Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Microtensiometer for Confocal Microscopy Visualization of Dynamic Interfaces
W tym Artykule
Podsumowanie
This manuscript describes the design and operation of a microtensiometer/confocal microscope to do simultaneous measurements of interfacial tension and surface dilatational rheology while visualizing the interfacial morphology. This provides the real-time construction of structure-property relationships of interfaces important in technology and physiology.
Streszczenie
Adsorption of surface-active molecules to fluid-fluid interfaces is ubiquitous in nature. Characterizing these interfaces requires measuring surfactant adsorption rates, evaluating equilibrium surface tensions as a function of bulk surfactant concentration, and relating how surface tension changes with changes in the interfacial area following equilibration. Simultaneous visualization of the interface using fluorescence imaging with a high-speed confocal microscope allows the direct evaluation of structure-function relationships. In the capillary pressure microtensiometer (CPM), a hemispherical air bubble is pinned at the end of the capillary in a 1 mL volume liquid reservoir. The capillary pressure across the bubble interface is controlled via a commercial microfluidic flow controller that allows for model-based pressure, bubble curvature, or bubble area control based on the Laplace equation. Compared to previous techniques such as the Langmuir trough and pendant drop, the measurement and control precision and response time are greatly enhanced; capillary pressure variations can be applied and controlled in milliseconds. The dynamic response of the bubble interface is visualized via a second optical lens as the bubble expands and contracts. The bubble contour is fit to a circular profile to determine the bubble curvature radius, R, as well as any deviations from circularity that would invalidate the results. The Laplace equation is used to determine the dynamic surface tension of the interface. Following equilibration, small pressure oscillations can be imposed by the computer-controlled microfluidic pump to oscillate the bubble radius (frequencies of 0.001-100 cycles/min) to determine the dilatational modulus The overall dimensions of the system are sufficiently small that the microtensiometer fits under the lens of a high-speed confocal microscope allowing fluorescently tagged chemical species to be quantitatively tracked with submicron lateral resolution.
Wprowadzenie
Air-water interfaces covered by surfactant films are ubiquitous in daily life. Surfactant-water injections are used to enhance oil recovery from depleted fields and are used as hydraulic fracturing solutions for shale gas and oil. Gas-liquid foams and liquid-liquid emulsions are common to many industrial and scientific processes as lubricants and cleaning agents and are common in food. Surfactants and proteins at interfaces stabilize antibody conformations during packaging, storage, and administration1,2,3,4,5, tear film stability in the eye6,7,8, and pulmonary mechanics9,10,11,12,13,14,15.
The study of surface-active agents or surfactants adsorbing to interfaces and their properties has a long history with many different experimental techniques16,17,18,19,20,21,22,23,24,25,26,27. A recent development is the capillary pressure microtensiometer (CPM), which allows the examination of interfacial properties on highly curved interfaces, at much smaller length scales, while using significantly fewer materials than other common methods9,23,24,25. Confocal fluorescence microscopy (CFM) can be used to study the morphology of lipids and proteins at the air-water interfaces in the CPM22 or on Langmuir troughs20,26,27,28,29. Here a CPM and CFM have been combined to connect morphological phenomena to dynamic and equilibrium interfacial properties to develop structure-function relationships for biological and technological interfaces.
There are numerous parameters of importance in interfacial surfactant systems accessible to the CPM-CFM. In the CPM, a 30-200 µm diameter air bubble is pinned to the tip of a glass capillary tube. In earlier versions of the CPM, the capillary pressure difference between the inside and outside of the bubble was controlled via a water column and oscillatory syringe pump9,30 ; the new version described here replaces these with a higher precision, computer-controlled microfluidic pump. The surface tension (γ) is determined via the Laplace equation, ΔP = 2γ/R, from the pressure drop across the interface set by the pump, ΔP, and optical analysis of the radius of curvature of the bubble, R. The dynamic surface tension of the interface can be determined with 10 ms time resolution following the generation of a new bubble in contact with a bulk liquid containing a soluble surfactant. The surfactant adsorption dynamics can be described by the classic Ward-Tordai equation10,31 to determine essential properties of the surfactant, including the diffusivity, surface coverage, and the relationship between bulk concentration and equilibrium surface tension. Once an equilibrium surface tension is achieved, the interfacial area can be oscillated to measure the dilatational modulus, 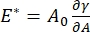 , by recording the changes in surface tension, induced by small changes in the bubble surface area, A32. For more complex interfaces that develop their own internal structures such as entangled polymers or proteins, the surface tension, , is replaced by a more general surface stress4,33,
, by recording the changes in surface tension, induced by small changes in the bubble surface area, A32. For more complex interfaces that develop their own internal structures such as entangled polymers or proteins, the surface tension, , is replaced by a more general surface stress4,33, 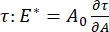 .
.
Lung stability during breathing may be directly tied to maintaining both a low surface tension and a high dilatational modulus at the alveolar air-liquid interface9,10. All internal lung surfaces are lined with a continuous, microns-thick film of epithelial lining fluid to maintain tissue hydration34. This epithelial lining fluid is primarily water, with salts and various other proteins, enzymes, sugars, and lung surfactant. As is the case for any curved liquid-vapor interface, a capillary pressure is induced with the pressure higher on the inside of the alveolus (or bubble). However, if the surface tension was constant everywhere within the lungs, the Laplace equation, ΔP = 2γ/R, shows that smaller alveoli would have a higher internal pressure relative to larger alveoli, forcing the gas contents of the smaller alveoli to flow to larger, lower pressure alveoli. This is known as "Laplace Instability"9,35. The net result is that the smallest alveoli would collapse and be filled with liquid and become difficult to re-inflate causing part of the lung to collapse, and other parts would over-inflate, both of which are typical symptoms of acute respiratory distress syndrome (ARDS). However, in a properly functioning lung, the surface tension changes dynamically as the air-epithelial fluid interface in the alveolus interfacial area expands and contracts during breathing. If 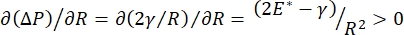 , or
, or 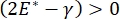 , the Laplace pressure decreases with decreasing radius and increases with increasing radius so as to eliminate the Laplace instability, thereby stabilizing the lung9. Hence,
, the Laplace pressure decreases with decreasing radius and increases with increasing radius so as to eliminate the Laplace instability, thereby stabilizing the lung9. Hence, 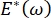 , and how it depends on frequency, monolayer morphology and composition, and alveolar fluid composition may be essential for lung stability. The CPM-CFM has also provided the first demonstrations of the effects of interfacial curvature on surfactant adsorption25, monolayer morphology22 and dilatational modulus9. The small volume (~1 mL) of the reservoir in the CPM allows for the quick introduction, removal, or exchange of the liquid phase and minimizes the required quantity of expensive proteins or surfactants10.
, and how it depends on frequency, monolayer morphology and composition, and alveolar fluid composition may be essential for lung stability. The CPM-CFM has also provided the first demonstrations of the effects of interfacial curvature on surfactant adsorption25, monolayer morphology22 and dilatational modulus9. The small volume (~1 mL) of the reservoir in the CPM allows for the quick introduction, removal, or exchange of the liquid phase and minimizes the required quantity of expensive proteins or surfactants10.
Contrast in a CPM-CFM image is due to the distribution of small fractions of fluorescently tagged lipids or proteins at the interface16,27. Two-dimensional surfactant monolayers often exhibit lateral phase separation as a function of surface tension or surface pressure, 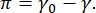 π is the difference between the surface tension of a clean fluid-fluid interface, γ0, and a surfactant-covered interface, γ. π can be thought as the 2-D "pressure" caused by the interactions of surfactant molecules at the interface that acts to lower the pure fluid surface tension. At low surface pressures, lipid monolayers are in a liquid-like disorganized state; this is known as the liquid expanded (LE) phase. As the surface pressure increases and the area per lipid molecule decreases, the lipids orient with each other and can undergo a first order phase transition to the long-range ordered liquid condensed (LC) phase16,20,27. The LE and LC phases can coexist at various surface pressures and can be visualized as fluorescently tagged lipids are excluded from the LC phase and segregate to the LE phase. Thus, the LE phase is bright and the LC phase is dark when imaged with CFM16.
π is the difference between the surface tension of a clean fluid-fluid interface, γ0, and a surfactant-covered interface, γ. π can be thought as the 2-D "pressure" caused by the interactions of surfactant molecules at the interface that acts to lower the pure fluid surface tension. At low surface pressures, lipid monolayers are in a liquid-like disorganized state; this is known as the liquid expanded (LE) phase. As the surface pressure increases and the area per lipid molecule decreases, the lipids orient with each other and can undergo a first order phase transition to the long-range ordered liquid condensed (LC) phase16,20,27. The LE and LC phases can coexist at various surface pressures and can be visualized as fluorescently tagged lipids are excluded from the LC phase and segregate to the LE phase. Thus, the LE phase is bright and the LC phase is dark when imaged with CFM16.
The goal of this manuscript is to describe the steps necessary to build and operate the combined confocal microscope microtensiometer. This will allow the reader to perform adsorption studies, measure surface tension, rheological behavior, and examine interfacial morphology simultaneously on a micron-scale air/water or oil/water interface. This includes a discussion of how to pull, cut and hydrophobize the required capillaries, instructions for using pressure, curvature, and surface area control modes, and interfacial transfer of insoluble surfactant to the microtensiometer curved interface.
Protokół
1. Preparation of capillary tubes
- Place the capillary into a capillary puller and run the desired pulling program to make two tapered capillaries with an outside diameter (OD) of ~1 µm at the tip.
NOTE: The OD of the capillary before pulling must be the OD specified to fit in the capillary holder in the microtensiometer cell. The inner diameter (ID) of the capillary can vary, but will affect the critical radius of the capillary following pulling. A pulling program is chosen so that the resulting taper initially reduces the capillary OD and ID quickly, then reaches a radius near the desired capillary OD and ID, and then reduces in diameter more slowly. This will create a greater capillary length that can be scored to yield a usable capillary of 30-100 µm in ID. - Score the tip of the capillary at the desired spot to obtain an ID of 30-100 µm and break off the tip. The capillary will now have an OD and ID of the desired radius at the tip (Figure 1A). The capillaries can be stored until step 2.
NOTE: The cut edge of the capillary must be a 90° clean break. Any defect in the cut edge will lead to bad pinning of the bubble to the capillary and poor surface property measurements. Tapered capillary tips are very delicate. They will be destroyed if they come into contact with anything other than the solutions (e.g., vial walls, air nozzle).
2. Hydrophobization of capillaries
- Gather pulled glass capillaries, acid cleaning solution, plastic tweezers, deionized (DI) water, hydrophobization solution (2% silane in ethanol), vacuum pump, and ethanol solution. See Table of Materials for details.
CAUTION: Acid cleaning solution is acutly toxic, causes skin and eye corrosion/irritation, is oxidizing. Hydrophobization solution is a skin/eye/respiratory irritant. Wear eye protection, lab coats, and gloves and work with solutions in a fume hood. - Acid-clean the capillary
NOTE: Acid-cleaning the capillary removes any organic residues inside the capillary and prepares the glass surface for the silanization reaction that renders the capillary hydrophobic.- Grab a capillary firmly near its wide end with the tweezers.
- Dip the tapered tip into the acid cleaning solution while attaching the hose from the vacuum pump to the wide end of the capillary. This will suck the solution into the capillary.
NOTE: A pipette tip may be attached to the end of the capillary hose to allow for a better fit with the capillary end. - Stop when the acid cleaning solution has filled about half of the capillary.
NOTE: After removal of the capillary tip from the acid cleaning solution, the solution on the exterior of the capillary often forms a bead near the capillary tip. Gently touch the capillary to the neck of the solution vial to remove excess solution. - Allow the acid cleaning solution to remain in the capillaries for at least 30 min, ensuring that the plug of the liquid remains at the tapered end of the capillary.
- Remove the acid cleaning solution from the capillary by firmly holding the capillary with the tweezers and using the vacuum hose to pull out the liquid from the large end of the capillary.
- Rinse the capillary
- Submerge the tapered end of the capillary into DI water ensuring it is submerged deep enough to cover any exterior that was submerged in the acid cleaning solution. While the tip is submerged, use the vacuum hose to pull DI water through the capillary. Remove the capillary from the water and remove the remaining water with the vacuum hose.
- Repeat the above step at least 4x.
- Perform step 2.3 again substituting ethanol for DI water.
- Apply suction continuously until the ethanol completely evaporates from the interior of the capillary. The capillary will become cloudy and cool to touch as the ethanol begins to evaporate but will clear after 30 to 45 s.
- Coat the capillary with the hydrophobization solution
- Briefly dip the wide end of the capillary into the ~2% silane in ethanol solution. Capillary action will cause the coating solution to rise within the capillary. Remove the capillary from the solution once a ~1 cm size plug has risen within the capillary.
- Orient the capillary so that the tapered tip faces downward, allowing the coating solution to fall with gravity toward the tapered tip.
- Allow the coating solution to remain in the capillary for at least 3 min.
NOTE: There must be no air bubbles in the plug of the coating solution that is in contact with the interior of the tapered tip. If there is an air bubble, then the capillary interior was likely not sufficiently dried in step 2.5. To remedy this, repeat steps 2.4-2.6 as needed.
- Rinse the capillaries with ethanol 1x in the same manner as step 2.3.
- Set the hydrophobic coating on the capillary
- Place clean and dry scintillation vials into a vacuum oven set to 120 °C. Place coated capillaries into the vials (ideally one capillary per vial) with wide ends resting on the base of the vial. Allow capillaries to remain in the oven for at least 6 h (overnight preferred) to achieve permanent bonding of the hydrophobic silane layer to the capillaries. The capillaries can be stored until step 4.
3. Sample preparation and storage
- Mix and store surfactant and fluorophore solutions in clean acid-washed vials to avoid contamination.
NOTE: Commercially available lipids must be of the highest purity and stored between use at - 20 °C. Old or contaminated lipids often cause results to be difficult to reproduce.
4. Setting up the microtensiometer
- Assemble the CPM cell as described in Figure 2.
- Place the large side of the capillary into the top of the CPM cell until it pushes through to the underside of the cell.
- Gently tighten the PEEK plug to secure the capillary, and then attach the tube from the microfluidic pump to the large side of the capillary. Be careful not to touch the tapered capillary tip.
- As necessary, attach the reservoir exchange and/or temperature control hoses to the respective inlets and outlets on the CPM cell (Figure 2); otherwise, plug the unused inlets and outlets.
- Attach the CPM cell to the confocal microscope stage, roughly aligning it with the CFM objective, CPM camera, and CPM light source (Figure 3).
- Open the gas flow to the microfluidic pump at the recommended operating pressure of the pump (150 mbar for the microfluidic pump used here) and ensure the flow to the capillary is open.
- Start running the CPM virtual interface (Supplemental Coding File 1: Microtensiometer Virtual Interface.vi) in Pressure Control mode with the capillary pressure oscillation frequency and amplitude set to zero (Figure 4-7). Figure 4 shows a screenshot of the virtual interface. For DI water and a capillary radius of ~35 µm, a pressure of ~20 mbar ensures that no water enters the capillary.
- Fill the CPM cell with water using a pipette.
- Focus on the capillary tip using the microtensiometer camera.
- Focus on the capillary tip with the CFM. If there is difficulty finding the capillary, use the CPM camera to find the CFM objective. This will help approximate the distance between the CFM objective and the bubble, achieving the correct working distance.
- After the annulus (green sector projection) is centered on the bubble, manually adjust the focus so that the bubble edge can be seen clearly (Figure 4-3).
NOTE: The position, start and end angle, and inner and outer radii of the annulus can be adjusted via the menu below the view window. - Click on Reset Bubble, and make sure that a new bubble is formed (one will be able to hear the old bubble pop, and the new bubble will be observable from the control panel viewing window; Figure 4-3). If the bubble does not pop, increase the Reset Pressure or increase the Reset Delay Time in the Bubble Reset tab below the viewing window. Check whether the surface tension is around 73 mN/m (for saline or water/air bubbles) (Figure 4-9).
- Take out the water via the direct-to-cell syringe (Figure 3-13), empty it, and reattach it. The sample is ready for loading to run the experiment.
5. Adsorption study
- Fill the cell with the desired sample using an autoclaved pipette keeping the CPM software in Pressure Control mode. Make sure the initial surface tension is around 73 mN/m when a new bubble interface is created.
- Determine the radius of the newly formed bubble and input that value into the centerline area control (Figure 4-7) and change the control type to area control by clicking on the Area Control tab (Figure 4-8).
NOTE: Constant pressure control can also be used, but this causes the bubble radius to change continuously as the surface tension of the interface changes. This changing area can complicate the analysis of surfactant adsorption rates and cause the bubble to pop during the study. - Start recording the confocal video.
- Click on Reset Bubble (Figure 4-5), and immediately click on Collect Data (Figure 4-6). The signaling light on the button will turn green.
- Adjust the data recording rate according to the concentration of the sample by sliding the bar shown in Figure 4-6. For slower adsorptions, use a slower recording rate. This can be adjusted in the middle of a run if a higher recording rate is desired early on, but a slower rate is preferable for long studies in order to reduce file size.
- After the end of the experiment (when a final surface tension plateau has been reached), save the file by choosing the correct file path (Figure 4-1) and clicking on the Save button (Figure 4-2).
- Stop and save the recording on the CFM as well.
6. Oscillation/relaxation study
- Fill the cell with the sample using an autoclaved pipette keeping the CPM software in Pressure Control mode. Make sure the surface tension is around 73 mN/m when a new bubble interface is created.
- Wait until the sample is fully adsorbed to the interface. This can be performed directly after an adsorption study instead of starting over with a new bubble interface.
- Decide whether oscillation will be a pressure oscillation, area oscillation, or curvature oscillation by selecting the appropriate tab (Figure 4-8) and entering the desired baseline value, oscillation% and oscillation frequency (Figure 4-7).
NOTE: Sawtooth, square, and triangular wave area oscillations are also accessible from the drop-down menu in the Other Area Oscillation tab. - Start the recording of the confocal video and click on Collect Data (Figure 4-6) on the CPM software.
- Start the oscillation. Be sure to record at least seven cycles for best results. Choose a data acquisition rate (Figure 4-6) to give an adequate number of data points for each oscillation cycle.
- If other oscillation amplitudes or frequencies are desired, change the values during the experiment.
- Save the results as in steps 5.6 and 5.7.
7. Solvent exchange study
- Fill the cell with the sample using an autoclaved pipette keeping the CPM software in pressure control mode. Make sure the surface tension is around 73 mN/m, when a new bubble interface is created.
NOTE: Adsorption and/or oscillation studies can be performed prior to the solvent exchange study. - Connect the inlet tube with the bottle of desired exchange solution (Figure 3-11) to the peristaltic pump (Figure 3-10).
- Start the recording of the video in confocal software and click on Collect Data (Figure 4-6) on the CPM software.
- Set the peristaltic pump speed. This will control the rate of fluid exchange and must be chosen based on the requirements for the experiment, i.e., how fast the solvent needs to be exchanged.
- If multiple fluids need to be exchanged, stop the peristaltic pump, and connect the inlet to another exchange solution.
- After the exchange has finished (~20 min), save the results as in step 5.6 and 5.7.
8. Insoluble surfactant adsorption
NOTE: If the surfactant to be adsorbed is not soluble in the reservoir liquid, this method can be used to transfer a monolayer from the air/water interface of the cell to the bubble surface. Many bilayer forming lipids are almost insoluble in saline solution and do not spontaneously absorb to the bubble when suspended in the reservoir solution.
- Fill the cell with the sample using an autoclaved pipette keeping the CPM software in Pressure Control mode. Make sure the surface tension is around 73 mN/m, when a new bubble interface is created.
- Deposit a monolayer of insoluble surfactant on air-water interface of the cell from a solution in a volatile organic solution. Using a syringe, deposit small droplets at the interface and allow the solvent to evaporate leaving the lipid behind as a thin film.
CAUTION: Chloroform is used as a solvent for phospholipids such as phosphatidylcholines and fatty acids. Spreading solutions are usually 0.01-0.02 mg of lipid per mL of the solvent. Chloroform is acutely toxic, can cause skin and eye irritation, and is carcinogenic. Wear appropriate eye protection, lab coat, and gloves and make the solution in a fume hood. - Decrease the surface area via the centerline pressure control (Figure 4-7) of the bubble until it is nearly flat. This prevents the bubble from popping after the surfactant has adsorbed.
- Remove the reservoir liquid from the cell via the direct-to-cell syringe until the air/water interface moves past the tip of the capillary. While a syringe pump can be used, this step can be achieved by manually using the syringe.
- Increase the reservoir liquid height to its initial level.
NOTE: After the tip is resubmerged, the bubble will be larger due to the surfactant that is now adsorbed on the interface. The monolayer will now be ready for oscillation or solvent exchange experiments.
9. Clean up
- Turn off the CFM.
- Change to the Pressure Control mode.
- Remove the sample from the cell using a pipette. Load the cell with DI water and turn up the pressure to ~50 mbar to cause bubbles to constantly escape the capillary and clean the capillary tip. Repeat this process 2x.
- Close the safety valve and turn off the CPM by clicking on the red button in the upper-left corner, turn off the light and blue pressure control panel, and close off the pressure source.
- Remove the cell from the confocal microscope stage. Rinse the cell out with ethanol and DI water. Remove the capillary tube from the CPM cell.
10. Cleaning the cell
- Disassemble the cell. Brush the inside wall with a toothbrush while rinsing under DI water. Submerge the parts in ethanol and sonicate it for ~30 min.
- Rinse all the parts with DI water a few times. Dry the parts by either blowing them with nitrogen gas or drying them inside a vacuum oven.
11. Oscillation analysis
- Run the Dilatational_Rheology_Analysis.m code (Supplemental Coding File 2), choosing the desired file saved from the CPM virtual interface. Sample data is included in the supplemental files.
- The pressure vs. time plot will appear as shown in Supplementary Figure 1. Left-click the point where the oscillation starts and left-click again where the oscillation ends. If the data contains multiple oscillations, repeat this process for all oscillations.
- When all start and end points have been left-clicked, right-click the mouse anywhere. For example, as shown in Supplementary Figure 1, one can left click at points 1, 2, 3, and 4, followed by a right-click.
NOTE: The code will calculate the dilatational modulus and phase angle and the results will be written to a new .csv file in the original file location. The results for the sample data can be seen in the code results given in the Supplemental Coding File 2. MATLAB will also generate several graphical representations of the data as shown in Supplementary Figure 2.
- When all start and end points have been left-clicked, right-click the mouse anywhere. For example, as shown in Supplementary Figure 1, one can left click at points 1, 2, 3, and 4, followed by a right-click.
Wyniki
A major source of measurement error arises from the capillaries that have defects either from the cutting process (Figure 5A,B) or the coating process (Figure 5D). Both types of defects lead to errors in determining the bubble shape and size by the optical image analysis system, leading to inaccurate surface tension values. It is important to carefully examine each new capillary after it is pulled and coated under the optical microscope before i...
Dyskusje
The combined CPM/CFM is a powerful tool for examining interfacial dynamics, equilibria, and morphology. This protocol describes the steps necessary for obtaining data with CPM/CFM.
Figure 2 shows the cell design with channels for the capillary, solvent, and heat exchange indicated. The inlet for solvent exchange should be at the bottom of the cell while the outlet should be at the top, allowing for the cell to not overflow during the exchange. In practice, the inl...
Ujawnienia
The authors have no conflicts of interest to disclose.
Podziękowania
All the confocal microscopy images were obtained using the Nikon A1RHD Multiphoton upright confocal microscope. We acknowledge the guidance and assistance of the support staff, especially Guillermo Marques, at the University Imaging Center at the University of Minnesota. This work was supported by NIH Grant HL51177. SI was supported by a Ruth L. Kirschstein NRSA Institutional Research Training Grant F32 HL151128.
Materiały
| Name | Company | Catalog Number | Comments |
| 1.5 O.D. Tygon tubing | Fischer Scientific | Tubing | |
| A1RHD Multiphoton upright confocal microscope | Nikon | Confocal Microscope | |
| Acid Cleaning Solution | Sulfuric acid and Alnochromix diluted with water 50% by volume, wait until clear befor diluting | ||
| Alnochromix | Alconox | 2510 | Mixed with sulfuric acid to package instructionand diluted to make acid cleaning solution |
| Ceramic glass cutter | Sutter Instruments | ||
| Chloroform | Sigma-Aldrich | 650471 | HPLC Plus |
| Curosurf | Chiesi | Lung Surfactant | |
| Di Water | 18.5 MΩ - cm | ||
| Ethanol | any | 200 proof used for hydrophobization, denatured used for cleaning | |
| Fiber-Lite Model 190 fiber optic illuminator | Dolan-Jenner Industries Inc. | 281900100 | Light source; other light sources should work as well |
| Flow EZ F69 mbar w/Link Module | Fluigent | LU-FEZ-0069 | Microfluidic Pump |
| Fluigent SDK VIs | Fluigent | Required for CPM virtual Interface | |
| Fluoroelastomer gaskets | Machined from 1 mm thick Viton sheet, See figure 3 | ||
| Gas filter | Norgren | F07-100-A3TG | Put between microfluidic pump and pressure regulator |
| Gas regulator | Norgren | 10R0400R | Steps down pressure from sorce to range of pump, connected to gas filter range 2-120 psi |
| Glass Capilary | Sutter Instruments | B150-86-10 | Borosilicate glass O.D. 1.5 mm I.D. 0.86 mm |
| Glass Slide | any | 75 mm x 25 mm | |
| Glass Syringe | Hamilton | 84878 | 25 μL glass syringe |
| Hydrophobizing Agent | Sigma-Aldrich | 667420 | 1H,1H,2H,2H-Perfluoro-octyltriethoxysilane 98%, other hydrophobic triethoxysilane can be substituted |
| Insoluble surfactant | Avanti | 850355C-200mg | 16:0 DPPC in chloroform |
| LabVIEW Software | National Instruments | 2017 | |
| Longpass Filter | ThorLabs | FEL0650 | 650 nm Longpass filter, wavelength must remove excitation lazer frequence |
| Lyso-PC | Avanti | 855675P | 16:0 Lyso PC 1-palmitoyl-2-hydroxy-sn-glycero-3-phosphocholine |
| Masterflex L/S variable speed analog consol pump system w/ Easy-Load II pump head | Masterflex | HV-77916-20 | Peristaltic Pump |
| MATLAB | Mathworks | R2019 | |
| Micropipette Puller P-1000 | Sutter Instruments | Capillary Puller | |
| Microtensiometer Cell and Holder | Cell machined from PEEK, holder machined from aluminum, See Figure 3 and 4 | ||
| Microtensiometer Objective | Nikon | Fluor 20x/0.50W DIC M/N2 ∞/0 WD 2.0 mm | |
| NI Vision Development Module | National Instruments | Required for CPM virtual Interface | |
| PEEK finger tight fittings | IDEX | F-120x | 10-32 Coned Ports |
| PEEK plug | IDEX | P-551 | 10-31 Coned Ports |
| pippette tips | Eppendorf | 22492225 | 100 μL - 1000 μL, Autoclaved |
| Plastic Forceps | Thermo Scientific | 6320-0010 | |
| Plastic Syringe | Fischer Scientific | 14-955-459 | 10 mL |
| Plumbing parts | Fischer Scientific | 3-way valves and other plumbing parts to connect tubing. | |
| Research Plus 1-channel 100 μL–1000 μL | Eppendorf | 3123000063 | Micro pipetter |
| Sulfuric Acid | any | Used for acid cleaning solution | |
| T Plan SLWD 20x/0.30 OFN25 WD 30 mm | Nikon | Confocal Microscope Objective | |
| Texas Red DHPE triethylammonim salt | Thermo Fischer Scientific | 1395MP | Fluorophore |
| Vaccum Pump | Gast | DOA-P704-AA |
Odniesienia
- Freer, E. M., Yim, K. S., Fuller, G. G., Radke, C. J. Interfacial rheology of globular and flexible proteins at the hexadecane/water interface: Comparison of shear and dilatation deformation. Journal of Physical Chemistry B. 108 (12), 3835-3844 (2004).
- Freer, E. M., Yim, K. S., Fuller, G. G., Radke, C. J. Shear and dilatational relaxation mechanisms of globular and flexible proteins at the hexadecane/water interface. Langmuir. 20 (23), 10159-10167 (2004).
- Kannan, A., Shieh, I. C., Fuller, G. G. Linking aggregation and interfacial properties in monoclonal antibody-surfactant formulations. Journal of Colloid and Interface Science. 550, 128-138 (2019).
- Kannan, A., Shieh, I. C., Leiske, D. L., Fuller, G. G. Monoclonal antibody interfaces: Dilatation mechanics and bubble coalescence. Langmuir. 34 (2), 630-638 (2018).
- Li, J. J., et al. Interfacial stress in the development of biologics: Fundamental understanding, current practice, and future perspective. The AAPS Journal. 21 (3), 44 (2019).
- Bhamla, M. S., Giacomin, C. E., Balemans, C., Fuller, G. G. Influence of interfacial rheology on drainage from curved surfaces. Soft Matter. 10 (36), 6917-6925 (2014).
- Fuller, G. G., Vermant, J. Complex fluid-fluid interfaces: Rheology and structure. Annual Review of Chemical and Biomolecular Engineering. 3, 519-543 (2012).
- Rosenfeld, L., et al. Structural and rheological properties of meibomian lipid. Investigative Ophthalmology & Visual Science. 54 (4), 2720-2732 (2013).
- Barman, S., Davidson, M. L., Walker, L. M., Anna, S. L., Zasadzinski, J. A. Inflammation product effects on dilatational mechanics can trigger the Laplace instability and acute respiratory distress syndrome. Soft Matter. 16 (29), 6890-6901 (2020).
- Barman, S., et al., Ramachadran, A., et al. . Recent Advances in Rheology: Theory, Biorheology, Suspension and Interfacial Rheology. , (2022).
- Alonso, C., Zasadzinski, J. A. A brief review of the relationship between monolayer viscosity, phase behavior, surface pressure and temperature using a simple monolayer viscometer. The Journal of Physical Chemistry B. 110 (44), 22185-22191 (2006).
- Alonso, C., et al. More than a monolayer: Relating lung surfactant structure and mechanics to composition. Biophysical Journal. 87 (6), 4188-4202 (2004).
- Alonso, C., Bringezu, F., Brezesinski, G., Waring, A. J., Zasadzinski, J. A. Modifying calf lung surfactant by hexadecanol. Langmuir. 21 (3), 1028-1035 (2005).
- Alonso, C., Waring, A. J., Zasadzinski, J. A. Keeping lung surfactant where it belongs: Protein regulation of two-dimensional viscosity. Biophysical Journal. 89 (1), 266-273 (2005).
- Zasadzinski, J. A., et al. Inhibition of pulmonary surfactant adsorption by serum and the mechanisms of reversal by hydrophilic polymers: Theory. Biophysical Journal. 89 (3), 1621-1629 (2005).
- McConnell, H. M. Structures and transitions in lipid monolayers at the air-water-interface. Annual Reviews of Physical Chemistry. 42, 171-195 (1991).
- McConnell, H. M., Moy, V. T. Shapes of finite two-dimensional lipid domains. Journal of Physical Chemistry. 92 (15), 4520-4525 (1988).
- Zasadzinski, J. A., Stenger, P., Shieh, I., Dhar, P. Overcoming rapid inactivation of lung surfactant: analogies between competitive adsorption and colloid stability. Biochemica et Biophysica Acta. 1798 (4), 801-828 (2010).
- Zasadzinski, J. A., Nag, K., et al. . Surfactant Progress. , (2008).
- Valtierrez-Gaytan, C., et al. Spontaneous evolution of equilibrium morphology in phospholipid-cholesterol monolayers. Science Advances. 8 (14), (2022).
- Williams, I., Zasadzinski, J. A., Squires, T. M. Interfacial rheology and direct imaging reveal domain-templated network formation in phospholipid monolayers penetrated by fibrinogen. Soft Matter. 15 (44), 9076-9084 (2019).
- Sachan, A. K., Zasadzinski, J. A. Interfacial curvature effects on the monolayer morphology and dynamics of a clinical lung surfactant. Proceedings of the National Academy of Sciences of the United States of America. 115 (2), 134-143 (2018).
- Alvarez, N. J., Anna, S. L., Saigal, T., Tilton, R. D., Walker, L. M. Intefacial dynamics and rheology of polymer grafter nanoparticles at air-water and xylene-water interfaces. Langmuir. 28 (21), 8052-8063 (2012).
- Alvarez, N. J., Vogus, D. R., Walker, L. M., Anna, S. L. Using bulk convection in a microtensiometer to approach kinetic-limited surfactant dynamics at fluid-fluid interfaces. Journal of Colloid and Interface Science. 372 (1), 183-191 (2012).
- Alvarez, N. J., Walker, L. M., Anna, S. L. Diffusion-limited adsorption to a spherical geometry: The impact of curvature and competitive time scales. Physical Review. E, Statistical, Nonlinear, and Soft Matter Physics. 82, 011604 (2010).
- Shieh, I., Waring, A. J., Zasadzinski, J. A. Visualizing the analogy between competitive adsorption and colloid stability to restore lung surfactant function. Biophysical Journal. 102 (4), 777-786 (2012).
- Shieh, I., Zasadzinski, J. A. Visualizing monolayers with a water-soluble fluorophore to quantify adsorption, desorption and the double-layer. Proceedings of the National Academy of Sciences of the United States of America. 112 (8), 826-835 (2015).
- Lipp, M. M., Lee, K. Y. C., Takamoto, D. Y., Zasadzinski, J. A., Waring, A. J. Coexistence of buckled and flat monolayers. Physical Review Letters. 81, 1650-1653 (1998).
- Lipp, M. M., Lee, K. Y. C., Waring, A., Zasadzinski, J. A. Fluorescence, polarized fluorescence, and Brewster angle microscopy of palmitic acid and lung surfactant protein B monolayers. Biophysical Journal. 72 (6), 2783-2804 (1997).
- Alvarez, N. J., Walker, L. M., Anna, S. L. A microtensiometer to probe the effect of radius of curvature on surfactant transport to a spherical interface. Langmuir. 26 (16), 13310-13319 (2010).
- Ward, A. F. H., Tordai, L. Time dependents of boundary tensions of solutions. 1. The role of diffusion in time-effects. Journal of Chemical Physics. 14, 453-461 (1946).
- Lucassen, J., Vanden Tempel, M. Dynamic measurements of dilatational properties of a liquid interface. Chemical Engineering Science. 27 (6), 1283-1291 (1972).
- Lin, G. L., et al. Interfacial dilatational deformation accelerates particle formation in monoclonal antibody solutions. Soft Matter. 12 (14), 3293-3302 (2016).
- Bastacky, J., et al. Alveolar lining layer is thin and continuous: low temperature scanning electron microscopy of rat lung. Journal of Applied Physiology. 79 (5), 1615-1628 (1995).
- Adamson, A. W., Gast, A. P. . Physical Chemistry of Surfaces, Sixth ed. , 784 (1997).
- del Rio, O. I., Kwok, D. Y., Wu, R., Alvarez, J. M., Neumann, A. W. Contact angle measurements by axisymmetric drop shape analysis and an automated polynomial fit program. Colloids and Surfaces A Physicochemical and Engineering Aspects. 143 (2-3), 197-210 (1998).
- Kanthe, A., et al. No ordinary proteins: Adsorption and molecular orientation of monoclonal antibodies. Science Advances. 7 (5), 14 (2021).
- Manikantan, H., Squires, T. M. Surfactant dynamics: hidden variables controlling fluid flows. Journal of Fluid Mechanics. 892, 115 (2020).
- Narayan, S., et al. Dilatational rheology of water-in-diesel fuel interfaces: effect of surfactant concentration and bulk-to-interface exchange. Soft Matter. 17 (18), 4751-4765 (2021).
- Meng, G. N., Paulose, J., Nelson, D. R., Manoharan, V. N. Elastic instability of a crystal growing on a curved surface. Science. 343 (6171), 634-637 (2014).
- Kotula, A. P., Anna, S. L. Insoluble layer deposition and dilatational rheology at a microscale spherical cap interface. Soft Matter. 12 (33), 7038-7055 (2016).
- Lipp, M. M., Lee, K. Y. C., Zasadzinski, J. A., Waring, A. J. Phase and morphology changes in lipid monolayers induced by SP-B protein and its amino-terminal peptide. Science. 273 (5279), 1196-1199 (1996).
- Pocivavsek, L., et al. Stress and fold localization in thin elastic membranes. Science. 320 (5878), 912-916 (2008).
- Pocivavsek, L., et al. Lateral stress relaxation and collapse in lipid monolayers. Soft Matter. 4 (10), 2019-2029 (2008).
- Kim, K., Choi, S. Q., Squires, T. M., Zasadzinski, J. A. Cholesterol nanodomains: their effect on monolayer morphology and dynamics. Proceedings of the National Academy of Sciences of the United States of America. 110 (33), 3054-3060 (2013).
- Kim, K., Choi, S. Q., Zasadzinski, J. A., Squires, T. M. Interfacial microrheology of DPPC monolayers at the air-water interface. Soft Matter. 7 (17), 7782-7789 (2011).
- Kim, K., Choi, S. Q., Zasadzinski, J. A., Squires, T. M. Nonlinear chiral rheology of phospholipid monolayers. Soft Matter. 14 (13), 2476-2483 (2018).
- Kotula, A. P., Anna, S. L. Regular perturbation analysis of small amplitude oscillatory dilatation of an interface in a capillary pressure tensiometer. Journal of Rheology. 59, 85-117 (2015).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone