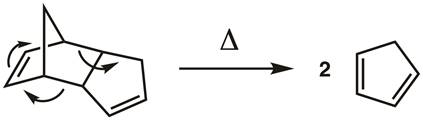Structure Of Ferrocene
Przegląd
Source: Tamara M. Powers, Department of Chemistry, Texas A&M University
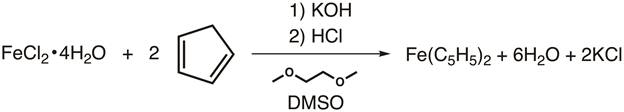
In 1951, Kealy and Pauson reported to Nature the synthesis of a new organometallic compound, ferrocene.1 In their original report, Pauson suggested a structure for ferrocene in which the iron is singly bonded (sigma bonds) to one carbon atom of each cyclopentadiene ligand (Figure 1, Structure I).1,2,3 This initial report led to wide-spread interest in the structure of ferrocene, and many leading scientists participated in the structure elucidation of this interesting new molecule. Wilkinson and Woodward were quick to suggest an alternative formulization where the iron is "sandwiched" between two cyclopentadiene ligands, with equal binding to all 10 carbon atoms (Figure 1, Structure II).4 Here, we will synthesize ferrocene and decide, based on experimental data (IR and 1H NMR), which of these structures is observed. In addition, we will study the electrochemistry of ferrocene by collecting a cyclic voltammogram. In the course of this experiment, we introduce the 18-electron rule and discuss valence electron counting for transition metal complexes.
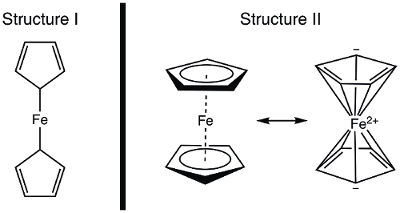
Figure 1. Two proposed structures of ferrocene.
Procedura
1. Cracking the Cyclopentadiene Dimer (Figure 3)
Cyclopentadiene undergoes a Diels-Alder reaction with itself to give dicyclopentadiene. This reaction is reversible, so cracking is accomplished using La Châtelier's principle to drive the reverse reaction by distilling the cyclopentadiene monomer (b.p. 42 °C) away from the dicyclopentadiene dimer (b.p. 170 °C). The dimerization reaction is slow when the cyclopentadiene is kept cold, but it must be
Wyniki
Ferrocene Characterization:
1H NMR (chloroform-d, 300 MHz, δ, ppm): 4.15 (s).
The 1H NMR spectrum of ferrocene clearly shows a single resonance, consistent with structure II.
A CV of ferrocene is given below. The E1/2 value obtained for the oxidation of ferrocene was +90 mV (acetonitrile, scan rate 100 mV/s, 0.1 M (Bu4N)PF6, glassy carbon working electrode). The ferrocene/ferrocenium redox couple is commonly used as a reference in cyclic voltam
Wniosek i Podsumowanie
In this video, we discussed ferrocene and the role it played in the development of organometallic chemistry. Ferrocene was synthesized and characterized by 1H NMR and IR spectroscopy. Both spectra are consistent with the 18 e− Structure II, where the iron is "sandwiched" between two cyclopentadiene ligands, with equal binding to all 10 carbon atoms (Figure 1, Structure II). Oxidation of ferrocene to ferrocenium cation was observed electrochemically.
Przejdź do...
Filmy z tej kolekcji:

Now Playing
Structure Of Ferrocene
Inorganic Chemistry
79.8K Wyświetleń

Synthesis Of A Ti(III) Metallocene Using Schlenk Line Technique
Inorganic Chemistry
31.6K Wyświetleń

Glovebox and Impurity Sensors
Inorganic Chemistry
18.7K Wyświetleń

Purification of Ferrocene by Sublimation
Inorganic Chemistry
54.7K Wyświetleń

The Evans Method
Inorganic Chemistry
68.7K Wyświetleń

Single Crystal and Powder X-ray Diffraction
Inorganic Chemistry
105.1K Wyświetleń

Electron Paramagnetic Resonance (EPR) Spectroscopy
Inorganic Chemistry
25.6K Wyświetleń

Mössbauer Spectroscopy
Inorganic Chemistry
22.0K Wyświetleń

Lewis Acid-Base Interaction in Ph3P-BH3
Inorganic Chemistry
39.0K Wyświetleń

Application of Group Theory to IR Spectroscopy
Inorganic Chemistry
45.9K Wyświetleń

Molecular Orbital (MO) Theory
Inorganic Chemistry
35.5K Wyświetleń

Quadruply Metal-Metal Bonded Paddlewheels
Inorganic Chemistry
15.3K Wyświetleń

Dye-sensitized Solar Cells
Inorganic Chemistry
16.0K Wyświetleń

Synthesis of an Oxygen-Carrying Cobalt(II) Complex
Inorganic Chemistry
51.8K Wyświetleń

Photochemical Initiation Of Radical Polymerization Reactions
Inorganic Chemistry
17.1K Wyświetleń
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone