Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
High-throughput, Robust and Highly Time-flexible Method for Surface Sterilization of Arabidopsis Seeds
W tym Artykule
Podsumowanie
A high-throughput protocol for the surface sterilization of Arabidopsisthaliana (Arabidopsis) seeds is provided, optimizing the liquid handling steps with a simple suction device constructed with a vacuum pump. Hundreds of seed samples can be surface-sterilized in one day.
Streszczenie
Arabidopsis is by far the plant model species most widely used for functional studies. The surface sterilization of Arabidopsis seeds is a fundamental step required towards this end. Thus, it is paramount to establish high-throughput Arabidopsis seed surface sterilization methods to handle tens to hundreds of samples (e.g., transgenic lines, ecotypes, or mutants) at once. A seed surface sterilization method based on the efficient elimination of liquid in tubes with a homemade suction device constructed from a common vacuum pump is presented in this study. By dramatically reducing labor-intensive hands-on time with this method handling several hundreds of samples in one day is possible with little effort. Series time-course analyses further indicated a highly flexible time range of surface sterilization by maintaining high germination rates. This method could be easily adapted for surface sterilization of other kinds of small seeds with simple customization of the suction device according to the seed size, and the speed desired to eliminate the liquid.
Wprowadzenie
Arabidopsis is a diploid plant species belonging to the Brassicaceae family. Its relatively short life cycle (two months per generation under long-day growing conditions), small plant size, and self-pollination with the production of hundreds of seeds per plant have made it the first fundamental plant model species1,2. In addition, its genome was fully sequenced3, extensive reverse genetics tools (saturated T-DNA, transposon, and chemically mutagenized populations) are available4,5,6, and effective Agrobacterium-mediated transformation is well-established to obtain sufficient transgenic lines for further downstream work7. Thus, during the last two decades, great advances have been achieved using Arabidopsis as a model species to dissecting diverse aspects of plant biology at the molecular level, including natural, genetic and phenotypic variation8,9.
To functionally characterize genes of interest in Arabidopsis, seed surface sterilization to eliminate fungal and bacterial contaminants is the prerequisite step for many downstream protocols requiring axenic cultures. Genetic transformation for the overexpression10, knock-down (RNA-I11) or knock-out (genome editing12,13) of gene function, subcellular localization14, promoter activity15,16, protein-protein17 and protein-DNA interaction18, to cite only the most common applications, all necessitate a seed surface sterilization step. Thus, despite its relative simplicity, seed surface sterilization plays a fundamental role in many functional analyses.
So far, two major categories of seed surface sterilization methods have been developed based either on gas- or on liquid-phase sterilization19. While the throughput of gas-phase seed surface sterilization is medium to high, using the hazardous reagent chlorine gas as a surface sterilization agent has hindered its wide application. Methods based on liquid-phase sterilization, on the contrary, rely on milder chemicals like ethanol and bleach solutions for surface sterilization, and they are more widely used despite they have an inherently lower throughput than chlorine fumigation. In general, two different methods which use liquid reagents are commonly used. One largely used method is based on washing with ethanol and bleach at different concentrations for different duration of time20,21. Another method is based on the application of bleach only21,22. Both methods are mainly applied for small-scale seed surface sterilization. However, in many experiments, it is necessary to screen many Arabidopsis transgenic lines derived from one transformation15,23 or screen in parallel many transgenic lines generated from different transformations24,25. To the best of our knowledge, no liquid-based method for high-throughput seed surface sterilization has been published, which constitutes, although little-recognized, an important bottleneck for functional genomics approaches. Therefore, developing safe, robust, and high-throughput methods for seed surface sterilization is a necessary and critical step towards the success of the functional characterization of many genes at once.
To this end, in the current study, an improved method for surface sterilization of Arabidopsis seeds is presented. This method is safe, low cost, highly robust, and high-throughput, allowing handling 96 independent lines within one hour from the beginning of seed surface sterilization until the end of seed sowing in Petri dishes. The method demonstrated relies on widely available, basic laboratory instrumentation like a vacuum pump, consumable glassware, and plastic ware. This improved method provides the scientific community a safe, simple, and affordable approach to streamline seed surface sterilization with a throughput adequate to modern functional genomics approaches in Arabidopsis and other non-model plant species.
Protokół
1. Reagents and media preparation
- Prepare 70% ethanol solution: Add 737 mL of 95% technical ethanol to 263 mL of distilled water. Mix thoroughly.
NOTE: Prepare 70% ethanol solution on a non-sterile working bench.
CAUTION: Ethanol is highly flammable and can cause serious irritation to the eyes. Keep away from flames and heat sources. In case of contact with eyes, rinse with abundant water. - Prepare 5% bleach solution: Add 5 mL of household bleach (containing ~3.5% of Sodium hypochlorite, NaClO) to 95 mL of sterile distilled water. Add a few drops of non-ionic detergent (e.g., Tween 20) and mix thoroughly.
NOTE: Prepare 5% bleach solution inside the laminar hood.
CAUTION: Sodium hypochlorite, the active component of bleach, is highly irritating. It is highly corrosive and can cause serious damages to the gastrointestinal tract. In case of contact, rinse immediately with abundant water. In case of ingestion, call the poison control center or a medical physician for treatment advice. - Prepare half-strength Murashige and Skoog (½ MS) medium26.
- Add 2.2 g of MS medium powder (including vitamins) and 10 g of sucrose in 800 mL of distilled water. Adjust the pH of the solution using 1 M KOH and bring the volume up to 1 L using distilled water. Aliquot 500 mL into a 1 L bottle and add 4g of agar to prepare a solid medium. Autoclave the solution.
- After autoclaving, cool down the medium to 50-53 °C in a water bath and pour it into Petri dishes under the laminar flow hood. To prepare the selective medium, add 1000 µL/L of 50 mg/mL kanamycin stock solution (mix 500 mg of Kanamycin sulfate monohydrate in 10 mL of distilled water, filter sterilize and store at -20 °C) to the medium (50-53 °C). Mix well by swirling, and pour into Petri dishes as mentioned before.
2. Aspirator setup
NOTE: Instrument setup is summarized in Figure 1.
- Connect the vacuum pump inlet to one end of a polyethylene (PE) tube of suitable size. Connect the other end of the tube to the outlet of the two-way lid of the decantation bottle. Wrap the tubing's junction tightly with a sealing film (Table of Materials) to ensure air-tight connection.
- Connect a second PE tube to the inlet (the hole protruding to the inside of the bottle) of the screwcap on the decantation bottle. Fit the other side of the tube to the outlet of an aquarium valve. If necessary, wrap with a sealing film along the junction to eliminate air leakage.
- Just before use, fit a sterile 200 µL pipette tip to the inlet of the aquarium filter under the laminar flow hood.
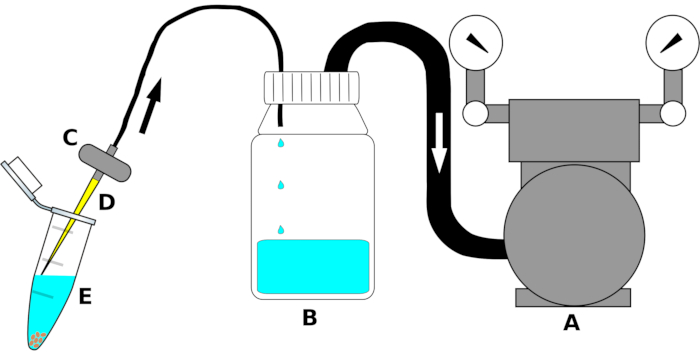
Figure 1: Schematic drawing of the suction device for high-throughput removal of sterilization liquids. For clarity, the single parts are not drawn to scale. Letter (A) indicates the vacuum pump, (B) the reservoir bottle to collect liquids (ethanol, bleach, or sterile water), (C) the valve to avoid reflux of the liquids, (D) the sterile 200 µL pipette tip, and (E) the 1.5 mL microcentrifuge tube containing seeds and sterilization liquid. Arrows indicate the direction of the airflow. Please click here to view a larger version of this figure.
3. High-throughput liquid surface sterilization of seeds
NOTE: The overall procedure and minimal time required for surface sterilization of Arabidopsis thaliana (L.) Heynh wild-type (Col-0) (Arabidopsis) seeds with 96 independent samples are summarized in Figure 2.
- Label with a permanent marker two batches of 48 x 1.5 mL microcentrifuge tubes with progressive numbers.
- Add 100-200 Arabidopsis seeds to each of the 96 sterile 1.5 mL microcentrifuge tubes (roughly 1-2 mm above the bottom of the conical end of the tube).
- Aliquot around 1000 µL of 70% ethanol into each tube using a 10 mL sterile serological pipette inside the laminar flow hood (seed batch one, 48 tubes) and carefully close the lids.
NOTE: Dispensing the solutions does not need to be extremely accurate, as long as the dispensed volume is several times higher than the volume of seeds. Alternatively, perform this step outside of the laminar hood (non-sterile condition). - Shake the tubes at an oscillation frequency of 8.0 Hz for at least 3 min in a shaker.
- Remove the adapters from the shaker and transfer them into the basket of a benchtop microcentrifuge.
- Quickly spin down the seeds using the pulse function (present in most benchtop centrifuges) to reach 1880 x g (~15 s).
NOTE: Longer time or higher centrifugation forces negatively affects seed germination. - Transfer the 48 tubes from the adapters to one rack and open all tubes under the laminar flow hood. Avoid contaminations by not touching the part of the lids fitting into the tubes. If lids are too close to each other, divide the tubes into two racks for easier handling.
- Fit a sterile 200 µL yellow tip onto the aquarium valve inlet of the homemade aspirator under the laminar flow hood and switch on the pump.
- Insert the yellow tip just above the level of the seeds to avoid touching the seeds when sucking the liquid. Alternatively, quickly position the tip at the bottom of the tube; if a seed blocks the suction of the liquid, eliminate the yellow tip and insert a new one.
- Aliquot into each tube around 1000 µL of 5% bleach using a 10 mL sterile serological pipette inside the laminar flow hood.
- Tightly close all the lids and put all the tubes back into the shaker adapters. Shake the tubes at an oscillation frequency of 8.0 Hz for at least 3 min in the shaker.
- Quickly spin down the seeds using the pulse function of the benchtop centrifuge for the time necessary to reach 1880 x g (~15 s).
- Fit a new sterile 200 µL yellow tip onto the aquarium valve connected to the vacuum pump under the laminar flow hood, and switch on the pump.
- Insert the yellow tip above the level of the seeds to avoid touching the seeds when sucking the bleach solution.
- Aliquot into each tube around 1000 µL of sterilized H2O using a 10 mL sterile serological pipette in the laminar flow hood.
NOTE: Combine the two batches of seeds to minimize the operation time. - Fit a new sterile 200 µL yellow tip onto the aquarium valve connected to the vacuum pump under the laminar flow hood and switch on the pump.
- Insert the yellow tip just above the level of the seeds to avoid touching the seeds when sucking the H2O.
- Aliquot into each tube around 500 µL of sterilized H2O using a 10 mL sterile serological pipette and close all the lids in the laminar flow hood. The seeds are ready to be sown. If needed, keep the tubes at room temperature for a few hours maximum or at 4 °C overnight.
- Fill the reservoir bottle used to collect liquid with an adequate amount of water and autoclave it. Afterward, discard the liquid into a normal sink.
NOTE: Autoclave the liquid to kill all the seeds inside the reservoir.
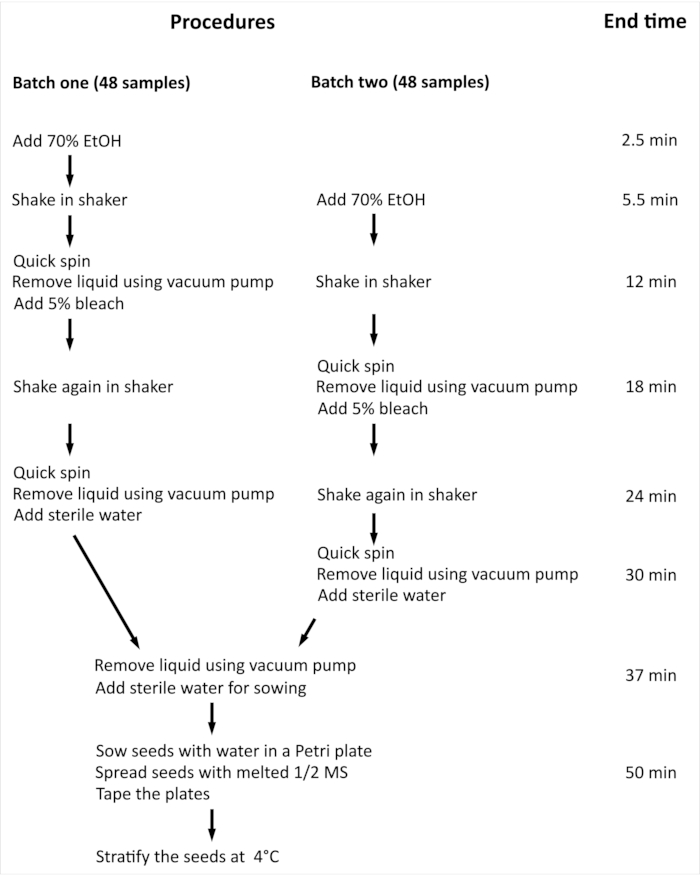
Figure 2: Overview of the procedure and minimal time required for surface sterilization of Arabidopsis seeds with 96 independent samples. In the presented experiment, 96 independent samples are handled in two equal-sized batches. The entire procedure is the same for both batches, and they are processed in parallel, but batch two is processed with one step delay compared to batch one. Please click here to view a larger version of this figure.
4. Plating and scoring of Arabidopsis on ½ MS plates
- Transfer the seeds and 300-400 µL of sterile H2O into a Petri dish by gentle pipetting with a 1000 µL pipette.
- After transferring 10 tubes, pour into each plate around 1.5-2.0 mL of melted ½ MS medium without antibiotics.
NOTE: Melt in advance and then keep the ½ MS melted medium in a thermostatic bath set at 50-53 °C to avoid solidification. Ensure that the temperature does not exceed 58 °C to avoid decreasing the germinability of the seeds. - Quickly swirl the plate to distribute the seeds inside it. Tape the plates on opposite sides.
- Wrap the plates in a plastic or aluminum foil and then place them in a fridge (4 °C) for 3 days in the dark to obtain uniform germination.
- Transfer the plates into a growth chamber set at 23 °C under long-day conditions (16 h light/8 h dark) with a light intensity of 100-120 µmol·m-2·s-1 and 60% relative humidity.
- After two days, score the plants by the presence of radicles. Detect the radicle emergence and green cotyledon formation (full opening of the two cotyledons) to evaluate seed germination.
5. Statistical analyses
NOTE: Here, Tukey's pairwise test was used for statistical analyses.
- Consider the P-values below 0.01 as statistically significant. Perform all the experiments at least with five biological replicates.
Wyniki
In order to assess the time required for the entire seed sterilization procedure, the time differences for liquid handling 96 samples in the current protocol were calculated and compared with traditional pipetting methods. The result indicates that the current protocol saves time, cutting the liquid handling time to one-fourth of that with the traditional protocols (Table 1). The table further highlights that the liquid removal time in the current protocol saves more time than that of the traditional met...
Dyskusje
Sterilization of seeds is the fundamental step for functional studies in Arabidopsis. Although it is frequently carried out for many different purposes, limited studies on high-throughput seed surface sterilization in Arabidopsis are available.
So far, one of the methods with the highest throughput is using chlorine gas generated by mixing bleach with concentrated HCl. Although this method requires limited hands-on time, it uses a gas highly toxic to human beings27. In ...
Ujawnienia
All authors declare no conflicts of interest.
Podziękowania
This research was funded by the Autonomous Province of Trento through core funding of the Ecogenomics group of Fondazione E. Mach.
Materiały
| Name | Company | Catalog Number | Comments |
| Aquarium valve | Amazon | B074CYC5SD | Kit including 2 valves and thin-walled tubings. The valve prevents the liquids to go back to the sterile tip |
| Arabidopsis Col-0 wild-type seeds | Nottingham Arabidopsis Stock Center | N1093 | Wild type seeds (sensitive to kanamycin) |
| Arabidopsis transgenic line AdoIspS-79 seeds | NA | NA | Transgenic line overexpressing an isoprene synthase gene from Arundo donax transformed in the Col-0 background, resistant to kanamycin (Li et al. (2017) Mol. Biol. Evol., 34, 2583–2599). Available on request from the authors |
| Microcentrifuge | Eppendorf | EP022628188 | Benchtop microcentrifuge used for spinning down the seeds |
| Murashige & Skoog medium including vitamins | Duchefa | M0222 | Standard medium for plant sterile culture |
| Pipette controller | Brand | 26300 | Used to operate the serological pipette |
| Polyethylene tube 1 | Roth | 9591.1 | Tube for connection from vacuum pump to decantation bottle (inner diameter: 7 mm; outer diameter: 9 mm) |
| Polyethylene tube 2 | Roth | 9587.1 | Tube for connection from decantation bottle to the aquarium valve (inner diameter: 5 mm; outer diameter: 7 mm) |
| Screw cap with connectors | Roth | PY86.1 | 2-way dispenser screw cap GL45 in polypropylene for decanting bottle |
| Serological pipette | Brand | 27823 | Graduated glass (reusable) serological pipette. Disposable pipettes can be used instead |
| Shakeret al. | Qiagen | 85300 | TissueLyser II bead mill used normally for tissue homogenization. Without the addition of beads to the tubes it works as shaker. |
| Technical ethanol | ITW Reagents (Nova Chimica Srl) | 212800 | Ethanol 96% v/v partially denatured technical grade |
| Tween 20 | Merck Millipore | 655205 | Non-ionic detergent acting as surfactant |
| Universal tubing connectors | Roth | Y523.1 | Can be used to improve/simplify tubing connections |
| Vacuum pump | Merck Millipore | WP6222050 | Used for making the suction device |
Odniesienia
- Somerville, C., Koornneef, M. A fortunate choice: The history of Arabidopsis as a model plant. Nature Reviews Genetics. 3 (11), 883-889 (2002).
- Koornneef, M., Meinke, D. The development of Arabidopsis as a model plant. Plant Journal. 61 (6), 909-921 (2010).
- Initiative, T. A. G. Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature. 408 (6814), 796-815 (2000).
- Krysan, P. J., Young, J. C., Sussman, M. R. T-DNA as an insertional mutagen in Arabidopsis. Plant Cell. 11 (12), 2283-2290 (1999).
- Speulman, E., et al. A two-component enhancer-inhibitor transposon mutagenesis system for functional analysis of the arabidopsis genome. Plant Cell. 11 (10), 1853-1866 (1999).
- Jander, G., et al. Ethylmethanesulfonate saturation mutagenesis in Arabidopsis to determine frequency of herbicide resistance. Plant Physiology. 131 (1), 139-146 (2003).
- Zhang, X., Henriques, R., Lin, S. -. S., Niu, Q. -. W., Chua, N. -. H. Agrobacterium-mediated transformation of Arabidopsis thaliana using the floral dip method. Nature Protocols. 1 (2), 641-646 (2006).
- Togninalli, M., et al. AraPheno and the AraGWAS catalog 2020: A major database update including RNA-Seq and knock-out mutation data for Arabidopsis thaliana. Nucleic Acids Research. 48 (1), 1063-1068 (2020).
- Lan, Y., et al. AtMAD: Arabidopsis thaliana multi-omics association database. Nucleic Acids Research. 49 (1), 1445-1451 (2021).
- Xu, J., Trainotti, L., Li, M., Varotto, C. Overexpression of isoprene synthase affects ABA-and drought-related gene expression and enhances tolerance to abiotic stress. International Journal of Molecular Sciences. 21 (12), 1-21 (2020).
- Czarnecki, O., et al. Simultaneous knock-down of six non-family genes using a single synthetic RNAi fragment in Arabidopsis thaliana. Plant Methods. 12 (1), 1-11 (2016).
- Yan, L., et al. high-efficiency genome editing in arabidopsis using YAO promoter-driven CRISPR/Cas9 system. Molecular Plant. 8 (12), 1820-1823 (2015).
- Liu, Y., Gao, Y., Gao, Y., Zhang, Q. Targeted deletion of floral development genes in Arabidopsis with CRISPR/Cas9 using the RNA endoribonuclease Csy4 processing system. Horticulture Research. 6 (1), (2019).
- Grefen, C., et al. Subcellular localization and in vivo interactions of the Arabidopsis thaliana ethylene receptor family members. Molecular Plant. 1 (2), 308-320 (2008).
- Gazzani, S., et al. Evolution of MIR168 paralogs in Brassicaceae. BMC Evolutionary Biology. 9 (1), (2009).
- Lee, S., Korban, S. S. Transcriptional regulation of Arabidopsis thaliana phytochelatin synthase (AtPCS1) by cadmium during early stages of plant development. Planta. 215 (4), 689-693 (2002).
- Long, Y., et al. In vivo FRET-FLIM reveals cell-type-specific protein interactions in Arabidopsis roots. Nature. 548 (7665), 97-102 (2017).
- Freire-Rios, A., et al. Architecture of DNA elements mediating ARF transcription factor binding and auxin-responsive gene expression in Arabidopsis. Proceedings of the National Academy of Sciences of the United States of America. 117 (39), 24557-24566 (2020).
- Rivero, L., et al. Handling arabidopsis plants: Growth, preservation of seeds, transformation, and genetic crosses. Methods in Molecular Biology. 1062, 3-25 (2014).
- Chen, J. H., et al. Drought and salt stress tolerance of an arabidopsis glutathione S-transferase U17 knock-out mutant are attributed to the combined effect of glutathione and abscisic acid. Plant Physiology. 158 (1), 340-351 (2012).
- Li, D. Z., et al. Comparative analysis of a large dataset indicates that internal transcribed spacer (ITS) should be incorporated into the core barcode for seed plants. Proceedings of the National Academy of Sciences of the United States of America. 108 (49), 19641-19646 (2011).
- Mathur, J., Koncz, C. Establishment and maintenance of cell suspension cultures. Arabidopsis Protocols. Methods in Molecular Biology. 82, 27-30 (1998).
- Li, M., Cappellin, L., Xu, J., Biasioli, F., Varotto, C. High-throughput screening for in planta characterization of VOC biosynthetic genes by PTR-ToF-MS. Journal of Plant Research. 133 (1), 123-131 (2020).
- Li, M., et al. In planta recapitulation of isoprene synthase evolution from ocimene synthases. Molecular Biology and Evolution. 34 (10), 2583-2599 (2017).
- Li, M., et al. Evolution of isoprene emission in Arecaceae (palms). Evolutionary Applications. 14, 902-914 (2020).
- Murashige, T., Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiologia Plantarum. 15 (3), 473-497 (1962).
- Bent, A. Arabidopsis thaliana floral dip transformation method. Methods in Molecular Biology. 343, 87-104 (2006).
- Lundberg, D. S., et al. Defining the core Arabidopsis thaliana root microbiome. Nature. 488 (7409), 86-90 (2012).
- Tkacz, A., Cheema, J., Chandra, G., Grant, A., Poole, P. S. Stability and succession of the rhizosphere microbiota depends upon plant type and soil composition. ISME Journal. 9 (11), 2349-2359 (2015).
- Singh, N., Gaddam, S. R., Singh, D., Trivedi, P. K. Regulation of arsenic stress response by ethylene biosynthesis and signaling in Arabidopsis thaliana. Environmental and Experimental Botany. 185, 104408 (2021).
- Lindsey, B. E., Rivero, L., Calhoun, C. S., Grotewold, E., Brkljacic, J. Standardized method for high-throughput sterilization of Arabidopsis seeds. Journal of Visualized Experiments: JOVE. (128), e56587 (2017).
- Acemi, A., Özen, F. Optimization of in vitro asymbiotic seed germination protocol for Serapias vomeracea. The EuroBiotech Journal. 3 (3), 143-151 (2019).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone