Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
A Reporter Based Cellular Assay for Monitoring Splicing Efficiency
W tym Artykule
Podsumowanie
This protocol describes a minigene reporter assay to monitor the impact of 5´-splice site mutations on splicing and develops suppressor U1 snRNA for the rescue of mutation-induced splicing inhibition. The reporter and suppressor U1 snRNA constructs are expressed in HeLa cells, and splicing is analyzed by primer extension or RT-PCR.
Streszczenie
During gene expression, the vital step of pre-mRNA splicing involves accurate recognition of splice sites and efficient assembly of spliceosomal complexes to join exons and remove introns prior to cytoplasmic export of the mature mRNA. Splicing efficiency can be altered by the presence of mutations at splice sites, the influence of trans-acting splicing factors, or the activity of therapeutics. Here, we describe the protocol for a cellular assay that can be applied for monitoring the splicing efficiency of any given exon. The assay uses an adaptable plasmid encoded 3-exon/2-intron minigene reporter, which can be expressed in mammalian cells by transient transfection. Post-transfection, total cellular RNA is isolated, and the efficiency of exon splicing in the reporter mRNA is determined by either primer extension or semi-quantitative reverse transcriptase-polymerase chain reaction (RT-PCR). We describe how the impact of disease associated 5′ splice-site mutations can be determined by introducing them in the reporter; and how the suppression of these mutations can be achieved by co-transfection with U1 small nuclear RNA (snRNA) construct carrying compensatory mutations in its 5′ region that basepairs with the 5′-splice sites at exon-intron junctions in pre-mRNAs. Thus, the reporter can be used for the design of therapeutic U1 particles to improve recognition of mutant 5′ splice-sites. Insertion of cis-acting regulatory sites, such as splicing enhancer or silencer sequences, into the reporter can also be used to examine the role of U1 snRNP in regulation mediated by a specific alternative splicing factor. Finally, reporter expressing cells can be incubated with small molecules to determine the effect of potential therapeutics on constitutive pre-mRNA splicing or on exons carrying mutant 5′ splice sites. Overall, the reporter assay can be applied to monitor splicing efficiency in a variety of conditions to study fundamental splicing mechanisms and splicing-associated diseases.
Wprowadzenie
Pre-mRNA splicing is an essential processing step that removes non-coding introns and precisely ligates coding exons to form mature mRNA. Recognition of consensus sequences at exon-intron junctions, referred to as 5′-splice site and 3′-splice site, by components of the splicing machinery initiates the splicing process. The U1 small nuclear ribonucleoprotein (snRNP) recognizes the 5′-splice site by base pairing of the U1 snRNA to the pre-mRNA1. Genetically inherited mutations that alter 5′-splice site sequences are associated with many diseases2,3. It is predicted that the loss of basepairing of U1 snRNA with the mutant 5′-splice sites causes aberrant splicing, which can compromise translation of the affected transcript. A potential therapeutic approach to correct the splicing defects involves suppression of mutations by modified U1 snRNA carrying compensatory nucleotide changes in its 5′-region that basepairs with the 5′-splice site. Such modified U1 snRNAs, also referred to as exon specific U1 snRNAs, have been found to be effective in reversing splicing defects, resulting in increased protein expression from the rescued mRNA4,5,6,7,8.
Here, we describe the U1 snRNP complementation assay, a reporter-based cellular splicing assay that allows assessment of the effect of 5′-ss mutations on splicing of an exon and can also be used for the development of modified U1 snRNAs to enable the rescue of exon inclusion. We also provide protocols for monitoring of the spliced reporter transcripts by primer extension and RT-PCR, and for determining the expression of modified U1 snRNAs by primer extension and RT-qPCR.
Protokół
1. Reagents and buffers
NOTE: All sterilization using vacuum filters should be performed with 0.2 µm polyethersulfone (PES) membrane in a biosafety cabinet.
- Prepare RNase-free water by adding 1.0 mL of diethylpyrocarbonate (DEPC) to 1.0 L of deionized water, mix for at least 1 hr at room temperature (RT), autoclave twice, and then cool to RT before use.
- Prepare Dulbecco's Modified Eagle Medium (DMEM) by mixing one packet of DMEM powder (13.4 g), 3.7 g of sodium bicarbonate, 100 mL of fetal bovine serum (FBS), penicillin, and streptomycin to ~800 mL of sterile deionized water. The final concentration of penicillin and streptomycin in DMEM should be 50 U/mL and 50 µg/mL, respectively. Adjust the pH to 7.4 and then make up the volume to 1.0 L with sterile deionized water. Sterilize by filtration and store at 4 °C.
- Prepare 10x phosphate buffered saline (10x PBS) by adding 25.6 g of disodium hydrogen heptahydrate (Na2HPO4·7H2O), 2 g of potassium dihydrogen phosphate (KH2PO4), 2 g of potassium chloride (KCl), and 80 g of sodium chloride (NaCl) to 800 mL of deionized water. Mix to dissolve and make up the volume to 1.0 L. Sterilize by filtration and store at 4 °C.
- Prepare 0.5 M ethylene diamine tetra-acetic acid (EDTA) by dissolving 186.1 g of Na2•EDTA•2H2O into ~800 mL of deionized water. Adjust pH to 8.0 and then make up the volume to 1.0 L with sterile deionized water. Sterilize by filtration and store at 4 °C.
- Prepare 1x trypsin-EDTA solution by mixing 100 mL of 10x trypsin (2.5%), 2 mL of 0.5 M EDTA, and add 1x PBS up to 1.0 L. Sterilize by filtration and aliquot into 50 mL conical tubes. Store at 4 °C for 1-2 weeks or freeze at -20 °C for long-term use.
- Prepare 2x formamide DNA/RNA loading dye by mixing 14.4 mL of formamide and 0.6 mL of 0.5 M EDTA for a final volume of 15 mL. Add bromophenol blue and xylene cyanol powder to a final concentration of 0.02% and store at 4 °C.
NOTE: Formamide is toxic and corrosive. Read the material safety data sheets for additional safety recommendations. - Prepare 5x Tris/Borate/EDTA (5x TBE) buffer by mixing 54.0 g of tris base, 27.5 g of boric acid, and 20 mL of 0.5 M EDTA into ~800 mL of deionized water. Mix to dissolve and make up the volume to 1.0 L with deionized water.
- Prepare urea-polyacrylamide gel electrophoresis (urea-PAGE) solution by mixing 200 mL of 5x TBE, 250 mL of 40% 19:1 bis/acrylamide, and 450.5 g of urea. Then add deionized water up to 1.0 L. Mix until the ingredients are completely dissolved, then sterilize by filtration, and store at 4 °C in an amber glass bottle.
NOTE: Bis/acrylamide is toxic. Read the material safety data sheets for additional safety procedures. - Prepare 10% ammonium persulfate (APS) by dissolving 1 g of APS in 10 mL of deionized water and store at 4 °C.
2. Cotransfection of HeLa cells with the reporter and U1 snRNA plasmids
NOTE: The transfection of Hela cells must be performed under sterile conditions in a biological safety cabinet. The outer surface of all materials must be sprayed with 70% ethanol before being introduced into the biological safety cabinet.
- Maintain Hela cells in DMEM in a 37 °C incubator with 5% CO2 by passaging every 2-3 days when the cells are about 80-90% confluent.
- For passaging HeLa cells, aspirate the spent medium and then incubate cells with 3 mL of 0.25% trypsin containing 1 mM EDTA at 37 °C for 3 min.
- After incubation, add 7 mL of fresh DMEM. Transfer the cell suspension to a 10 mL tube, centrifuge at 1,000 x g for 5 min.
- Resuspend the cell pellet in 10 mL of fresh DMEM, and then plate the cells on a new tissue culture dish at 20% confluence.
- For transient transfections, count Hela cells with a clean hemocytometer slide and prepare a suspension with a density of 2.5 x 105 cells/mL.
- Seed 1.0 mL of 2.5 x 105 cells/mL Hela cell suspension into each well of a 12-well plate and incubate overnight at 37 °C.
- The next day, aspirate the spent medium and add 0.8 mL of fresh DMEM with serum.
- Prepare Solution I by adding 0.2 µg of Dup51 or Dup51p reporter plasmid, 1.8 µg of pcDNA, pNS6U1, or pNS6U1-5a plasmid, and 100 µL of transfection medium into a new 1.5 mL microcentrifuge tube.
- Prepare a master mix of Solution II for all samples to be transfected by adding 100 µL of transfection medium and 4.0 µL of transfection reagent per sample.
- Prepare the transfection mix by adding 100 µL of Solution II into each microcentrifuge tube containing Solution I.
- Vortex the transfection mixes for 15 s, centrifuge in a tabletop microcentrifuge at 3,000 x g for 10 s at RT, and then incubate at RT for 5 min.
- Add all 200 µL of the transfection mix into one well of the 12-well HeLa cell plate to achieve a final volume of 1.0 mL per well and incubate the plate at 37 °C for 48 hrs.
- After incubation, extract RNA from the transfected HeLa cells with commercially available guanidine thiocyanate and phenol solution.
NOTE: This reagent contains phenol and this step should be performed in a fume hood. The use of DEPC treated water is recommended for resuspension of extracted RNA.- Aspirate the spent medium and add 500 µL of the reagent into each well. Incubate at RT for 5 min.
- Homogenize by pipetting up and down. Then transfer the solution to a new 1.5 mL microcentrifuge tube.
- Add 100 µL of chloroform and vortex for 15 s.
- Centrifuge at 12,000 x g for 15 min at RT.
- Transfer 200 µL of the RNA containing, top aqueous layer to a new 1.5 mL microcentrifuge tube.
- Add 2 µg of glycogen and 200 µL of isopropanol to each RNA sample. Mix by inverting the tubes.
- Collect the RNA precipitate by centrifugation at 12,000 x g for 10 min at 4 °C.
- Remove and discard the supernatant without disturbing the RNA pellet.
- Wash the pellet twice by adding 1.0 mL of 70% ethanol, inverting the tubes, and centrifuging as described in Step 2.13.7.
- Air dry the pellet for ~10-20 min at RT and resuspend the RNA in 10-20 µL of RNase-free water.
- Determine RNA concentration by measuring absorbance at 260 nm as described by Desjardins and Conklin9.
- Proceed with primer extension or store RNA samples in -20 °C. Isolated RNA can be stored at -20 °C for 6-12 months.
3. 32P-labeling of oligonucleotides
NOTE: Steps involving the use of 32P-ATP and 32P-labeled oligonucleotides must be performed behind an acrylic shield by trained individuals with approval from authorized institutional entities. The protocol described below can be used for labeling of oligonucleotides, Dup3r and U17-26-R, and markers for urea-PAGE. Use of DEPC treated water is recommended for resuspension of oligonucleotides and size exclusion beads.
- To a 1.5 mL microcentrifuge tube, add oligonucleotide, T4 polynucleotide kinase (T4 PNK), T4 PNK buffer, and 32P-ATP as described in Table 1. Add 32P-ATP last to the mixture; this is important.
NOTE: For the addition of radioactive solutions, use of filter tips is recommended. - Incubate in a water bath at 37 °C for 30 min.
- While the labeling reactions are incubating, resuspend the size exclusion beads with a 25 kDa molecular weight cut off by gently vortexing for ~10 s.
NOTE: The size exclusion beads should be prepared according to the manufacturer's instructions and stored as a 50% suspension in 25% ethanol at 4 °C. - Prepare columns by transferring 600 μL of the resuspended beads to a disposable mini column placed in a 1.5 mL collection tube and returning the bead stock to 4 °C.
- Centrifuge at 2,000 x g for 1 min at RT and discard the flow through.
- Wash the beads by adding 300 µL of RNase-free water to the column.
- Repeat steps 3.5. and 3.6. twice and transfer the mini-column to a new 1.5 mL centrifuge tube.
- Add the kinase reaction mix to the size-exclusion bead column and centrifuge at 5000 x g for 1 min at RT.
- Collect and save the flow through, which has the 32P-labeled oligonucleotide, and discard all tips and tubes into an acrylic waste box.
- Add 20 µL of RNase-free water to dilute 32P-labeled oligonucleotide to a final concentration of 2.5 µM.
NOTE: Dilute the labeled markers as needed for loading on urea-PAGE gels. - Store the labeled oligonucleotide in an acrylic microtube box at -20 °C or proceed with primer extension analysis.
4. Analysis of the spliced reporter transcripts by primer extension
NOTE: It is recommended to clean surfaces and equipment with an RNase inactivating reagent before use.
- Add 2.0 µg of RNA extracted from the Hela cells into separate 200 μL microcentrifuge tubes and add RNase-free water to make up the volume to 6.55 µL.
- Prepare Master Mix I with the diluted 32P-Dup3r and dNTPs as shown in Table 2 and add 0.9 µL of the mix to each PCR tube containing RNA samples.
- Incubate the tubes, first at 65 °C for 5 min and then on ice for 1 min.
- Prepare Master Mix II with 5x First Strand Buffer, dithiothreitol (DTT), RNase inhibitor, and reverse transcriptase as shown in Table 2.
- Add 2.55 µL of the mix to each tube containing RNA and Master Mix 1; the total volume of the reaction should be 10 µL. Keep the tubes at RT for 10 min.
- Transfer the tubes to a dry bath or a thermal cycler and incubate, first at 50 °C for 60 min and then at 70 °C for 15 min.
- After incubation, add 10 µL of 2x formamide RNA loading dye to each sample and store in an acrylic box at -20 °C or proceed with the separation of fragments by urea-PAGE using a 14-cm long gel and visualization of gel image as described below in Step 8.
- Perform densitometric scanning of the gel image with an image analysis software and use the band intensities of included and skipped products to calculate the percentage of exon 2 inclusion as shown below.

5. Analysis of the spliced reporter transcripts by fluorescent RT-PCR
NOTE: The RT-PCR analysis described below uses random hexamers for cDNA synthesis and a combination of unlabeled Dup8f and Cy5-labeled Dup3r oligonucleotides for PCR amplification of the spliced products.
- For cDNA synthesis, add 2.0 µg of RNA extracted from the transfected Hela cells into separate 200 μL microcentrifuge tubes and add RNase-free water to make up the volume to 11.0 µL.
- Prepare Master Mix I, containing random hexamers and dNTP as shown in Table 3 and add 2.0 μL of the mix to each sample. Incubate, first at 65 °C for 5 min then on ice for 1 min.
- Prepare Master Mix II, containing First Strand Buffer, RNase inhibitor, DTT, and reverse transcriptase as shown in Table 3 and add 7.0 μL of the mix to each tube containing RNA and Master Mix I.
- Keep the tubes at RT for 10 min, and then incubate at 50 °C for 60 min and 70 °C for 15 min.
NOTE: Completed cDNA reactions may be stored at -20 °C. - For PCR, transfer 1.0 μL (100 ng/μL) of each cDNA sample to new PCR tubes.
- Prepare a master mix consisting of Dup8f, Cy5-Dup3r, dNTPs, Taq buffer, Taq polymerase, and water as described in Table 4 and add 11.5 μL of the mix to each tube containing cDNA.
- Perform PCR using a thermal cycler with an initial denaturation step at 94 °C for 3 min; followed by 20 cycles of denaturation (94 °C for 30 s), annealing (65 °C for 30 s), and extension (72 °C for 15 s), and a termination step at 72 °C for 5 min.
- Add 12.5 µL of 2x formamide DNA loading dye to each tube and heat at 95 °C for 5 min.
- Store the PCR reaction at -20 °C or proceed with urea-PAGE as described below in Step 8.1-8.4.
- After electrophoresis, remove the glass plates from the electrophoresis apparatus and scan using a fluorescence imager to visualize the gel.
- Perform densitometric scanning of the gel image and use band intensity of the included and the skipped products to calculate percentage of exon 2 inclusion as described in Step 4.8.
6. Analysis of variant U1 snRNA expression by primer extension
- Add 2.0 µg of RNA extracted from the Hela cells into separate 200 μL microcentrifuge tubes and add RNase-free water to make up the volume to 4.325 µL and then add dATP, as shown in Table 5.
- Add 10,000 CPM of the 32P-U17-26-R oligonucleotide to each tube.
NOTE: To prepare a 10,000 cpm/μL solution of 32P-U17-26-R (from Step 3), dilute the labeled oligonucleotide (1:20 dilution), determine cpm in 1.0 μL using a scintillation counter, and further dilute with deionized water to prepare a solution of 10,000 cpm/μL in a new 1.5 mL microcentrifuge tube. - Incubate at 65 °C for 5 min, and then on ice for 1 min.
- Prepare a master mix with 5x First Strand Buffer, RNase inhibitor, DTT, and reverse transcriptase as shown in Table 5 and add 1.8 µL of the mix to each sample.
- Incubate, first at RT for 10 min and then at 42 °C for 10 min.
- After incubation, add 10 µL of 2x formamide RNA loading dye into each sample and store in an acrylic box at -20 °C or proceed with separation of fragments by urea-PAGE using a 38-cm long gel (see Step 8).
7. Analysis of variant U1 snRNA expression by RT-qPCR
- Dilute the cDNA stock prepared as described above in Steps 5.1 - 5.4 to a concentration of 0.2 ng/µL.
- Pipette 5.0 µL of diluted cDNA into individual wells of a 96-well qPCR plate in triplicate. Add deionized water instead of cDNA for no-template control (NTC).
- Prepare two separate primer mixes consisting of the forward and reverse primers for U1 and U2 snRNAs, and water as shown in Table 6.
NOTE: Sequences for U1 and U2 snRNA specific primers are provided in Table 7. - Add 5.0 µL of the U1 and U2 snRNA primer mix to the sample and NTC wells.
- Add 10.0 µL of real-time PCR mix to each well.
- Seal the plates with an optical film, then centrifuge at 1,000 x g for 2 min at RT to collect the reactions to the bottom of the wells.
- Perform qPCR with an initial denaturation step at 95 °C for 10 min, followed by 40 cycles of a 2-step protocol consisting of denaturation (95 °C for 15 s) and annealing/extension (62 °C for 60 s) while collecting the threshold quantification cycle (Cq) values of target amplicons.
- Finish the qPCR reaction by checking for a single peak in the dissociation curve for U1 and U2 snRNA reactions.
- From the Cq values, calculate delta Cq (ΔCq) for U1 and U2 snRNAs as compared to the pcDNA control.
- Determine variant U1 snRNA expression as relative quantity (RQ) of U1 as compared to U2 using the ΔΔCq value as shown below for all samples.

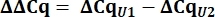
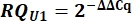
8. Setup and running of Urea-PAGE gels
NOTE: Assembly of glass plates and the gel running apparatus should be performed according to manufacturer's instructions. The casting of the 10% urea-PAGE gel can be performed according to a previously described protocol by Summer et al.10. Steps involving the preparation of markers and samples, and of running and visualization of gels are described below. Optionally, to prevent the gel from sticking to glass plates, the inner surface may be coated with silicone solution by adding 1 mL of the solution onto the surface and evenly spreading over the entire surface with tissue. Once dry, the plates should be washed with deionized water and dried again.
CAUTION: Unpolymerized acrylamide is neurotoxic and must be handled with protections recommended in the material safety data sheet.
- Prepare the primer extension samples and markers by heating at 95 °C for 5 min and then centrifuging in a tabletop microcentrifuge at 3,000 x g for 5 s at RT.
- Prior to loading the markers and samples, flush out the wells with 1x TBE buffer to remove the settled urea.
- Load 10 μL/sample/well and run the gel at 300-500V for 2-3 hrs or until the xylene cyanol reaches the bottom.
NOTE: About 1,000 cpm of the 32P-labeled marker can be loaded. - After electrophoresis, remove the glass plates from the electrophoresis apparatus.
- Carefully separate the two plates so that the gel lays flat on either glass surface and transfer the gel onto a filter paper and cover it with plastic wrap.
- Vacuum dry the gel on the filter paper at 80 °C for 30 min using a gel dryer.
- Place the dried gel in a phosphor imaging cassette and keep at RT overnight.
NOTE: The phosphor screen should be erased using a light box prior to use. - To visualize the gel image, remove the screen and scan using a phosphor imager.
Wyniki
The splicing reporter Dup51, a three exon-two intron minigene, was derived from the human β-globin gene and has been described previously (Figure 1A)11,12 . We created a mutant reporter, Dup51p, by introducing Usher syndrome associated 5´-splice site mutations that occur in exon 3 of the protocadherin 15 (PCDH15) gene13. The 5´-splice site sequence at the exon 2-intron 2 junction was ch...
Dyskusje
The assay can be adapted for splicing analysis in cell lines other than HeLa, however, factors affecting transfection efficiency, such as cell confluency and quantity of DNA may need to be optimized. The reporter to U1 construct ratio is another critical parameter that may need to be determined depending upon the expression levels observed in other cell types. The quality of extracted RNA is critical for splicing analysis; therefore, the use of RNase-free water and decontamination of surfaces with RNase inactivating agen...
Ujawnienia
The authors have nothing to disclose.
Podziękowania
This work was supported by funds to S.S. from the National Institutes of Health (R21CA170786 and R01GM127464) and the American Cancer Society (the Institutional Research Grant 74-001-34-IRG) and to S.S. and W.M. from the Valley Research Partnership Program (P1-4009 and VRP77). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Materiały
| Name | Company | Catalog Number | Comments |
| Reagent Grade Deionized Water | ThermoFisher Scientific | 23-751628 | |
| Diethyl pyrocarbonate (DEPC) | Sigma-Aldrich | D5758-25ML | |
| Dulbecco's Modified Eagle Medium (DMEM) powder packet | Gibco | 12100-046 | |
| Sodium Bicarbonate | ThermoFisher Scientific | S233-500 | |
| Fetal Bovine Serum (FBS), Australian Source, Heat Inactivated | Omega Scientific | FB-22 | |
| Penicillin-Streptomycin (P/S) | Sigma-Aldrich | P4458-100ML | |
| Sodium Hydroxide, Standard Solution 1.0N | Sigma-Aldrich | S2567-16A | |
| Hydrochloric Acid, Certified ACS Plus, 36.5 to 38.0% | ThermoFisher Scientific | A144-500 | |
| Disposable PES Bottle Top Filters | ThermoFisher Scientific | FB12566510 | |
| EDTA Disodium Salt Dihydrate | Amresco | 0105-2.5KG | |
| 2.5% Trypsin (10x), no phenol red | ThermoFisher Scientific | 15090046 | |
| Sodium Chloride | Fisher Bioreagent | BP358-212 | |
| Potassium Chloride | Fisher Bioreagent | BP366-1 | |
| Disodium Hydrogen Phosphate Heptahydrate | Fisher Bioreagent | BP332-1 | |
| Potassium Dihydrogen Phosphate | Fisher Bioreagent | BP362-1 | |
| Transfection medium - Opti-MEM™ I Reduced Serum Medium, no phenol red | ThermoFisher Scientific | 11058021 | |
| Transfection Reagent - Lipofectamine™ 2000 | ThermoFisher Scientific | 13778150 | |
| TRIzol™ Reagent | ThermoFisher Scientific | 15596018 | |
| Chloroform (Approx. 0.75% Ethanol as Preservative/Molecular Biology) | ThermoFisher Scientific | BP1145-1 | |
| Ethanol, Absolute (200 Proof), Molecular Biology Grade, Fisher BioReagents | ThermoFisher Scientific | BP2818-4 | |
| Isopropanol, Molecular Biology Grade, Fisher BioReagents | ThermoFisher Scientific | BP2618-212 | |
| Glycogen (5 mg/ml) | ThermoFisher Scientific | AM9510 | |
| Direct-zol RNA Miniprep Kit | Zymo Research | R2052 | |
| ATP, [γ-32P]- 6000Ci/mmol 150mCi/ml Lead, 1 mCi | PerkinElmer | NEG035C001MC | |
| T4 Polynucleotide Kinase | New England Biolabs | M0201L | |
| Size exclusion beands - Sephadex® G-25 | Sigma-Aldrich | G2580-10G | |
| Size exclusion mini columns | USA Scientific | 1415-0600 | |
| pBR322 DNA-MspI Digest | New England Biolabs | N3032S | |
| Low Molecular Weight Marker, 10-100 nt | Affymetrix | 76410 100 UL | |
| Rnase inactivating reagents - RNaseZAP™ | Sigma-Aldrich | R2020-250ML | |
| dNTP Mix (10 mM ea) | ThermoFisher Scientific | 18427013 | |
| RNaseOUT™ Recombinant Ribonuclease Inhibitor | ThermoFisher Scientific | 10777019 | |
| Reverse Transcriptase - M-MLV Reverse Transcriptase | ThermoFisher Scientific | 28025013 | used for primer extension |
| Taq DNA Polymerase | ThermoFisher Scientific | 10342020 | |
| Random Hexamers (50 µM) | ThermoFisher Scientific | N8080127 | |
| Real time PCR mix - SYBR™ Select Master Mix | ThermoFisher Scientific | 4472903 | |
| SuperScript™ III Reverse Transcriptase | ThermoFisher Scientific | 18080093 | used for cDNA preparation |
| Dithiothreitol (DTT) | ThermoFisher Scientific | 18080093 | |
| 5X First-Strand Buffer | ThermoFisher Scientific | 18080093 | |
| Formamide (≥99.5%) | ThermoFisher Scientific | BP228-100 | Review Material Safety Data Sheets |
| Bromophenol Blue sodium salt | Sigma-Aldrich | 114405-5G | |
| Xylene Cyanol FF | Sigma-Aldrich | 2650-17-1 | |
| Tris Base (White Crystals or Crystalline Powder/Molecular Biology) | ThermoFisher Scientific | BP152-5 | |
| Boric Acid (Crystalline/Electrophoresis) | ThermoFisher Scientific | BP168-500 | |
| Acrylamide: Bis-Acrylamide 19:1 (40% Solution/Electrophoresis) | ThermoFisher Scientific | BP1406-1 | Review Material Safety Data Sheets |
| Urea (Colorless-to-White Crystals or Crystalline Powder/Mol. Biol.) | ThermoFisher Scientific | BP169-212 | |
| Ammonium peroxodisulphate (APS) ≥98%, Pro-Pure, Proteomics Grade | VWR | M133-25G | |
| Sigmacote | Sigma-Aldrich | SL2-100ML | |
| N,N,N',N'-Tetramethylethylenediamine (TEMED) ≥99%, Ultrapure | VWR | 0761-25ML | Review Material Safety Data Sheets |
| Adjustable Slab Gel Systems, Expedeon | VWR | ASG-400 | |
| Vertical Gel Wrap™ Glass Plate Sets, 16.5 x 14.5cm | VWR | NGP-125NR | |
| Vertical Gel Wrap™ Glass Plate Sets, 16.5 x 22.0cm | VWR | NGP-200NR | |
| Vertical Gel Wrap™ Glass Plate Sets, 16.5 x 38.7cm | VWR | NGP-400NR | |
| GE Storage Phosphor Screens | Sigma-Aldrich | GE28-9564 | |
| Typhoon™ FLA 7000 Biomolecular Imager | GE Healthcare | 28-9610-73 AB | |
| Beckman Coulter LS6500 Liquid Scintillation Counter | GMI | 8043-30-1194 | |
| C1000 Touch Thermal Cycler | ThermoFisher Scientific | ||
| QuantStudio 6 Flex Real-Time PCR Systems | ThermoFisher Scientific |
Odniesienia
- Zhuang, Y., Weiner, A. M. A compensatory base change in U1 snRNA suppresses a 5' splice site mutation. Cell. 46 (6), 827-835 (1986).
- Scotti, M. M., Swanson, M. S. RNA mis-splicing in disease. Nature Review Genetics. 17 (1), 19-32 (2016).
- Ward, A. J., Cooper, T. A. The pathobiology of splicing. Journal of Pathology. 220 (2), 152-163 (2010).
- Scalet, D., et al. Disease-causing variants of the conserved +2T of 5' splice sites can be rescued by engineered U1snRNAs. Human Mutatation. 40 (1), 48-52 (2019).
- Yamazaki, N., et al. Use of modified U1 small nuclear RNA for rescue from exon 7 skipping caused by 5'-splice site mutation of human cathepsin A gene. Gene. 677, 41-48 (2018).
- Yanaizu, M., Sakai, K., Tosaki, Y., Kino, Y., Satoh, J. I. Small nuclear RNA-mediated modulation of splicing reveals a therapeutic strategy for a TREM2 mutation and its post-transcriptional regulation. Science Reports. 8 (1), 6937 (2018).
- Balestra, D., et al. Splicing mutations impairing CDKL5 expression and activity can be efficiently rescued by U1snRNA-based therapy. International Journal of Molecular Sciences. 20 (17), 20174130 (2019).
- Donadon, I., et al. Exon-specific U1 snRNAs improve ELP1 exon 20 definition and rescue ELP1 protein expression in a familial dysautonomia mouse model. Human Molecular Genetics. 27 (14), 2466-2476 (2018).
- Desjardins, P., Conklin, D. NanoDrop microvolume quantitation of nucleic acids. Journal of Visualized Experiments. (45), e2565 (2010).
- Summer, H., Gramer, R., Droge, P. Denaturing urea polyacrylamide gel electrophoresis (Urea PAGE). Journal of Visualized Experiments. (32), e1485 (2009).
- Dominski, Z., Kole, R. Selection of splice sites in pre-mRNAs with short internal exons. Molecular Cell Biology. 11 (12), 6075-6083 (1991).
- Amir-Ahmady, B., Boutz, P. L., Markovtsov, V., Phillips, M. L., Black, D. L. Exon repression by polypyrimidine tract binding protein. RNA. 11 (5), 699-716 (2005).
- Le Guedard-Mereuze, S., et al. Sequence contexts that determine the pathogenicity of base substitutions at position +3 of donor splice-sites. Human Mutation. 30 (9), 1329-1339 (2009).
- Sharma, S., Wongpalee, S. P., Vashisht, A., Wohlschlegel, J. A., Black, D. L. Stem-loop 4 of U1 snRNA is essential for splicing and interacts with the U2 snRNP-specific SF3A1 protein during spliceosome assembly. Genes and Development. 28 (22), 2518-2531 (2014).
- Steitz, J. A., et al. . Functions of the abundant U-snRNPs. Structure and function of major and minor small nuclear ribonucleoprotein particles. , 115-154 (1988).
- Fortes, P., et al. Inhibiting expression of specific genes in mammalian cells with 5' end-mutated U1 small nuclear RNAs targeted to terminal exons of pre-mRNA. Proceedings of the National Academy of Sciences U.S.A. 100 (14), 8264-8269 (2003).
- Roca, X., et al. Widespread recognition of 5' splice sites by noncanonical base-pairing to U1 snRNA involving bulged nucleotides. Genes and Development. 26 (10), 1098-1109 (2012).
- Roca, X., Krainer, A. R. Recognition of atypical 5' splice sites by shifted base-pairing to U1 snRNA. Nature Structural Molecular Biology. 16 (2), 176-182 (2009).
- Taladriz-Sender, A., Campbell, E., Burley, G. A. Splice-switching small molecules: A new therapeutic approach to modulate gene expression. Methods. 167, 134-142 (2019).
- Hamid, F. M., Makeyev, E. V. A mechanism underlying position-specific regulation of alternative splicing. Nucleic Acids Research. 45 (21), 12455-12468 (2017).
- Martelly, W., et al. Synergistic roles for human U1 snRNA stem-loops in pre-mRNA splicing. RNA Biology. , 1-18 (2021).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone