AC-DC Electropenetrography for the Study of Probing and Ingestion Behaviors of Culex tarsalis Mosquitoes on Human Hands
In This Article
Summary
Procedures are described for studying the probing and ingestion behaviors of Culex tarsalis mosquitoes on human hands using AC-DC electropenetrography.
Abstract
Mosquitoes transmit pathogens that negatively affect human and animal health. A greater understanding of their blood-feeding biology and interactions with hosts and pathogens could be exploited to develop new targets for controlling mosquito-borne diseases. Unfortunately, probing (i.e., biting) behaviors of mosquitoes are poorly understood because they occur inside host tissues. Here, a non-invasive procedure is described for using AC-DC electropenetrography (EPG) to indirectly visualize and quantify mosquito feeding behaviors by recording changes in electrical signals generated when probing and ingesting on human hands. Thin gold wires are attached to the mosquitoes using conductive silver glue and connected to the EPG instrument. The human host holds a substrate voltage probe in their hand. Probing of the host by the wired mosquito completes the electrical circuit, and electrical signals are recorded on a computer as "waveforms" that can then be measured and enumerated for analysis. This methodology has been used to characterize the probing and ingestion behaviors of Aedes aegypti and Culex tarsalis mosquitoes and can be applied to other mosquito species. EPG can be used to study the effects of pathogens, insecticides, and other factors on mosquito feeding behaviors.
Introduction
Mosquitoes cause suffering and economic loss worldwide due to their roles as disease vectors and nuisance pests1. Anopheles mosquitoes transmit malaria, which annually causes an estimated 219 million cases globally, more than 400,000 deaths2, and 12 billion US dollars in direct costs3. Aedes mosquitoes are the most notable vectors of public health importance because they have a nearly global presence and transmit agents of Chikungunya, dengue fever, lymphatic filariasis, Rift Valley Fever, yellow fever, and Zika, which claim lives, overwhelm health systems, and result in chronic suffering, life-long morbidity, and disability4,5. In addition, Culex mosquitoes transmit agents of equine encephalitis, Japanese encephalitis, lymphatic filariasis, Saint Louis encephalitis, and West Nile fever, which impact both human and animal health3,4.
Vector control with insecticide-based approaches is the leading method for controlling vector-borne diseases globally6,7. However, the number of insecticides available for mosquito control is limited. In addition, insecticide resistance is evolving in many species, especially in Aedes mosquitoes, impacting our ability to combat vector-borne diseases and driving the need for new control strategies6,8. A better understanding of mosquito biology, mosquito-host-pathogen interactions, and the processes that regulate pathogen transmission can facilitate the development of new tools for vector control6.
Probing and ingestion behaviors in host tissues can affect pathogen transmission and disease pathology9,10,11,12. For instance, more microfilariae were ingested when Aedes aegypti fed directly from capillaries rather than pooled blood outside ruptured vessels13. Thus, fundamental and applied research on this topic can aid in developing, refining, and evaluating mosquito control strategies and other countermeasures for pathogen transmission. However, significant knowledge gaps exist in the details of cryptic blood-feeding behaviors that occur below the surface of the host's skin because these behaviors often require invasive procedures on live animal models or employ transparent artificial substrates or liquid diets9,11,14,15. Non-invasive electropenetrography (EPG; also called electrical penetration graph) techniques have been used to study feeding behaviors of plant-feeding insects related to plant pathogen transmission, host plant resistance, and insecticide modes of action because they allow researchers to indirectly observe, record, and quantify mouthpart movements and behaviors that occur inside host tissues10,12.
Since the 1950s, electronic penetration monitoring techniques have been applied to blood-feeding arthropods, including mosquitoes16,17,18,19,20. However, EPG was never widely adopted for blood-feeding arthropods because biological activities corresponding to the waveforms were never verified21. In addition, the designs and electronics of early instruments resulted in over-emphasis of some electrical signals, high noise-to-signal ratios, low resolution and distortion of waveforms, and irritation to vertebrate hosts12,22. A more recent instrument, the AC-DC EPG monitor23, has variable settings and improved signal processing, making it better suited to record blood-feeding arthropods and decipher the biological origins of the waveforms in experimental correlation studies10,12.
For EPG, an electrical signal is applied to the host through a substrate voltage probe (i.e., reference electrode). The arthropod is attached to a recording electrode and placed on the host. When the arthropod inserts its mouthparts (i.e., stylets) into the host, the electrical signal is conveyed to the AC-DC EPG monitor for signal processing. The electrical signals are then displayed on a computer as "waveforms" corresponding to specific behaviors such as mouthpart movements, ingestion, salivation, and pathogen acquisition/inoculation10,12. Sequential and nonsequential response variables related to the duration and count of the waveforms24,25 at different hierarchical levels (families, types, and subtypes) can then be calculated based on the frequency and duration of the waveforms to gain insight into the feeding behaviors that occur within and between species, and in response to biotic and abiotic factors, such as hosts, pathogens, pesticides, and environmental conditions10,12.
So far, the AC-DC EPG monitor has been used to characterize the probing and ingestion behaviors of Ae. aegypti26 and Culex tarsalis27 mosquitoes feeding on human hands, and Dermacentor variabilis and Amblyomma americanum ticks feeding on bovine calves28. In addition, a murine model for EPG29 was recently developed to facilitate pathogen studies using this instrument. Human hands are ideal for the initial mosquito waveform characterization because human hosts can report when they feel the mosquito probing as well as other behaviors, making the correlation of waveforms with behaviors easier (Table 1). In addition, a better understanding of the plasticity in feeding behaviors among mosquitos in different human populations could contribute to the development of more specialized management interventions for mosquitoes and the pathogens they transmit.
Table 1: Description of waveform families used to classify electrical signals recorded from Culex tarsalis on human hands using AC-DC EPG. The probing phase and biological activities corresponding to each waveform family. See Cooper et al.27 for more information. Abbreviation: AC = alternating current, DC = direct current, EPG = electropenetrograph. Please click here to download this Table.
To facilitate the use of EPG to study mosquito biology and host-vector interactions, methodology for quantifying the feeding behaviors of Cx. tarsalis mosquitoes on human hands using the AC-DC EPG monitor are presented here. These protocols build upon the fundamental AC-DC EPG procedures described in online resources30 and assume that users have a basic working knowledge of AC-DC EPG and associated software31. The procedures outlined can be modified for other mosquito and host species and provide a foundation to facilitate hypothesis-driven research of mosquito feeding behaviors.
Table 2: Definitions of common AC-DC EPG terminology. Definitions are based on those given in online resources30,31 and scientific literature32. Abbreviation: AC = alternating current, DC = direct current, EPG = electropenetrograph. Please click here to download this Table.
Protocol
This protocol (IRB-10729) was approved by the Institutional Review Board (IRB) for Human Research at Kansas State University27 and is intended for use with pathogen-free mosquitoes only. Human volunteers gave their informed consent to participate in the study and publish their data. Before initiating any work with animals, humans, or biohazards, ensure all procedures have been reviewed and approved by the appropriate institutional regulatory committees or oversight individuals. See the Table of Materials for details about all equipment and materials used in this protocol.
1. Initial preparation and setup
- Set up the AC-DC EPG monitor (Figure 1A), Faraday cage (Figure 1B,C), and software as previously described30,31 using the modifications described below (steps 1.1.1-1.1.5). Refer to online resources30 for step-by-step tutorials on setting up and using the equipment and software. Save the desired setup as default in the hardware management and waveform reviewing software. Refer to Table 2 for a list of common AC-DC EPG terminology and their definitions.
- Connect a head stage amplifier (head amp) and a substrate voltage probe to Channel 1 of the control box.
NOTE: Recording from a single channel is presented even though the AC-DC monitor can simultaneously accommodate four insects and hosts. - Ensure that Channel 1 on the control box is switched on and the corresponding Pre-1 and Post-1 channels on the switch interface box are switched on.
NOTE: Channels not being used must remain switched off. - Use the hardware management software to enable Channels 1 and 3 on the DI-710 A/D Board (but not others) to record the pre and post rectified signals.
- Format the hardware management software to view/overlap the pre- and post-rectified waveforms (e.g., 1 = 1 for the pre-signal and 2 = 3 for the post-signal).
- Set the sampling rate on the hardware management software to 100 samples per second per channel (i.e., S/s/CHAN: 100).
- Connect a head stage amplifier (head amp) and a substrate voltage probe to Channel 1 of the control box.
- Construct insect stubs26,27,29,30:
- Preheat the soldering iron to the recommended temperature for the specific rosin core solder.
- Use a wire cutter-stripper tool to strip the plastic coating ~6 cm from the end of a 20 AWG copper wire roll, but avoid severing or marking the copper wire bundle inside (Figure 2A). Then, cut off the exposed copper wire bundle and unravel into individual strands (Figure 2B).
- Use needle-nosed pliers to wrap one strand of the copper wire around the shaft of a suitable escutcheon pin underneath the head (i.e., flat top) so that most of the copper wire extends past the head of the pin. Fold the wire's tail over the pin's head to secure the wire in place (Figure 2C).
NOTE: Fit test escutcheon pins to identify ones that fit snuggly into the port of the head amp (when it is turned off, unplugged, and set to 106 Ohms (Ω)). - Clasp the shaft of the escutcheon pin in the helping hands soldering aid so the copper wire points upward. Then, apply a small dab (~0.2 cm ball) of water-soluble flux to the junction of the escutcheon pin and copper wire (Figure 2D).
CAUTION: Flux is toxic and must be handled with care. - Uncoil ~10 cm of solder and straighten it out. Hold the hot soldering iron against the head of the pin for a few seconds until the flux melts and the metal is hot. Then, press the tip of the solder to the head of the pin next to the soldering iron until the solder melts and completely coats the junction, fusing the pin and wire into an insect stub.
- While the insect stub cools, clean the tip of the soldering iron with steel wool and a damp sponge.
- Once cool, remove the insect stub from the helping hands. Rinse the insect stub in 70% ethanol and then water to remove residual flux. Trim the copper wire to the desired length (~3 cm, Figure 2E).
- Repeat steps 1.2.3-1.2.7 until 20-30 insect stubs are constructed.
- Assemble an insect aspirator26,27:
- Pass the tip of a glass Pasteur pipette through a flame, rotating the pipette slightly. Repeat until the end of the pipette is the appropriate size to fit snuggly around the insect's abdomen.
- Cover the large end of the modified Pasteur pipette with mosquito netting, then insert it directly into the silicone tubing connected to a laboratory aspirator and secure the junction with electrical tape to form an insect aspirator (Figure 2H).
NOTE: The Pasteur pipette and netting can be inserted into a short piece of plastic tubing fitted with a quick disconnector for easy switching between different sizes (Figure 2H).
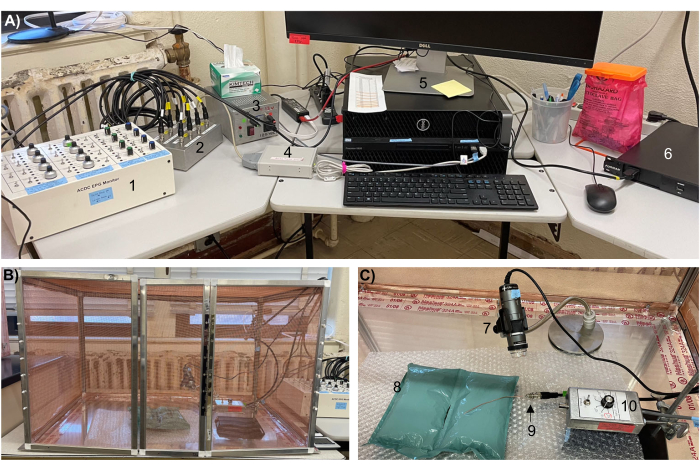
Figure 1: The AC-DC EPG monitor equipment setup for recording on human hands. (A) Components of the EPG, including the (1) control box, (2) switch interface box, (3) power supply, (4) DI-710 A/D Board, (5) computer, (6) power regulator, and (B) Faraday cage containing (C) a digital microscope (7) on a gooseneck stand, (8) bubble wrap and air pillows, (9) substrate voltage probe, and (10) head stage amplifier on a laboratory stand. Abbreviations: AC = alternating current, DC = direct current, EPG = electropenetrograph. Please click here to view a larger version of this figure.
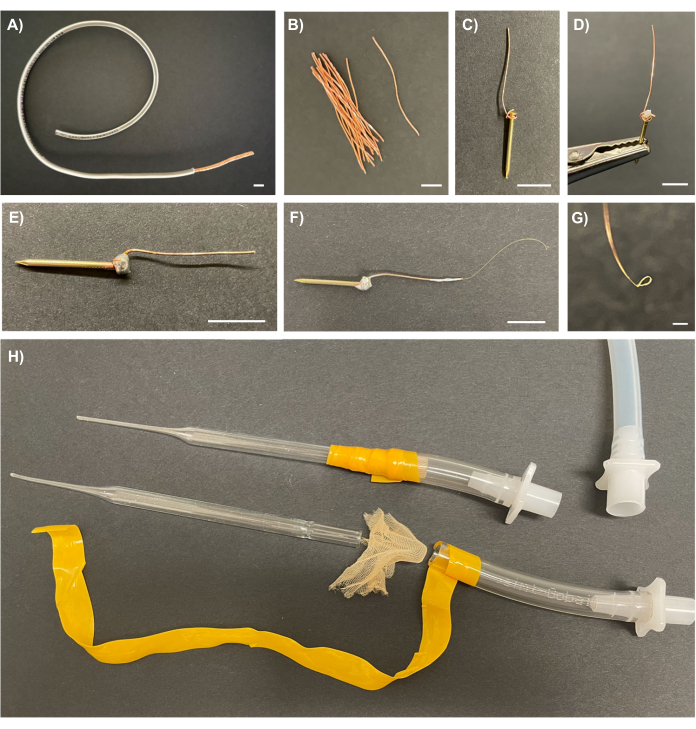
Figure 2: Recording electrodes for wiring mosquitoes and an insect aspirator for manipulating mosquitoes during wiring. (A) 20 AWG copper wire with 6 cm of plastic coating removed to expose the copper wire bundle inside. (B) Individual strands of the copper wire bundle. (C) One strand of copper wire wrapped around the head of a brass escutcheon pin with the wire's tail folded over the pin's head. (D) A dab of flux on the junction of the brass escutcheon pin and copper wire held by a helping hands soldering aid in preparation for soldering. (E) A completed insect stub constructed from a brass nail soldered to a copper wire. (F) A recording electrode constructed from a gold wire wrapped around the tip of the copper wire of an insect stub and secured in place with silver glue. (G) A close-up of the gold loop at the tip of the recording electrode. (H) An assembled (top) and disassembled (bottom) insect aspirator made from modified Pasteur pipettes, mosquito netting, plastic tubing, electrical tape, and male quick disconnectors, next to the laboratory aspirator tube fitted with the female quick disconnector (top right). Scale bars = 1 cm (A-F), 200 µm (G). Please click here to view a larger version of this figure.
2. Wiring of mosquitoes
- Prepare mosquitoes and silver glue (the afternoon/evening before EPG):
- Rear and maintain Cx. tarsalis KNWR strain mosquitoes as previously described27.
- Fast adult female mosquitoes, 5-17 days post-eclosion, for 8-12 h to enhance probing rates but allow access to water to avoid mortality.
- Prepare fresh silver glue26,27,28,29,30:
- Add 510 mg of 4-8 µm silver flake to a 20 mL beaker. Tare the balance and add 1,100 mg of non-toxic school glue to the beaker on top of the silver flake.
- Tare the balance, add 510 mg of 8-10 µm silver flake to the beaker on top of the glue, and then add 1,000 µL of water using a pipette.
- Gently add a stir bar to the beaker and place it on the stir plate. Begin stirring slowly, gradually turn up to the 'fast' setting, and stir for 4 min. Ensure all contents are incorporated.
- Use a transfer pipette (with the tip cut off) to transfer the silver glue to 1.5 mL microcentrifuge tubes.
- Wrap the tops of the silver glue tubes with Parafilm and store on the benchtop until use.
NOTE: Silver glue has the correct consistency for up to 2 days, but making fresh glue the evening before use is recommended30.
- Prepare recording electrodes26,27,29,30:
- Vigorously vortex the silver glue before use.
- Wrap a foam block with fresh plastic wrap to hold the prepared insect stubs and wired mosquitoes.
NOTE: The foam must be large enough to allow the wired mosquitoes to stand on the surface without touching each other but small enough to fit inside a storage box. CAUTION: Excretory droplets released after blood feeding may contain pathogens. Discard the plastic wrap/foam block appropriately after use. - Remove tarnish (and old silver glue if reusing insect stubs) from the tip of the copper wire by scraping the top 1 cm of the copper wire with a razor blade until shiny on all sides. Prepare one insect stub for each mosquito, plus a few extras.
- Cut 0.025 mm diameter gold wire into 4-5 cm long pieces. Always hold the wire in self-closing forceps masked with tape to avoid denting the wire.
NOTE: Gold wire is much easier to see against a black background. - Place the end of the gold wire along the clean tip of the copper wire of the insect stub and gently wrap the gold wire 3x around the copper wire.
- Use a dissection probe to apply silver glue to hold the gold wire in place. Ensure there is no tail or loose end. Place the insect stub in the foam until the glue dries.
- Gently stroke the gold wire attached to the insect stub to smooth and shape it into a long, gentle arc (Figure 2F). Trim the wire to the desired length (~2.5-3 cm).
- Working under a dissection microscope, use fine-point forceps to make a gold loop at the tip of the gold wire, and bend the loop at a 90° angle as described below (steps 2.2.8.1-2.2.8.4; Figure 2E,F). Ensure the gold loop is fully closed. Return the insect stub to the foam block.
- Using the non-dominant hand, hold the insect stub by the brass nail, with the gold wire tip touching the microscope stage. Using the dominant hand, grasp the very end of the gold wire with fine-point forceps.
- Using the fine-point forceps, cross the tip of the gold wire over the copper wire of the insect stub near where the gold wire is glued on. Touch the end of the fine-point forceps and gold wire down against the microscope stage.
- Gently pull on the insect stub until the gold wire forms a small gold loop around the tip of the fine point forceps (do not pull too tight). Release the gold wire and carefully remove the forceps.
- While still holding the insect stub in the non-dominant hand, grasp the gold loop with the fine point forceps so that the forceps are at a perpendicular angle to the neck of the wire and the tip of the forceps span the middle of the gold loop, thus grasping both sides of the loop. Gently pull on the insect stub while lightly rotating the forceps away from the insect stub until the loop is bent at a 90° angle to the neck of the wire (Figure 2G).
- Attach recording electrodes to mosquitoes26,27,29,30 (the morning of EPG):
- Anesthetize fasted mosquitoes with CO2 gas until all movement stops. Position the mosquitoes on their backs on the anesthesia pad, with their abdomens facing the researcher.
- Place a ~2 cm piece of laboratory tape under a dissection microscope, sticky side down. Place a 0.5 cm drop (~ 10 µL) of silver glue on the tape.
- Aspirate the abdomen of an anesthetized mosquito into the modified Pasteur pipette using the suction only long enough to seat the abdomen in place. Hold the mosquito under the dissecting scope above the silver glue using the aspirator.
NOTE: The wings can be inside or outside the tip of the modified Pasteur pipette, but having them inside will help prevent the mosquito from falling out. Avoid bending or damaging the wings. - Working quickly, dip a minutien pin in the silver glue and apply a thin layer to the mosquito's pronotum (i.e., the dorsal surface of the thorax). Immediately proceed to the next step if possible (or re-anesthetize the mosquito if needed).
NOTE: It is recommended to remove scales on the pronotum by rubbing the pin back and forth to help ensure glue penetration of pores in the cuticle. Avoid getting glue on any other part of the mosquito. - Quickly dip the gold loop at the tip of the recording electrode in the silver glue and place it on the silver glue spot on the mosquito's pronotum. Rock the loop back and forth to ensure that it sits flush against the pronotum. Hold the wire until the glue dries (~20-25 s).
- Replace the insect stub in the foam at an angle that allows the wired mosquito to stand comfortably when it wakes up. Proceed with wiring the next mosquito.
NOTE: It is advisable to take notes about the quality of each wiring job. - Once the desired number of mosquitoes is wired, place the foam block in a storage box. Maintain the mosquitoes under normal rearing conditions until use.
3. EPG setup and preparation (the morning before EPG)
- Turn on the AC-DC EPG monitor (by flipping the switch on the power supply) at least 30 min prior to recording to allow for instrument equilibration and any troubleshooting.
NOTE: Channels in use on the control box and switch interface box can be left switched on. - Turn on the computer and open the hardware management software.
NOTE: If the signals do not appear as flat thin lines, troubleshooting is required (see discussion section). - Perform the touch test27,30 to verify equipment functionality:
- While grounded (i.e., touching the Faraday cage with bare skin), turn the dial on the head amp to 106 Ω and insert the shaft of an insect stub into the port.
NOTE: Conducting the touch test at a higher input resistance (Ri) level may damage the head amp. - While grounded, switch the head amp on and firmly grasp the copper wire of the insect stub between the index finger and thumb three times. If the EPG is working correctly, three large peaks will appear in both the pre- and post-signals on the screen. If this is not the case, troubleshooting is required (see discussion section).
- While grounded (i.e., touching the Faraday cage with bare skin), turn the dial on the head amp to 106 Ω and insert the shaft of an insect stub into the port.
- Adjust the instrument settings to 150 mV alternating current (AC) with an Ri of 107 Ω, a hardware gain of 20-40x, a negative offset, a native baseline, and a software gain of 8-16x27,30, as described below (steps 3.4.1-3.4.3).
- Set the voltage, current type, hardware gain (range, gain, and multiplier), offset, and baseline (zero) using the switches and dials on Channel 1 of the control box.
NOTE: The appropriate hardware gain setting depends on the quality of the wiring job and silver glue, so variation in optimal gain settings from mosquito to mosquito and day-to-day is expected. The hardware gain may need to be adjusted mid-recording if the signal peaks out (defined in Table 2 and shown in Figure 3A,C) or is too small to visualize. Any hardware gain changes will permanently affect the recording (shown in Figure 3A,C). - Set the Ri level using the dial on the head amp.
- Set and adjust the software gain using the hardware management software (e.g., select the pre or post signal and then left-click to raise the software gain or right-click to lower the software gain).
NOTE: Any gain changes made in the software will NOT permanently affect the recording.
- Set the voltage, current type, hardware gain (range, gain, and multiplier), offset, and baseline (zero) using the switches and dials on Channel 1 of the control box.
- Set up a new recording in the hardware management software, but do not start recording until step 4.3 in the next section.
4. EPG on human hands26,27 :
- After informed consent is obtained (as specified by the home institution's IRB), have the host sit beside the Faraday cage with one arm inside while grasping the substrate voltage probe and holding it below the head amp.
NOTE: The arm must rest comfortably on bubble wrap or a similar non-conductive substrate. The hand and substate voltage probe must not contact the Faraday cage or head amp while recording. To avoid damage, it is imperative that the volunteer does not touch the head amp once it is turned on. If the head amp is touched accidentally, perform the touch test described above to assess its functionality. - Once the host is in position, turn the head amp to the side. Remove a wired mosquito from the storage box and connect it to the instrument by inserting the brass pin of the insect stub into the port on the head amp. Leave the mosquito dangling.
NOTE: Keep a spray bottle of 70% ethanol nearby to control any mosquitoes that detach from the wire. - Start the recording using the hardware management software. Position the head amp above the hand so that the mosquito can comfortably stand and probe.
- Switch the head amp on while grounded. Start a 10 min timer. Adjust the host hand position as needed to assist in probing success.
NOTE: When using mosquitoes inclined to probe immediately, turn the head amp on before positioning the mosquito on the hand. A thin wooden dowl or nonconductive paintbrush handle can be used to bend the copper wire of the insect stub to further adjust the position of the mosquito. A magnifying glass or digital microscope on a gooseneck stand can aid in observing the mosquito during EPG, especially for behavioral correlation studies. - Use the hardware management software to make timestamped observations (e.g., event markers) during the recording regarding host responses, mosquito behaviors, gain changes, etc.). Adjust the position of the signals on the screen and the software gain as needed to keep both signals clearly visible on the screen. Adjust the hardware gain if the signal(s) peaks out (Figure 3A,C and Table 2) or if the waves appear too small when the software gain is maxed out. Adjust the offset if rectifier foldover is observed (Figure 3C,C' and Table 2). Refer to the software manual31 and online resources30 for detailed instructions on how to make these adjustments.
- To adjust the position of the signals on the screen, select the pre or post signal (e.g., by clicking on
1 = 1 or 2 = 3) and then drag the signal to the desired position on the screen (ideally near the middle of the screen but not overlapping). - To adjust the software gain, use the hardware management software described in step 3.4.3.
- To adjust the hardware gain, start by turning the Gain Dial for Channel 1 to the right or left to raise or lower the gain, respectively. If that adjustment is not sufficient to eliminate peaking out (gain too high; Figure 3A,C) or allow the details of the signal to be clearly visualized (gain too low), flip the Channel 1 Range and Multiplier Switches to adjust the gain further.
NOTE: Using event markers to record when the hardware gain was adjusted is recommended to aid waveform data interpretation. - To adjust the offset, flip the Channel 1 Offset Switch on the control box to neg, then turn the Channel 1 Offset Dial until the peaks in the pre and post signals occur in the same direction on the computer screen. If the Offset Dial is turned too far, the post-signal will flip again.
- To adjust the position of the signals on the screen, select the pre or post signal (e.g., by clicking on
- If the mosquito does not probe within 10 min (or other defined time point), switch the head amp off. If the mosquito is mid-probe at the time mark, allow it to finish probing prior to turning the head amp off. Have the host carefully remove their hand, then remove the mosquito with the attached insect stub. Replace the mosquito in the storage box.
- Stop/close the recording using the hardware management software. Proceed with EPG on the next mosquito (Step 4.2).
NOTE: Remember to change the file name after each mosquito. - Switch off the head amp and power source at the session end and close the software.
- Review the Participant Debriefing Statement or similar with the volunteer as specified in the approved IRB.
- Dispose of mosquitoes per protocol requirements. Remove the plastic wrap from the foam block and discard it appropriately.
- After all data collection is complete, review and measure the waveforms and analyze the data as previously described24,25,26,27,29,30. Refer to online resources30 for step-by-step tutorials for using the waveform reviewing software, making figures depicting the waveforms, measuring the waveforms, exporting the data for analysis, conducting the statistical analysis, and a detailed explanation of common waveform response variables.
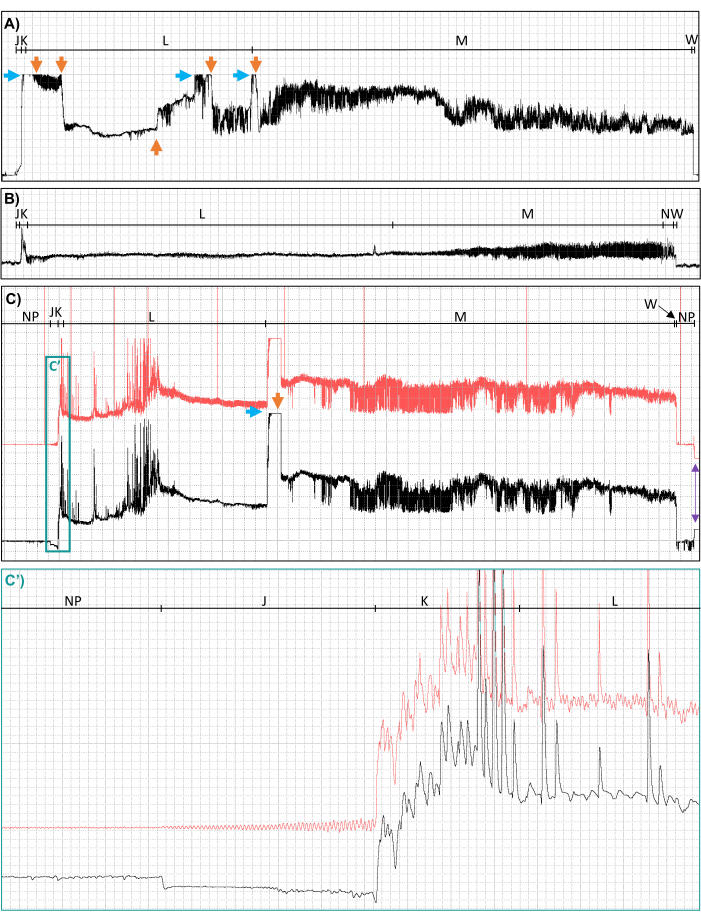
Figure 3: Examples of suboptimal recordings due to peaking out, control box gain changes, unclear voltage changes from inferior wiring, rectifier foldover, and noise. Waveforms from Culex tarsalis mosquitoes feeding to repletion on human hands, distorted by (A) peaking out (blue horizontal arrow) and subsequent control box gain changes from 100x to 80x to 40x to 60x to 40x to 20x (orange vertical arrows), (B) unclear voltage changes, and (C) rectifier fold over in the post- (black) signal compared to the pre- (red) signal (see waveform J, closeup shown in panel C'), line noise (11 Hz sine wave visible in pre-rectification (red) signal only, closeup shown in panel C'), peaking out (blue horizontal arrow), and a control box gain change from 500x to 200x (orange vertical arrow). Waveform names are shown along the top of each panel. The purple arrows in the lower right corner of panel C indicate when the head amp was turned off after the probe was complete and provide another example of rectifier foldover. Red horizontal lines associated with the pre-rectification signal in panel C indicate when Event Markers were used to record time-stamped notes. Ri levels, applied signals, time scales, and software gains are (A) 107 Ω, 150 mV DC, 13.2 s/div, 8x; (B) 107 Ω, 150 mV DC, 4.4 s/div, 128x; and (C) 107 Ω, 150 mV AC, 7.4 s/div, 2x; (C') 0.2 s/div, 8x. Abbreviations: AC = alternating current, DC = direct current, Ri = input resistance, mV = millivolt, Ω = Ohms, s/div = seconds per division. This figure was prepared with data from Cooper et al.27. Please click here to view a larger version of this figure.
Representative Results
AC-DC EPG recordings of Cx. tarsalis mosquitoes' blood feeding on human hands (Figure 4) generated interpretable waveforms suitable for analysis and publication27. Interpretable waveforms contained distinct, distinguishable repeating patterns that could be classified into waveform families (Table 1 and Figure 4), types, and subtypes when the compression (defined in Table 2) and software gain were adjusted in waveform reviewing software27,31. All waveform families (J, K, L, M, N) and types (L1, L2, M1, M2) previously described in Ae. aegypti feeding on human hands26 were identified in the waveforms generated by Cx. tarsalis feeding on human hands, verifying the validity of the methods described here. In addition, three new waveform types (M3, N1, N2) and numerous subtypes of L1, L2, M1, and M2 were identified27. In addition, the voltage drop back to baseline when the mouthparts are withdrawn from the host was named waveform family W27.
Systematically varying the instrument settings (current type and Ri levels) while following the procedures outlined here allowed for the determination of the optimal settings for recording Cx. tarsalis feeding on human hands, the electrical origins of each waveform, the average duration in seconds of complete and incomplete probes per mosquito, and for statistical comparison of the waveform duration in seconds per insect (WDI), the number of waveform events per insect (NWEI), and the waveform duration in seconds per event per insect (WDEI) across the settings27. Mosquitoes were also interrupted during different waveforms and dissected to determine which waveform was correlated with blood ingestion27. A similar investigation was performed for Ae. aegypti using comparable methodology26.
Due to EPG's sensitive and intricate nature, not all recordings were as pristine as those shown in Figure 4. Peaking out (Figure 3A,C), hardware gain changes (Figure 3A,C), inferior wiring jobs (Figure 3B), rectifier foldover in waveform J (Figure 3C,C'), and electrical noise (Figure 3C,C') occasionally distorted and masked the waveforms, rendering them ill-suited for use in publication quality figures and making scoring and analysis difficult. Thus, front-end effort is recommended to perfect wiring skills, optimize gain and voltage settings, adjust the offset, and control electrical noise. Interestingly, rectifier foldover was only observed in waveform family J for Cx. tarsalis (Figure 3C,C') and did not recur once the offset was initially adjusted, even when other species of mosquitoes (Ae. aegypti and Culex quinquefasciatus) were used (data not shown). In addition, troubleshooting revealed that the uncontrolled electrical noise shown in Figure 3 (panels C and C') resulted from a faulty Faraday cage. The recordings made in the faulty Faraday cage were still usable, but noise issues were resolved when an improved Faraday cage was built (Figure 1B).
Even when all precautions and front-end optimizations were taken, slight variations in the quality of the electrode attachment to the mosquito and other unknown factors that distorted the signal and altered the signal-to-noise ratio periodically made it necessary to adjust the hardware gain mid-recording to ensure that the signal was in the visible range and the resolution of the peaks in each waveform were sufficient for waveform identification and characterization. Unfortunately, adjusting the hardware gain mid-recording can result in abrupt voltage shifts (Figure 3A, C), rendering the waveforms ill-suited for publication but still usable (in whole or part) for measurement and analysis.
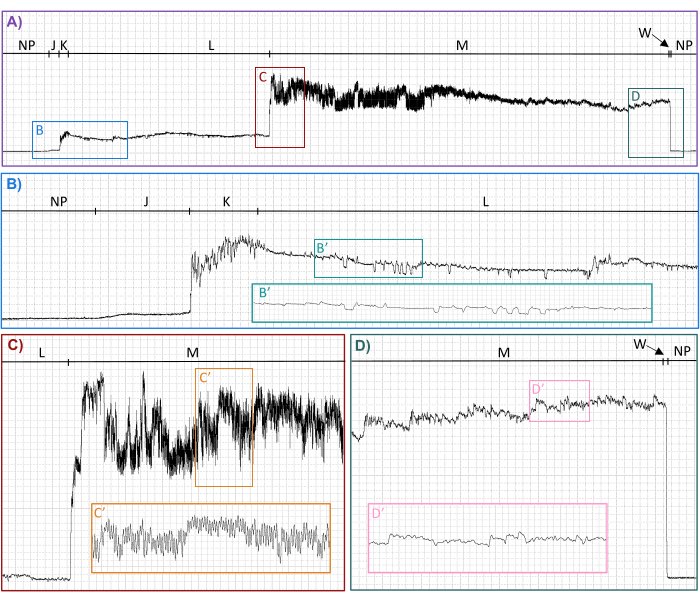
Figure 4: Representative waveforms from a Culex tarsalis probe on a human hand recorded with an Ri of 107 Ω using an applied signal of 250 mV AC. Family-level (NP, J, K, L, M, W) waveform names are shown along the top of each panel. Lower panels show enlargements of select regions in panel A. Inserted panels show enlargements of indicated regions. Time scales and software gains are (A) 7.0 s/div,16x; (B) 1.0 s/div, 64x; (B') 0.2 s/div, 64x; (C) 1.0 s/div, 64x; (C') 0.2 s/div, 32x; (D) 1.0 s/div, 64x; (D') 0.2 s/div, 64x. Abbreviations: AC = alternating current, Ri = input resistance, mV = millivolt, Ω = Ohms, s/div = seconds per division. This figure was prepared with data from Cooper et al.27. Please click here to view a larger version of this figure.
Discussion
When using the AC-DC EPG, it is critical to have a basic understanding of hardware and software operation30 and carefully follow the protocols described herein to avoid damaging the equipment. Most notably, avoid touching the head amp when it is turned on unless grounded on the Faraday cage. Recording as far back in the Faraday cage as possible is recommended30. The cage doors do not necessarily need to be closed to obtain interpretable waveforms unless recording in an environment with excessive ambient electrical noise. Nothing motorized can be in the Faraday cage. It is important to note that the Faraday cage itself must be electrically grounded solely through the head amp for it to work properly. This is accomplished by connecting the head amp to a metal laboratory stand with the paint sanded off one foot of the stand to allow a conductive pathway for the electricity captured by the Faraday cage to flow to the laboratory stand, into the metal casing of the head amp, down the cord to the control box, through the power supply into a properly grounded wall outlet, and into the earth through the building ground30. A multimeter can be used to check the quality of the connection among the components inside the cage by measuring the resistivity (which is the inverse of conductivity) between various points. A wireless AM radio can also be placed inside the Faraday cage to verify that the sound is significantly reduced (or ideally eliminated) when the doors are closed, confirming that the Faraday cage is adequately capturing airborne electrical signals (E. Backus per. Comm.).
Proper and standardized preparation of the silver glue and attachment of the recording electrodes to insects are critical to obtaining usable recordings. Insect cuticle is an insulator and acts as a capacitor if the silver glue does not adequately penetrate the pores of the cuticle to create a pathway for electricity to travel from inside the insect to the gold wire on the surface of the pronotum30. Prepare the silver glue at the same time before each EPG session, work with fresh silver glue from the tube, and replace it with vigorously vortexed silver glue frequently during the wiring procedure. Ensure the gold loop is positioned against the thorax so the entire loop is in contact with the cuticle and not tilted to one side or separated by a thick layer of silver glue and scales to help form a strong electrical connection as well as prevent detachment and escape of the mosquito from the wire.
The protocols described here must be optimized when working with different mosquito species. The optimal EPG settings (Ri level, voltage, current type, and hardware gain) for recording on a given arthropod must be experimentally determined12,30. However, once the offset is adjusted, it will not need to be readjusted when working with similar mosquitoes. Optimal settings have been determined for AC-DC EPG with Ae. aegypti26 and Cx. tarsalis27. Waveform libraries (defined in Table 2) for additional species will facilitate the adoption of EPG for mosquitoes. It is also necessary to optimize the anesthesia for the mosquito of interest, as blood-feeding behavior can be differentially affected based on mosquito species and anesthesia choice. For instance, Cx. tarsalis require a 4 h recovery period after 15 min of anesthesia with CO2, whereas for Ae. aegypti, a 15 min recovery period is sufficient after a 15 min anesthesia period with ice. Culex tarsalis, however, are difficult to knock down with cold exposure, so CO2 is predominantly used despite the long recovery period.
While the AC-DC EPG monitor is straightforward to use after reviewing online resources30,31 or training with an experienced user, some troubleshooting is required. Initially, the author's EPG recordings of mosquito probes had an unexpected 11 Hz sign wave in the pre-signal that had not been observed or reported by Wayadande and colleagues26. Consultation with E. Backus indicated that the Faraday cage was likely the issue because of interfering ground loops. A new Faraday cage constructed from a highly conductive copper screen connected to an aluminum frame bolted to a large aluminum floor plate supported by rubber feet to isolate it from the table below (Figure 1B), solved the 11 Hz noise signal (Figure 3C,C'). Additionally, occasionally, when the EPG power supply was first turned on, the pre-and post-rectification signals would appear as thick, fuzzy lines instead of straight, smooth, thin lines. This was usually fixed by turning off the instrument and checking all the cord connections. If that failed, unplugging the instrument for 1-3 min usually helped. Very rarely, the pre-and post-signals become thick and fuzzy during operation, which was addressed by turning off the instrument and plugging it into a different outlet or using a voltage regulator and power conditioner to plug the EPG into before connecting it to the wall. Ideally, a dedicated line for the EPG monitor would eliminate line noise stemming from refrigerators and other equipment plugged into the same electrical circuit as the EPG30.
Currently, there are two main limitations to using EPG to study mosquitoes. First, few correlation studies have been performed to verify which probing behaviors correspond to the waveforms. While there are strong hypotheses about what is happening during each waveform family (Table 1) and some of the waveform types (M1 and M2), few of these hypotheses have been experimentally confirmed, except that blood ingestion is correlated with waveform family M26,27. Most importantly, waveforms corresponding to salivation events must be identified because many pathogens are transmitted in the saliva. Correlation of EPG waveforms with biological activities in mosquitoes will be required to meaningfully interpret significant differences in waveform duration and counts between treatment groups. Second, probing and ingestion rates during EPG can be much lower than expected27. Individually tethering mosquitoes to short wires and placing them directly on the host could prevent long-distance host-seeking and social feeding behaviors, which may affect probing and ingestion behaviors. Correctly wired insects can walk and fly normally. Still, mosquitoes often pull against the wires and sometimes get tangled or hold themselves at unnatural angles, indicating that being wired affects their behavior to some degree. The authors tested many strategies to enhance probing rates during EPG, and fasting the mosquitoes overnight, using older individuals, exposing them to human breath immediately before placing them on the hand, and applying human saliva to the hand were the most effective, as well as using hosts that constantly made fine-scale adjustments to the position of their hand to ensure the mosquito was in the ideal position to probe27.
Despite these limitations, AC-DC EPG has numerous advantages over other approaches for studying probing and ingestion behaviors. Systems like the BiteOScope11, the flyPAD15, and the Automated Biomaterial Platform14 that were designed to quantify probing and ingestion behaviors and salivary secretions during blood feeding utilize transparent artificial substrates and liquid diets that lack normal host tissue systems and host cues. While these synthetic platforms are useful for many experimental purposes, a clear advantage of AC-DC EPG is the ability to study these arthropod behaviors and variables of interest within natural host systems. Further, the user-defined adaptability of the AC-DC EPG system offers an additional advantage over other EPG instruments22,33 and EPG-like electronic monitoring devices16,21, which do not have adjustable Ri levels, current types, and voltage levels that are needed to obtain waveforms with the maximum amount of information and minimize adverse effects on a broad range of the hosts and arthropods22,23. Electromyography (EMG) techniques are also used to study blood feeding. However, the electrical circuit usually only includes the arthropod and only muscle potentials nearest the recording electrode are recorded, so the waveforms do not provide information on detailed activities occurring at the host-insect interface like cell ruptures, stylet locations and activities, and streaming potentials through the mouthparts28,34. Intravital microscopy techniques can be used to visualize detailed mouthpart movements and activities in living host tissues directly, but these techniques involve invasive procedures on live animal models9. Only the AC-DC EPG monitor can provide highly detailed information in real-time about probing, ingestion, and salivation behaviors that occur in living opaque host tissues without the need for invasive procedures and is adaptable for use in a wide variety of arthropods10,12.
This is the first detailed step-by-step procedure for wiring mosquitoes, constructing an insect aspirator, and performing EPG on human hosts. These protocols are expected to facilitate the adoption of AC-DC EPG on mosquitoes feeding on human hosts. Examples of future research efforts employing AC-DC EPG may include studies on fundamental mechanisms of blood feeding and host tissue damage10, including identification of stereotypical sequences of probing behaviors preceding blood ingestion, investigation of blood flow through mouthparts, and comparison of feeding plasticity within and between arthropod species/developmental stages26. EPG can also be used to quantify the effects of insecticides, antifeedants, and repellents12. The procedures presented here for using AC-DC EPG to study mosquitoes on human hands will accelerate these studies and enable novel insights into mosquito blood-feeder biology.
Disclosures
The authors have no conflicts of interest to disclose. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture. The conclusions in this report are those of the authors and do not necessarily represent the views of the USDA.
Acknowledgements
We are grateful to Prof. Astri Wayadande from Oklahoma State University for pioneering the wiring procedure for mosquitoes, and to Dr. Elaine Backus at the USDA-ARS in Parlier, CA, and Mr. Andrew Dowell at EPG Technologies Inc. in Gainesville, FL, for assistance in troubleshooting the EPG equipment. We also thank Dr. Dustin Swanson and Mr. William Yarnell at the USDA-ARS Insectary in Manhattan, KS, for providing mosquitoes, and Miss Miriam Ruhinda at Kansas State University in Manhattan, KS for her assistance with filming the procedural videos. This work was made possible by access to the Integrated Molecular Entomology Core (IEMC) at Kansas State University. This research was supported by the USDA Research, Education, and Economics Workforce Development Agreement (#58-3022-0-002) and the Hatch Multistate Project (NE1943). This manuscript is contribution No. 24-194-J from the Kansas Agricultural Experiment Station, Kansas State University, Manhattan, KS, USA.
Materials
| Name | Company | Catalog Number | Comments |
| Instrumentation | |||
| AC-DC 4-channel electropenetrograph | EPG Technologies, Inc. | For EPG, includes the power supply, control box, switch interface box, four head stage amplifiers, four substrate voltage probes, and associated cords | |
| Air pillows | Amazon.com | for the human host to rest their hand/arm on during EPG | |
| DI-710 Data acquisition hardware (AD Board) | DATAQ Instruments Inc. | for digitizing the analogue EPG signals so they can be visualized on a computer | |
| Dimmable Led Magnifying Lamp, Large 5X Hands Free Magnifying Glass with Light and Stand for Reading Hobbies Crafts Sewing Workbench | Amazon.com | for behavioral observation during EPG; only use unplugged during EPG or noise may result | |
| Dino-Lite Edge Digital Microscope (250x magnification) | Dino-Lite | AF4915ZT | for behavioral observation during EPG |
| Dino-Lite Flex-arm Desktop Stand | Dino-Lite | MS22B | for holding and manipulating the camera |
| Eisco Steel Retort Base Stand and Rod Set | Fisher Scientific | S41740 | for holding the head stage amplifier & grounding the Faraday cage (must be made of metal) |
| EPG Online Workshop | UF IFAS Extension | https://ifas-crec-entomology.catalog.instructure.com/courses/epg-workshop-2022 | AKA, "online resources" for learning EPG (instrument set up, data collection and analysis, Faraday construction, silver glue preparation, insect stub construction, etc.) |
| Furman P-1800 AR Power Regulator/Power Conditioner | Amazon.com | for reducing line noise | |
| Small Bubble Cushioning Wrap | Amazon.com | for use as a barrier between the host and Faraday cage | |
| Troemner Talboys Labjaws Regular Clamp Holder | Fisher Scientific | 02-217-121 | for holding the head stage amplifier & grounding the Faraday cage (must be made of metal and not coated in plastic) |
| Windows computer | Best Buy | for EPG & statistical analysis | |
| Software | |||
| Backus 2.0 Data Analysis SAS program | UF IFAS Extension | https://crec.ifas.ufl.edu/extension/epg/data-analysis/ | for statistical analysis of waveform data |
| Ebert 1.0 File Manipulator and Error Checker SAS programs | UF IFAS Extension | https://crec.ifas.ufl.edu/extension/epg/data-analysis/ | for preperation of files for statistical analysis of waveform data |
| SAS version 9.4 | SAS Institute Inc. | for statistical analysis of waveform data | |
| WinDaq Lite | DATAQ Instruments Inc. | for recording and reviewing EPG data. Includes the WinDaq Hardware Manager software (AKA, "hardware management software") and WinDaq Waveform Browser software (AKA, "waveform reviewing software"). For use on Windows computers only. | |
| Faraday Cage | |||
| Aluminum screen frame | Home Depot | MSCF5167 | for the walls of the Faraday cage |
| Aluminum Sheet, 0.125 (1/8) thick 3003-H14 | Metals Depot | S318 | for the base of the Faraday cage |
| Corner braces, 1" | Home Depot | 339547 | for the walls of the Faraday cage |
| Flat head Phillips machine screws, #6-32 x 1" | Home Depot | 459999 | for Faraday cage construction |
| Flat washers, #6 | Home Depot | 638977 | for Faraday cage construction |
| Fluke 15B+ Digital Multimeter, for Electrical Applications, Measures AC/DC Voltage and Current Measurements up to 1000V and 10A, Along with Resistance, Continuity, Diode, and Capacitance Capabilities | Amazon.com | for testing the Faraday Cage | |
| Portable Radio AM FM, Goodes Transistor Radio with Loud Speaker, Headphone Jack, 2AA Battery Operated Radio for Long Range Reception, Pocket Radio for Indoor, Outdoor and Emergency Use | Amazon.com | for testing the Faraday Cage | |
| Pure copper screen, 16 x 16 mesh with 0.11 wire diameter, 36" x 12' | Metro Screenworks | for the walls of the Faraday cage | |
| Round head combo machine screws, #6-32 x 3/4" | Home Depot | 528466 | for Faraday cage construction |
| Sand paper, 1/2" by 1", 150 Grit | Home Depot | 19036-20-CC | for removing the paint from the bottom of the laboratory stand |
| Screen frame corners, 5/16" | Home Depot | WCORN516 | for the walls of the Faraday cage |
| Screen spline, 0.14" | Home Depot | 3034719 | for the walls of the Faraday cage |
| Insect Stubs | |||
| Brass Escutcheon Pins | Home Depot | 801294 | for constructing insect stubs |
| Copper wire, 20 AWG | Amazon.com | for constructing insect stubs | |
| Harris water soluble flu | Home Depot | 355398 | for helping solder coat the insect stub wire and form a strong connection |
| Needle-nosed pliers | Home Depot | for bending wire when constructing insect stubs | |
| Pro's Kit 900-015 Helping Hands Soldering Aid | Amazon.com | for holding escutcheon pins during soldering | |
| Solder (with rosin core) | Home Depot | 296497 | for constructing insect stubs |
| Wire cutter stripper tool | Home Depot | for stripping and cutting copper wire when constructing insect stubs | |
| YIHUA 928D-III Soldering Iron, 110W High Power, fully Digital Display Soldering Iron | Amazon.com | for constructing insect stubs; comes with a stand, sponge, and steel wool for cleaning | |
| Insect Aspirator | |||
| Accuris ASPIRE Laboratory Aspirator | Accuris instruments | V0020 | for applying suction to the insect aspirator; includes the handheld vacuum controller, the fine-tip (40 mm) stainless steel adaptor, and the pipette tip adapter |
| Chemglass Life Sciences Pasteur pipettes, 5 3/4" long | Fisher Scientific | NC0230004 | for use as an insect aspirator when wiring mosquitoes |
| Commercial Electric Colored Vinyl Electrical Tape | Home Depot | for attaching the modified Pasteur pipette to the vacuum line | |
| Mosquito netting utility fabric, 10 x 10 cm square | Joann Fabrics | 16651622 | for preventing mosquitoes from being aspirated into the vacuum pump |
| Saint Gobain Performance Plastics Tube Tygon 1/4 x 7/16 | Fisher Scientific | 50-206-8914 | for quick and easy switching between different modified Pasteur pipettes |
| Thermo Scientific Nalgene HDPE Quick Disconnects (3/8 to 7/16 in. I.D.) | Fisher Scientific | 15-315B | for quick and easy switching between different modified Pasteur pipettes |
| Silver Glue | |||
| Elmer's Glue-All Multi Purpose White Glue | Amazon.com | for silver glue preparation | |
| Silver Flake 99.9% pure, 4-8 µm | ThermoFisher | 786.09 | for silver glue preparation |
| Silver Flake 99.95% pure, 8-10 µm | Inframat Advanced Materials | 47MR-12F | for silver glue preparation |
| Insect Anesthesia | |||
| CO2 bubbler kit, 1000 mL, #9 Rubber Stopper | FlyStuff | 59-181 | for preventing insect desiccation while using the flypad |
| Flypad frame, standard size | FlyStuff | 59-118 | for prevent mosquitoes from falling off the flypad |
| Flystation Brush | FlyStuff | 59-205 | for reducing static when working on a fly pad |
| Flystuff Flypad standard size | FlyStuff | 59-114 | AKA "anesthesia pad" for anesthetizing mosquitoes |
| Funnel (4 inch diameter) | Amazon.com | for gassing down cups of mosquitoes before transferring to the flypad | |
| Plexi glass or a flat plastic lid (cut to size) | Home Depot | MC-27 | for covering the flypad if the mosquitoes start to wake up |
| Shut Off Plastic Tubing Hose Clamps Single Position and Adjustable Open (variety 1/8" to 1/2") | Amazon.com | for diverting gas between the flypad and funnel | |
| Single Stage CO2 regulator | FlyStuff | 59-142 | for regulating the flow of gas from the CO2 tank |
| “T” Fitting for 1/8in ID Tubing | FlyStuff | 59-123 | for connecting both the flypad and funnel to the CO2 tank |
| Tubing, clear | FlyStuff | 59-124C | for connecting the funnel and flypad to the regulator on the CO2 tank |
| Insect Wiring | |||
| Black cardstock, (65Lb, 176 g/m2) 8.5" x 11" | Michael's Stores Inc. | 327826 | to make gold wire easier to visualize |
| Black construction paper square 3.5" x 4.0" | Staples | 956221 | to make gold wire easier to visualize |
| Cotton balls | Amazon.com | for fasting mosquitoes | |
| Craft foam #12 block (3.1 cm x 17.2 cm x 30.2 cm) | Joann Fabric and Craft Store | 1941079 | for holding wired mosquitoes |
| Deflecto Ribbon Dispenser, Clear | Amazon.com | for storing gold wire | |
| Featherweight entomology forceps | Fisher Scientific | S72110 | for insect manipulation |
| Fine forceps (FST by Dumont: #5 91150-20, Inox-Electronic) | Fine Science Tools | 91150-20 | for making a gold loop when wiring mosquitoes |
| Fine point paint brush | Amazon.com | for manipulating mosquitoes during wiring and EPG; must have a plastic or wooden handle if used for EPG | |
| Fine-tip standard transfer pipets, narrow, 5.8 mL | Thomas Scientific | 7761D21 | for silver glue preparation |
| Gold wire, 99.99% pure, 0.025 mm thickness | Goodfellow | AU005121 | for wiring mosquitoes |
| Minutien pins, tip 0.2 mm, 1 cm | Fisher Scientific | NC9681411 | for applying glue to the mosquitoes |
| Paper cups | Rigid Paper Tube | for fasting mosquitoes | |
| Pin vice | Fisher Scientific | 50-996-811 | for holding the minutien pin during insect wiring |
| Plastic storage box with lid (7 inch x 9 inch x 14 inch) | Amazon.com | for transporting wired mosquitoes | |
| Plexi glass or a flat plastic lid (cut to size) | Home Depot | MC-27 | for covering the fly pad if the mosquitoes start to wake up |
| Self-closing forceps | Fisher Scientific | 08-906 | for holding and manipulating gold wire |
| Compressed Gas | |||
| CO2 gas tank | Matheson Gas | for anesthetizing mosquitoes with a flypad | |
| Common Lab Items | |||
| Biohazard disposable autoclave bags, 8 1/2" x 11" | Fisher Scientific | 03-410-591 | for disposal of blood-fed mosquitoes |
| Bunsen burner or propane torch | Amazon.com | for modification of Pasteur pipettes | |
| Colored laboratory tape | Daigger Supplies | EF9763R | for labeling, preparing silver glue, and masking forceps |
| Disposable pipette tips, 1000 µL | Fisher Scientific | for aliquoting water when making silver glue | |
| Dissection microscope | Leica Microsystems | for wiring mosquitoes | |
| Dissection probe | Fisher Scientific | NC9109673 | for stirring and applying silver glue |
| Erlenmeyer flask, 1000 mL | Fisher Scientific | for preventing insect desiccation while using the flypad | |
| Ethanol, 70% | Fisher Scientific | 04-355-122 | for recovering escaped mosquitoes |
| Glass petri dishes | Fisher Scientific | 08-747B | for anesthetizing mosquitoes with ice |
| Gloves | Fisher Scientific | for personal protection | |
| Kimwipes KimTech Science brand delicate task wipers | Fisher Scientific | 06-666 | for crushing mosquitoes |
| Lab coats | Fisher Scientific | for personal protection | |
| LiBa Spray Bottles, Refillable Empty Spray Bottles (rubbing alcohol safe) | Amazon.com | for recovering escaped mosquitoes | |
| Magnetic stir bar, 2 cm long | Fisher Scientific | for mixing up silver glue | |
| Mechanical Single-Channel pipette, 1000 µL | Fisher Scientific | 13-690-032 | for aliquoting water when making silver glue |
| Microcentrifuge tubes, 1.5 mL | Fisher Scientific | 780420 | for storing silver glue |
| Parafilm M | Fisher Scientific | 50-998-944 | for sealing tubes of silver glue |
| Plastic wrap | Amazon.com | for covering the foam block | |
| Pyrex 20 mL beaker | Fisher Scientific | 07-250-051 | for mixing up silver glue |
| Semi-Micro Balance | Fisher Scientific | 10-919-370 | for weighing glue and silver flake |
| Sharpie pen, fine point | Amazon.com | for labeling | |
| Single-edge razor blade | Electron Microscopy Sciences | 71980 | for cleaning insect stubs |
| Standard Disposable Transfer Pipettes | Fisher Scientific | 133-711-7mL | for transferring silver glue to a storage tube |
| Stir plate | Fisher Scientific | for mixing silver glue | |
| Timer/stop watch | Fisher Scientific | for timing how long the host is exposed to each insect | |
| Ultrapure (18 MΩ) water | Fisher Scientific | for silver glue preparation | |
| Utility scissors | Fisher Scientific | 08-945 | for cutting gold wire |
| Vortex mixer | Fisher Scientific | for mixing silver glue | |
| Study Organisms | |||
| Culex tarsalis mosquitoes | USDA ARS Center for Grain and Animal Health Research in Manhattan KS | for use as study subjects |
References
- Becker, N., et al. . Medical importance of mosquitoes in Mosquitoes and their control. , 25-42 (2010).
- World Health Organization. . Vector-borne diseases. , (2024).
- Woodstream Corporation, Inc. The economic cost of mosquito-borne diseases. AMosquito Magnet. , (2022).
- Bartlow, A. W., et al. Forecasting zoonotic infectious disease response to climate change: Mosquito vectors and a changing environment. Vet Sci. 6 (2), 40 (2019).
- Naslund, J., et al. Emerging mosquito-borne viruses linked to Aedes aegypti and Aedes albopictus: Global status and preventive strategies. Vector Borne Zoonotic Dis. 21 (10), 731-746 (2021).
- Shaw, W. R., Catteruccia, F. Vector biology meets disease control: Using basic research to fight vector-borne diseases. Nat Microbiol. 4 (1), 20-34 (2019).
- Wilson, A. L., et al. The importance of vector control for the control and elimination of vector-borne diseases. PLoS Negl Trop Dis. 14 (1), e0007831 (2020).
- Dusfour, I., et al. Management of insecticide resistance in the major Aedes vectors of arboviruses: Advances and challenges. PLoS Negl Trop Dis. 13 (10), e0007615 (2019).
- Choumet, V., et al. Visualizing non infectious and infectious Anopheles gambiae blood feedings in naïve and saliva-immunized mice. PLoS ONE. 7 (12), e50464 (2012).
- Backus, E. A., Cervantes, F. A., Guedes, R. N. C., Li, A. Y., Wayadande, A. C. AC-DC electropenetrography for in-depth studies of feeding and oviposition behaviors. Ann Entomol Soc Am. 112 (3), 236-248 (2019).
- Hol, F. J., Lambrechts, L., Prakash, M. BiteOscope, an open platform to study mosquito biting behavior. Elife. 9, e56829 (2020).
- Backus, E. A., Guedes, R. N., Reif, K. E. AC-DC electropenetrography: Fundamentals, controversies, and perspectives for arthropod pest management. Pest Manag Sci. 77 (3), 1132-1149 (2021).
- Gordon, R. M., Lumsden, W. H. R. A study of the behavior of the mouthparts of mosquitoes when taking up blood from living tissue; together with some observations on the ingestion of microfilariae. Ann Trop Med Parasitol. 33, 259-278 (1939).
- Janson, K. D., et al. Development of an automated biomaterial platform to study mosquito feeding behavior. Front Bioeng Biotechnol. 11, 1103748 (2023).
- Henriques-Santos, B. M., Xiong, C., Pietrantonio, P. V. Automated analysis of feeding behaviors of females of the mosquito Aedes aegypti using a modified flyPAD system. Sci Rep. 13, 20188 (2023).
- Kashin, P., Wakeley, H. G. An insect 'Bitometer. Nature. 208 (5009), 462-464 (1965).
- Kashin, P. Electronic recording of the mosquito bite. J Insect Physiol. 12, 281-286 (1966).
- Kashin, P. Development of an orally effective insect repellent. IIT Research Institute. , (1967).
- Kashin, P., Arneson, B. E. An automated repellency assay system. II. a new electronic "Bitometer-timer.". J Econ Entomol. 62 (1), 200-205 (1969).
- Shieh, J. -. N. . Salivation and engorgement parameters of sucking insect vectors: Implications in pathogen transmission. , (1994).
- Kimsey, R. B. Measuring and recording arthropod blood feeding. History, Development, and Applications of AC Electronic Insect Feeding Monitors. , 122-128 (1994).
- Backus, E. A., Devaney, M. J., Bennett, W. H., Walker, G. P., Backus, E. A. Comparison of signal processing circuits among seven AC electronic monitoring systems for their effects on the emf and R components of aphid (Homoptera: Aphididae) waveforms. Principles and Applications of Electronic Monitoring and Other Techniques in the Study of Homopteran Feeding Behavior. , 102-143 (2000).
- Backus, E. A., Bennett, W. H. The AC-DC correlation monitor: New EPG design with flexible input resistors to detect both R and emf components for any piercing-sucking hemipteran. J Insect Physiol. 55 (10), 869-884 (2009).
- Backus, E. A., Cline, A. R., Ellerseick, M. R., Serrano, M. S. Lygus hesperus (Hemiptera: Miridae) feeding on cotton: New methods and parameters for analysis of nonsequential electrical penetration graph data. Annals of the Entomological Society of America. 100, 296-310 (2007).
- Ebert, T. A., Backus, E. A., Cid, M., Fereres, A., Rogers, M. E. A new SAS program for behavioral analysis of electrical penetration graph data. Comput Electron Agr. 116, 80-87 (2015).
- Wayadande, A. C., et al. Waveforms from stylet probing of the mosquito Aedes aegypti (Diptera: Culicidae) measured by AC-DC electropenetrography. J Med Entomol. 57 (2), 353-368 (2020).
- Cooper, A. M. W., et al. An electropenetrography waveform library for the probing and ingestion behaviors of Culex tarsalis on human hands. Insect Sci. 31 (4), 1165-1186 (2024).
- Reif, K. E., Backus, E. A. AC-DC electropenetrography unmasks fine temporal details of feeding behaviors for two tick species on unsedated hosts. Sci Rep. 11 (1), 2040 (2021).
- Vaughan, L. D., et al. Electropenetrography as a tool to quantify probing and injection behaviors of mosquitoes (Aedes aegypti) in mice under biocontainment housing. Comp Med. 73 (6), 25 (2023).
- Electropenetrography (EPG) Online Workshop. Hosted by L. Diepenbrock at the University of Florida. Institute of Food and Agricultural Sciences Extension Available from: https://ifas-crec-entomology.catalog.instructure.com/courses/epg-workshop-2022 (2022)
- Dataq Instruments Inc. . WinDaq recording and playback software. , (2024).
- Backus, E. A., Walker, G. P., Backus, E. A. Our own jabberwocky: clarifying the terminology of certain piercing-sucking behaviors of homopterans. Principles and applications of electronic monitoring and other techniques in the study of homopteran feeding. , 102-143 (2000).
- Tjallingii, W. F., Walker, G. P., Backus, E. A. Comparison of AC and DC systems for electronic monitoring of stylet penetration activities by homopterans. Principles and Applications of Electronic Monitoring and Other Techniques in the Study of Homopteran Feeding Behavior. , 41-69 (2000).
- Mizrahi, J. . Advances in Applied Electromyography. , (2011).
Explore More Articles
This article has been published
Video Coming Soon
ABOUT JoVE
Copyright © 2025 MyJoVE Corporation. All rights reserved