1. One of these important nutrients is ATP, as the concentration of ieATP is 103 and 104 times higher than that found in normal tissues, in the range of several hundred µM to low mM2,3,4,5. As a key energy and signaling molecule, ATP plays a central role in cellular metabolism in cancerous and healthy cells6,7,8. Extracellular ATP is not only involved in cancer cell growth, but it also promotes drug resistance9. Previously unrecognized functions of ATP, such as hydrotropic activity, have recently been identified, thus implicating ATP involvement in diseases such as Alzheimer's10. Indeed, it seems our understanding of ATP and its functions in cancer cells, healthy cells, and other diseased cells is far from complete. However, due to ATP's instability and high turnover rates in cells, it is technically challenging to monitor ATP's movement across the cell membrane and into the cell.
To address this problem and fill the need of this research area, a method was developed in which nonhydrolyzable fluorescent ATP (NHF-ATP) (Figure 1) was used as a surrogate to visualize the internalization of ATP and observe the intracellular spatial localization of internalized ATP, both in vitro and in vivo11,12. NHF-ATP has been demonstrated to substitute for endogenous ATP to investigate ATP movement across animal cell membranes, both in cancer cell lines and in human tumor tissue xenografted on immunodeficient mice11,12. Moreover, administering macropinocytosis inhibitors to cells blocked eATP internalization, suggesting that intracellular uptake of eATP involves a macropinocytotic mechanism9,11,12. This protocol permits immunobased colabeling against cell-specific proteins and thus identification of which cell type internalizes NHF-ATP. Using in vivo tumor xenografts and high-resolution microscopy, NHF-ATP can be visualized spatially across the tissue sample and even within a single cell. These methods also permit quantitative analysis, such as the percentage of cellular uptake, number of macropinocytotic vesicles, and internalization kinetics. This paper describes in detail how NHF-ATP, working alone or together with endocytosis-tracer fluorescent dextrans13,14,15,16, can be used in different experimental settings to study ATP's internalization and intracellular localization, following internalization in cells.

Figure 1: Structures of nonhydrolyzable fluorescent ATP and tetramethylrhodamine labeled high molecular weight fluorescent dextran. (A) Structure of NHF-ATP. (B) Schematic representation of HMWFD. Abbreviations: ATP = adenosine triphosphate; NHF-ATP = nonhydrolyzable fluorescent ATP; TMR = tetramethylrhodamine; HMWFD = high molecular weight fluorescent dextran. Please click here to view a larger version of this figure.
Protokół
All procedures reported herein were performed in accordance with Ohio University's IACUC and with the NIH.
1. Selection of nonhydrolyzable fluorescent ATP (NHF-ATP) and dextrans
- Select a fluorophore-conjugated NHF-ATP (Figure 1A) and endocytosis tracers, high and low molecular weight fluorescent dextrans (TMR-HMWFD and TMR-LMWFD) (Figure 1B), based on the preferred emission wavelengths (e.g., imaging system equipped with appropriate filters) and the specific endocytosis process to be studied.
2. ATP localization studies, in vitro (Figure 2)
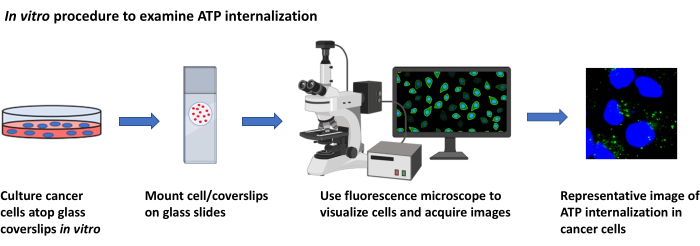
Figure 2: In vitro procedure to examine ATP internalization. Schematic representation of the protocol to visualize the internalization of extracellular ATP in cultured cancer cells using fluorescence microscopy. Please click here to view a larger version of this figure.
- Cell culture and preparation of cells
NOTE: Perform cell culture under sterile conditions in a tissue culture hood.- Prepare Dulbecco's Modified Eagle Medium (DMEM), containing 10% (v/v) fetal bovine serum (FBS) and 1% (v/v) penicillin/streptomycin (hereafter called DMEM/FBS), sterile phosphate-buffered saline (PBS), and 0.25% trypsin/ethylenediaminetetraacetic acid (EDTA), in a 37 °C water bath.
- Culture human cancer cells in DMEM/FBS in a 100 mm tissue culture dish. Maintain the cells in an incubator set to 37 °C with a 5% CO2 atmosphere.
- When the cells reach confluence, passage the cells by first removing the culture medium. Next, rinse the dish with 5 mL of sterile PBS, remove the PBS, and add 3 mL of 0.25% trypsin. Incubate at 37 °C in a 5% CO2 atmosphere for 5 min.
- Retrieve the dish, then add 6 mL of DMEM/FBS to stop the trypsinization. Transfer the cells in suspension to a 15 mL conical tube and centrifuge at 800 × g for 5 min to pellet the cells.
- After centrifugation, aspirate the supernatant and use 10 mL of DMEM/FBS to resuspend the cell pellet by pipetting.
- Count the cell density and viability using a hemocytometer. Use DMEM/FBS to dilute the cell suspension to a density of ~7.5 × 104 cells/mL.
- Preparation of coverslips and seeding cells
- Wash 12 mm coverslips with 70% ethanol and wipe them carefully with delicate task wipes. Sterilize the coverslips and one pair of forceps via autoclaving.
- In a tissue culture hood, use forceps to place one coverslip into each well of a 24-well tissue culture plate.
NOTE: Later, the coverslip, with cells, will be mounted directly onto a microscope slide for imaging. - Dispense 300 µL of the cell suspension (cells in DMEM/FBS), at a seeding density of ~2.5 × 104 cells per well, into the 24-well plate containing the sterilized coverslips.Incubate in sterile conditions at 37 °C with 5% CO2 flow.
- Starvation of cells
- Twenty-four hours after seeding, remove the DMEM/FBS from each well. Immediately add 300 µL of prewarmed serum-free DMEM into each well to serum-starve the cells for 15-18 h to induce uptake of extracellular nutrients.
NOTE: The 15-18 h starvation period is a critical parameter.
- Twenty-four hours after seeding, remove the DMEM/FBS from each well. Immediately add 300 µL of prewarmed serum-free DMEM into each well to serum-starve the cells for 15-18 h to induce uptake of extracellular nutrients.
- Preparation of NHF-ATP and HMWFD/LMWFD solutions
- Use an analytical balance to weigh high-molecular-weight (70 kDa) fluorescent TMR-dextran (TMR-HMWFD, 1 mg/mL), a tracer for visualizing macropinosomes, or NHF-ATP (10 µmol/L) in serum-free DMEM in a 1.5 mL microcentrifuge tube. Place the tubes, protected from light, in a 37 °C water bath for 15 min.
- Centrifuge at 12,000 × g for 5 min at room temperature. Carefully transfer the clear supernatant to a new 1.5 mL microcentrifuge tube, leaving any pellet or debris intact to remove indissoluble crystals.
- Add the solutions from step 2.4.1 to the cells in each well, and incubate the cells for 30 min at 37 °C.
NOTE: If HMWFD and NHF-ATP solutions are to be mixed for co-incubation with the cells, prepare both solutions at 2x the final concentrations. The solutions will be mixed later at a 1:1 ratio to achieve the final accurate working concentrations. Avoid light as the reagents are light-sensitive.
- Treatment of cells and fixation
- In a fresh 24-well plate, dispense 500 µL of prewarmed PBS into each of five wells.
- After cell incubation, carefully pick up each coverslip using forceps. Rinse each coverslip by dipping it in 500 µL of prewarmed PBS. Repeat five times using the five PBS-filled wells.
NOTE: Gentle washing of cells-on-coverslips is critical for the success of this experiment. - After the final PBS wash, tap the coverslip on a delicate task wipe to absorb extra PBS and transfer the coverslip immediately to cold (4 °C) 3.7% formaldehyde, preloaded in a 24-well plate. Fix the cells for 15 min at room temperature.
- While the cells are being fixed, pre-clean microscope slides with 70% ethanol. Remove the coverslips from the wells and mount them onto the slides, using 5 µL of aqueous mounting medium containing the nuclear stain 4′,6-diamidino-2-phenylindole (DAPI), per coverslip. Gently blot excess PBS with a paper towel or a delicate task wipe.
- Fluorescence microscopy and image acquisition
- Two to 24 hours after the steps above, capture images of cells and internalized HMWFD and/or NHF-ATP using an epifluorescence imaging system and data acquisition software.
NOTE: This sub-section describes steps to acquire images using a Nikon NiU microscope, equipped with epifluorescence imaging capability, and Nikon NIS Elements software. However, other comparable imaging systems and acquisition software may be used. Follow the operating instructions from the manufacturer.- Place the slide on the stage of an upright epifluorescence microscope in binocular mode. Access the imaging program.
- Select the 10x objective, adjust the stage to define focus, and scan the slide from left to right in a serpentine manner to identify the regions of interest.
NOTE: Identifying regions of interest will vary between cell types, with some cell lines/cancer types exhibiting diverse and distinct degrees of TMR-HMWFD and/or NHF-ATP uptake. - Select the 40x objective, and switch from binocular mode to image capture mode, using the toggle on the microscope.
- Click on the Live Quality icon on the imaging program to view and subsequently acquire images.
- Using the OC Panel on the Annotations and Measurements toolbar, define the exposure parameters for each filter cube or fluorescent channel.
NOTE: Select the appropriate exposure time for each channel, as signal intensities are different. For example, select an exposure time of 200 ms for DAPI, 2 s for HMWFD, and 4 s for NHF-ATP. Once the exposure time is determined per channel, use this setting for all images, per channel, with different treatment or conditions. - Once the exposure settings have been set for each channel, use the multichannel acquisition toolbar to acquire a 3-channel image with the defined exposure settings.
NOTE: Image acquisition through the multichannel ND acquisition mode enables automatic image capture for each channel of the same field of view. The shutter is closed automatically between turret changes. - Alternatively, acquire multichannel images manually by toggling between filter cubes, setting the exposure time, closing/opening shutter in between image acquisition for each channel, and overlaying each image taken for individual channels.
NOTE: The ND acquisition mode automates this process and provides merged images. - Save the image as .nd2 file (Nikon Elements format saves metadata). Save TIF files, including the merged channel image and individual channel images.
NOTE: TIF files can be used with a broader selection of software applications. - Use the Object Count feature on the Analysis toolbar to count the number of NHF-ATP-, TMR-HMWFD-, and/or TMR-LMWFD-positive cells on a saved .nd2 image file.
- Export the data to a spreadsheet through the analysis program.
- Two to 24 hours after the steps above, capture images of cells and internalized HMWFD and/or NHF-ATP using an epifluorescence imaging system and data acquisition software.
- Data quantification and analysis
- For each condition assayed, image 50 to 100 cells for quantification. Using the data analysis software (software included in the epifluorescence imaging system or other software), count and calculate the mean number of fluorescent vesicles per cell.
- Use appropriate statistical methods to analyze the quantified results.
3. ATP internalization in tumors, ex vivo (Figure 3)

Figure 3: In vivo procedure to examine ATP internalization. Schematic representation of the protocol to visualize the internalization of extracellular ATP in tumor xenografts using cryosectioning and fluorescence microscopy. Please click here to view a larger version of this figure.
- Preparation of cell cultures for implantation
- Grow cancer cells to 80% confluence at 37 °C in a 225 cm2 flask, using DMEM supplemented with FBS, to a final concentration of 10% (v/v) and penicillin/streptomycin at 1% (v/v).
- Wash the cells two times with 10 mL of PBS. Pre-warm 0.25% trypsin/EDTA to 37 °C. Add 8 mL of trypsin/EDTA and incubate at 37 °C for 2 min.
- Once the cells start detaching from the bottom of the flask, use a 10 mL sterile serological pipette to add 8 mL of DMEM/FBS. Aspirate two times to dislodge any adherent cells. Use the pipette to transfer the detached cells from the flask into a 50 mL conical tube.
- Add 10 mL of DMEM/FBS using a 10 mL pipette, and collect all remaining floating cells in the same 50 mL conical tube.
- Centrifuge the cell suspension at 600 × g, 4 °C for 4 min. Remove the supernatant and resuspend the cells in 1 mL of ice-cold PBS.
- Count the cell density using a hemocytometer. Keep the cell suspension on ice while counting.
- Centrifuge the cell suspension at 600 × g, 4 °C for 4 min. Remove the supernatant and suspend the cells in ice-cold PBS such that the cell density becomes 5 × 106 cells per 100 µL of PBS. Transfer the cell suspension to a 1.5 mL microcentrifuge tube.
- Subcutaneous injection of cancer cells for xenograft tumor development
- Use a latex-free syringe (1 mL) with a precision glide needle (27 G needle) for cancer cell injection.
- Transfer the cell suspension (5 × 106 in 100 µL of PBS)to a 1.5mL microcentrifuge tube. Draw the cells into the syringe.
- Select an injection site on the flank of an immunodeficient (nude) mouse, and gently clean the skin using 75% ethanol. Wipe off excess ethanol with a delicate task wipe.
- For subcutaneous injection, hold the needle at an approximately 10° angle to the skin. Insert the needle tip, bevel side up, just underneath the skin, so that only 1-2 mm of the needle is visible outside the skin. Dispense the cells from the syringe slowly over approximately 10 s.
- After injecting the entire volume, continue to hold the needle in place for 3-5 s, then withdraw the needle and use a finger to apply gentle but firm pressure to the injection site for 3-5 more seconds to prevent leaking of the injected content.
- Monitor and measure tumor growth using vernier calipers until the tumors reach a volume of 200-500 mm3.
- Preparation of HMWFD and NHF-ATP solutions to be used post-tumor resection
- Dissolve 300 µL of 16 mg/mL HMWFD in serum-free DMEM (culture medium), incubate in a 37 °C water bath for 30 min, and centrifuge at 12,000 × g for 5 min as described above. Transfer the solution to a 1.5 mL microcentrifuge tube.
- Add 40 µL of NHF-ATP analog stock (1 mM) to 160 µL of serum-free DMEM to prepare a 0.2 mM NHF-ATP solution.
- Preparation of experimental wells
NOTE: This experimental design will assay the intracellular internalization of HMWFD + NHF-ATP, indicative of uptake by macropinosomes.- Prepare the wells as follows: Well #1, Control: 200 µL of serum-free DMEM; Well #2, Control: 100 µL of 16 mg/mL LMWFD + 100 µL of serum-free DMEM = 200 µL of 8 mg/mL LMWFD; Well #3, Control: 100 µL of 0.2 mM NHF-ATP + 100 µL of serum-free DMEM = 200 µL of 0.1 mM NHF-ATP; Well #4; Experimental: 100 µL of 16 mg/mL HMWFD + 100 µL of 0.2mM NHF-ATP = 200 µL of 0.1 mM NHF-ATP and 8 mg/mL HMWFD.
- Preparation of tumor tissues
- Euthanize the mouse by cervical dislocation or according to the IACUC-approved protocol.
- Use a size 10 scalpel to slice the isolated tumors at a thickness of ~500-1,000 µm.
- Incubate the tumor slices in serum-free DMEM supplemented with 100 µM NHF-ATP and/or 8 mg/mL H/LMWFD in microcentrifuge tubes for 40 min at 37 °C with 5% CO2 flow.
NOTE: After incubation, tumor tissue metabolism causes the color of the medium to change. - Rinse the tissues in 37 °C prewarmed PBS (2 mL for each rinse in a 24-well plate).
- Transfer the tissue to a new 24-well plate with prewarmed fresh PBS, rinse and repeat four times with gentle shaking.
- Cryo-embedding (preparing frozen tissue blocks)
- Prepare identification labels for each tumor to be harvested. Cut a 2 cm piece of laboratory tape and fold in half, adhesive sides together, lengthwise. Use a marking pen to label the tag, e.g., with a mouse/tumor identification number.
- Prepare embedding molds by placing stainless steel tissue molds directly on dry ice.
NOTE: Dry ice may cause frostbite, burns, and asphyxiation. Wear insulated gloves when handling dry ice. Use dry ice in a well-ventilated area. Do not store dry ice in a tightly sealed container. Instead, store in a container (such as a Styrofoam cooler) that allows gas to escape. - While the mold chills, place a small pool of tissue-freezing medium into a 10 mm tissue culture plate. Ensure that the volume is enough to submerge the tumor tissue that will be harvested.
- Use a perforated spoon to scoop up the resected tumor tissue and immediately place the tissue into a freezing medium, ensuring that the tissue is submerged. Using the perforated spoon, gently roll the tissue in the freezing medium, ensuring that the medium is bathing all the tissue surfaces.
- Carefully move the tissue into the embedding mold containing the freezing medium. Place the corresponding label tag vertically into the freezing medium/mold to freeze in place. Ensure the written label is visible outside of the medium.
- When the freezing is complete (freezing medium turns opaque-white), remove the tissue block from the mold, place it on dry ice, and repeat for each tumor. Store the tissue blocks at -80 °C for several months before the cryosectioning procedure.
- Preparation of slides of tissues samples
- To maximize the chance of finding internalization-positive cells and having more representative tissue regions, collect serial cryosections at -18 to -20 °C using a cryostat.
- Prechill cryostat tools (blade, razor blade, anti-roll plate, tissue chuck holder, paintbrush) and equilibrate the tumor tissue blocks by placing them in a cryostat chamber at -18 to -20 °C. Set the blade holder angle to 5-10°. Carefully trim the tissue block, as needed, with a razor blade, and mount it onto the chuck holder using tissue freezing medium as "glue."
- Lock the chuck holder into the vertical position on the microtome unit, which advances to the set distance (e.g., 10 µm) with each turn of the hand crank. Position the anti-roll plate to rest just above the height of the blade. To prevent tissue curling before advancing the microtome, carefully slide the thumb over the bottom edge of the tissue block.
- As the microtome advances and the tissue section falls onto the metal plate, use a paintbrush to guide the tissue section and unroll the tissue, if necessary.
- Hover the microscope slide over the tissue section without touching so that the section will be attracted to the slide.
NOTE: Cryostat blades (high-profile, disposable) are extremely sharp and can cause serious injury. Use care when handling blades and operating the cryostat. Use a blade protector, if available. Proper training is required. - Slice the tumor into sections of 10 µm thickness. Immediately transfer the sliced sections onto a positively charged glass microscope slide.
NOTE: For serial sections, first collect a 10 µm-thick section onto the upper left corner of each of eight positively charged slides. Advance the cryostat through subsequent 100-200 µm of tissue, and discard the tissue. Immediately transfer all sliced sections onto glass microscope slides. - Next, collect another 10 µm-thick section, next to the previously placed tissue section, for each of the eight slides. Repeat this serial collection process until each of the eight slides contains eight tissue sections, each 100-200 µm apart. Keep the tissue sections in the dark to preserve fluorescence.
NOTE: Tissue sections on slides can be stored in a slide box at -80 °C for several months.
- To maximize the chance of finding internalization-positive cells and having more representative tissue regions, collect serial cryosections at -18 to -20 °C using a cryostat.
- Fixation of tissue slides
- CRITICAL STEP: Fix the tissue sections in 95% ethanol at -18 to -20 °C to for 5 min.
- Wash the fixed section for 5 min with room temperature PBS, and then mount the fixed tumor sections under a glass coverslip using 10 µL of an aqueous mounting medium with DAPI.
- Twelve to 24 h after mounting, examine the fixed tumor sections by fluorescence microscopy and acquire images, as described for the cultured cells above.
- Fluorescence microscopy and image acquisition
- Identify regions of interest and acquire images, as described in section 2.6.
- Data quantification and analysis
- Quantify the cells and apply appropriate statistical analyses, as in section 2.7.
4. ATP internalization in tumors, in vivo
- Prepare cell cultures for implantation as described in section 3.1.
- Subcutaneous injection of cancer cells for xenograft tumor development
- Generate xenografted tumors as described in section 3.2.
- ATP and/or dextran injection into xenograft tumors
- Prepare the treatment solutions of DMEM (vehicle) or 8 mg/mL HMWFD or LMWFD, with or without NHF-ATP (100 µM) in DMEM, as described above.
- Use a 1 mL syringe to collect 50 µL of one treatment solution and inject the solution directly into each xenograft tumor. Repeat the procedure for four biological replicates of each treatment.
- Tissue harvest and cryo-embedding
- Prepare identification labels for each tumor to be harvested. Cut a 2 cm piece of laboratory tape and fold in half, adhesive sides together, lengthwise. Use a marking pen to label the tag, e.g., with a mouse/tumor identification number.
- Approximately 5 min post-injection, euthanize the mouse by cervical dislocation or according to the IACUC approved protocol.
- Using a size 10 scalpel, make an incision adjacent to the tumor and approximately perpendicular to the direction of the needle injection. Use forceps and surgical scissors to resect the tumor tissue from the surrounding tissue.
- Divide the tumor into two to four 1 cm2 pieces, depending on the total tumor size.
- Prepare the embedding molds and embed the tissue, as described above in section 3.6. Ensure that harvest time, from intratumoral dextran injection to cryo-embedding, is no more than 7-8 min.
- Preparation of slides of tissues samples
- Collect serial tumor sections, as described in section 3.7.
- Fixation of tissue slides
- Fix the tissue, as described in section 3.8.
- Fluorescence microscopy and image acquisition
- Identify the regions of interest and acquire images, as described in section 2.6.
- Data quantification and analysis
- Quantify the cells and apply appropriate statistical analyses, as described in section 2.7.
Wyniki
In vitro study
Intracellular internalization of NHF-ATP was demonstrated by co-localization of NHF-ATP with HMWFD or LMWFD (Figure 4). The success of this procedure primarily relies on the use of appropriate concentrations of NHF-ATP and dextrans and on determining the appropriate type(s) of dextrans (poly-lysine vs. neutral). For example, to investigate macropinocytosis, HMWFD was chosen as it is internalized only by macropinosomes
Dyskusje
A method was developed for spatial, temporal, and quantitative analysis of the cellular internalization of nonhydrolyzable ATP. This method is broadly applicable for use in diverse biological systems, including various tumorigenic models, for which we provide technical instruction and representative data. To acquire interpretable data during in vivo ATP internalization studies (section 4 of the protocol), it is critical to limit the experimental time elapsed from intratumoral dextran injection to cryo-embedding....
Ujawnienia
The authors declare no competing interests.
Podziękowania
Cryosectioning was performed on-site at the Ohio University Histopathology Core. This work was supported partly by start-up funds (Ohio University College of Arts & Sciences) to C Nielsen; NIH grant R15 CA242177-01 and RSAC award to X Chen.
Materiały
| Name | Company | Catalog Number | Comments |
| A549 cells, human lung epithelial, carcinoma | National Cancer Institute | n/a | Less expensive source |
| Acetone | Fisher Scientific | S25904 | |
| Aluminum foil, Reynolds | Grainger | 6CHG6 | |
| Aqueous Mounting Medium, ProLong Gold Anti-fade Reagent | ThermoFisher | P36930 | |
| ATP analog | Jena Biosciences | NK-101 | |
| Autoclave, sterilizer | Grainger | 33ZZ40 | |
| Blades, cryostat, high profile | C. L. Sturkey, Inc. | DT554550 | |
| Calipers, vernier | Grainger | 4KU77 | |
| Cell medium, Ham's Nutrient Mixture F12, serum-free | Millipore Sigma | 51651C-1000ML | |
| Centrifuge, refrigerated with swinging bucket rotor | Eppendorf | 5810R | |
| Chloroform | Acros Organics | 423555000 | |
| Conical tube, 15 mL | VWR | 21008-216 | |
| Conical tube, 50 mL | VWR | 21008-242 | |
| Coverslips, glass, 12 mm | Corning | 2975-245 | |
| Cryostat, Leica CM1950 | Leica Biosystems | CM1950 | |
| Delicate task wipe, Kim Wipes | Kimberly-Clark | 34155 | |
| Dextran, Lysine fixable, High Molecular Weight (HMW) | Invitrogen | D1818 | MW = 70,000, Tetramethylrhodamine |
| Dextran, Neutral, High Molecular Weight (HMW) | Invitrogen | D1819 | |
| Dulbecco's Modified Eagle Medium (DMEM), serum-free | Fisher Scientific | 11885076 | |
| Dry ice | Local delivery | Custom order | |
| Epifluorescent imaging system, Nikon NiU and Nikon NIS Elements acquisition software | Nikon | Custom order | |
| Ethanol | Fisher Scientific | BP2818-4 | |
| Fetal bovine serum (FBS) | ThermoFisher | 16000044 | |
| Forceps, Dumont #7, curved | Fine Science Tools | 11274-20 | |
| Forceps, Dumont #5, straight | Fine Science Tools | 11254-20 | |
| Gloves (small, medium, large) | Microflex | N191, N192, N193 | |
| Gloves, MAPA Temp-Ice 700 Thermal (for handling dry ice) | Fisher Scientific | 19-046-563 | |
| Hemocytometer | Daigger | EF16034F EA | |
| Incubator, cell culture | Eppendorf | Galaxy 170 S | |
| Labelling tape | Fisher Scientific | 159015R | |
| Marking pen, Sharpie (ultra-fine) | Staples | 642736 | |
| Mice, immunodeficient (Nu/J) | Jackson Laboratory | 2019 | |
| Microcentrifuge, accuSpin Micro17 | Fisher Scientific | 13-100-675 | |
| Microcentrifgue tubes, Eppendorf tubes (1.5 mL) | Axygen | MCT-150-C | |
| Microscope slide box | Fisher Scientific | 50-751-4983 | |
| Needle, 27 gauge | Becton-Dickinson | 752 0071 | |
| Paintbrush | Grainger | 39AL12 | |
| Paper towels | Staples | 33550 | |
| Paraformaldehyde | Acros Organics | 416785000 | |
| Penicillin/Streptomycin | Gibco | 15140122 | |
| Perforated spoon, 15 mm diameter, 135 mm length | Roboz Surgical Instrument Co. | RS-6162 | |
| Phosphate buffered saline (PBS) | Fisher Scientific | BP3991 | |
| Pipet tips (10 μL) | Fisher Scientific | 02-707-438 | |
| Pipet tips (200 μL) | Fisher Scientific | 02-707-411 | |
| Pipet tips (1000 μL) | Fisher Scientific | 02-707-403 | |
| Pipets, serological (10 mL) | VWR | 89130-910 | |
| Pippetor, Gilson P2 | Daigger | EF9930A | |
| Pipettor Starter Kit, Gilson (2-10 μL, 20-200 μL, 200-1000 μL) | Daigger | EF9931A | |
| Platform shaker - orbital, benchtop | Cole-Parmer | EW-51710-23 | |
| Positively-charged microscope slides, Superfrost | Fisher Scientific | 12-550-15 | |
| Scalpel, size 10, Surgical Design, Inc. | Fisher Scientific | 22-079-707 | |
| Scissors, surgical - sharp, curved | Fine Science Tools | 14005-12 | |
| Software for image analysis, Nikon Elements | Nikon | Custom order | |
| Software for image analysis, ImageJ (FIJI) | National Institutes of Health | n/a | Download online (free) |
| Specimen disc 30 mm (chuck holder), cryostat accessory | Leica Biosystems | 14047740044 | |
| Staining tray, 245 mm BioAssay Dish | Corning | 431111 | |
| Syringe, 1 cc | Becton-Dickinson | 309623 | |
| Tape, laboratory, 19 mm width | Fisher Scientific | 15-901-5R | |
| Timer | Fisher Scientific | 14-649-17 | |
| Tissue culture dish, 100 x 15 mm diameter | Fisher Scientific | 08-757-100D | |
| Tissue culture flask, 225 cm2 | ThermoFisher | 159933 | |
| Tissue culture plate, 24-well | Becton-Dickinson | 353226 | |
| Tissue embedding mold, stainless steel | Tissue Tek | 4161 | |
| Tissue Freezing Medium, Optimal Cutting Temperature (OCT) | Fisher Scientific | 4585 | |
| Trypsin-EDTA (ethylenediaminetetraacetic acid), 0.25% | Gibco | 25200072 | |
| Water bath, Precision GP 2S | ThermoFisher | TSGP2S |
Odniesienia
- Pavlova, N. N., Thompson, C. B. The emerging hallmarks of cancer metabolism. Cell Metabolism. 23 (1), 27-47 (2016).
- Pellegatti, P., et al. Increased level of extracellular ATP at tumor sites: in vivo imaging with plasma membrane luciferase. PLoS ONE. 3, 25992008 (2008).
- Falzoni, S., Donvito, G., Di Virgilio, F. Detecting adenosine triphosphate in the pericellular space. Interface Focus. 3 (3), 20120101 (2013).
- Michaud, M., et al. Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science. 334, 1573-1577 (2011).
- Wilhelm, K., et al. Graft-versus-host disease is enhanced by extracellular ATP activating P2X7R. Nature Medicine. 16, 1434-1438 (2010).
- Vander Heiden, M. G., Cantley, L. C., Thompson, C. B. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 324, 1029-1033 (2009).
- Cairns, R. A., Harris, I. S., Mak, T. W. Regulation of cancer cell metabolism. Nature Reviews Cancer. 11, 85-95 (2011).
- Chen, X., Qian, Y., Wu, S. The Warburg effect: evolving interpretations of an established concept. Free Radical Biology & Medicine. 79, 253-263 (2015).
- Wang, X., et al. Extracellular ATP, as an energy and phosphorylating molecule, induces different types of drug resistances in cancer cells through ATP internalization and intracellular ATP level increase. Oncotarget. 8 (5), 87860-87877 (2017).
- Patel, A., et al. ATP as a biological hydrotrope. Science. 356, 753-756 (2017).
- Qian, Y., et al. Extracellular ATP is internalized by macropinocytosis and induces intracellular ATP increase and drug resistance in cancer cells. Cancer Letters. 351, 242-251 (2014).
- Qian, Y., Wang, X., Li, Y., Cao, Y., Chen, X. Extracellular ATP a new player in cancer metabolism: NSCLC cells internalize ATP in vitro and in vivo using multiple endocytic mechanisms. Molecular Cancer Research. 14, 1087-1096 (2016).
- Commisso, C., et al. Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature. 497, 633-637 (2013).
- Li, L., et al. The effect of the size of fluorescent dextran on its endocytic pathway. Cell Biology International. 39, 531-539 (2015).
- Yanagawa, Y., Matsumoto, M., Togashi, H. Enhanced dendritic cell antigen uptake via alpha2 adrenoceptor-mediated PI3K activation following brief exposure to noradrenaline. Journal of Immunology. 185, 5762-5768 (2010).
- Hoppe, H. C., et al. Antimalarial quinolines and artemisinin inhibit endocytosis in Plasmodium falciparum. Antimicrobial Agents & Chemotherapy. 48, 2370-2378 (2004).
- Chaudry, I. H. Does ATP cross the cell plasma membrane. Yale Journal of Biology & Medicine. 55, 1-10 (1982).
- Pant, H. C., Terakawa, S., Yoshioka, T., Tasaki, I., Gainer, H. Evidence for the utilization of extracellular [gamma-32P]ATP for the phosphorylation of intracellular proteins in the squid giant axon. Biochimica et Biophysica Acta. 582, 107-114 (1979).
- Chaudry, I. H., Baue, A. E. Further evidence for ATP uptake by rat tissues. Biochimica et Biophysica Acta. 628, 336-342 (1980).
- Koppenol, W. H., Bounds, P. L., Dang, C. V. Otto Warburg's contributions to current concepts of cancer metabolism. Nature Reviews Cancer. 11, 325-337 (2011).
- Dang, C. V. Links between metabolism and cancer. Genes & Development. 26, 877-890 (2012).
- Israelsen, W. J., Vander Heiden, M. G. ATP consumption promotes cancer metabolism. Cell. 143, 669-671 (2010).
- Koster, J. C., Permutt, M. A., Nichols, C. G. Diabetes and insulin secretion: the ATP-sensitive K+ channel (K ATP) connection. Diabetes. 54, 3065-3072 (2005).
- Szendroedi, J., et al. Muscle mitochondrial ATP synthesis and glucose transport/phosphorylation in type 2 diabetes. PLoS Medicine. 4, 154 (2007).
- Miyamoto, S., et al. Mass spectrometry imaging reveals elevated glomerular ATP/AMP in diabetes/obesity and identifies sphingomyelin as a possible mediator. EBioMedicine. 7, 121-134 (2016).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaPrzeglądaj więcej artyków
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone