When light of a particular wavelength strikes a metal surface, electrons are emitted. This is called the photoelectric effect. The minimum frequency of light that can cause such emission of electrons is called the threshold frequency, which is specific to the metal. Light with a frequency lower than the threshold frequency, even if it is of high intensity, cannot initiate the emission of electrons. However, when the frequency is higher than the threshold value, the number of electrons ejected is directly proportional to the intensity of the beam.
According to classical wave theory, a wave's energy depends on its intensity (which depends on its amplitude), not its frequency. One part of these observations was that the number of electrons ejected within a given time period was seen to increase as the brightness increased. In 1905, Albert Einstein was able to resolve the paradox by incorporating Planck's quantization findings into the discredited particle view of light.
Einstein argued that the quantized energies that Planck had postulated could be applied to the light in the photoelectric effect. The light striking the metal surface should not be viewed as a wave, but should instead be viewed as a stream of particles (later called photons) whose energy depended on their frequency, The amount of energy (E) in a light packet depends on its frequency (ν) according to the following equation:
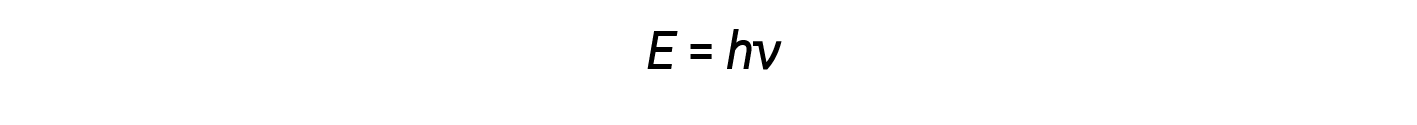
where h is Planck’s constant.
The photoelectric effect can be described by assuming that the light is quantized. A certain minimum energy is required to overcome the binding energy (Φ) experienced by an electron. This is also known as the work function (W) of the metal.
Since the electrons in the metal had a certain amount of binding energy keeping them there, the incident light needs to have more energy to free the electrons. Photons of low-frequency light do not contain enough energy to eject electrons from the metal. Even if the metal is exposed to such light for a long time, no emission of electrons is observed. An electron can only be emitted when a photon with energy greater than the work function strikes the metal.

The excess energy of the photon is converted into kinetic energy of the emitted electron.
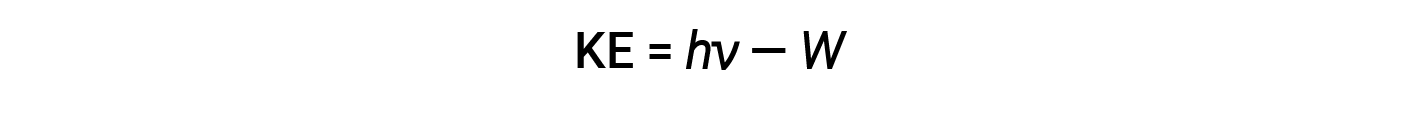
Therefore, electrons are ejected when they are hit by photons having sufficient energy (a frequency greater than the threshold). The greater the frequency of incident light, the greater the kinetic energy imparted by the collisions to the escaping electrons. Einstein also argued that the light intensity did not depend on the amplitude of the incoming wave, but instead corresponded to the number of photons striking the surface within a given time period. The number of ejected electrons increases with brightness. The greater the number of incoming photons, the more likely that they will collide with some of the electrons.
The photoelectric effect strongly suggests the particle behavior of light. Einstein won the Nobel Prize in Physics in 1921 for his explanation of the photoelectric effect. Although many light phenomena could be explained either in terms of waves or particles, certain phenomena, such as the interference patterns obtained when light passed through a double slit, were completely contrary to a particle view of light, while other phenomena, such as the photoelectric effect, were completely contrary to a wave view of light. Somehow, at a deep fundamental level still not fully understood, light is both wavelike and particle-like. This is known as wave-particle duality.
This text is adapted from Openstax, Chemistry 2e, Section 6.1: Electromagnetic Energy.
From Chapter 7:

Now Playing
7.4 : Photoelectric Effect
Electronic Structure of Atoms
28.8K Views

7.1 : The Wave Nature of Light
Electronic Structure of Atoms
47.4K Views

7.2 : The Electromagnetic Spectrum
Electronic Structure of Atoms
51.8K Views

7.3 : Interference and Diffraction
Electronic Structure of Atoms
28.5K Views

7.5 : The Bohr Model
Electronic Structure of Atoms
47.8K Views

7.6 : Emission Spectra
Electronic Structure of Atoms
47.4K Views

7.7 : The de Broglie Wavelength
Electronic Structure of Atoms
25.0K Views

7.8 : The Uncertainty Principle
Electronic Structure of Atoms
22.4K Views

7.9 : The Quantum-Mechanical Model of an Atom
Electronic Structure of Atoms
41.0K Views

7.10 : Quantum Numbers
Electronic Structure of Atoms
33.6K Views

7.11 : Atomic Orbitals
Electronic Structure of Atoms
32.2K Views

7.12 : The Pauli Exclusion Principle
Electronic Structure of Atoms
31.5K Views

7.13 : The Energies of Atomic Orbitals
Electronic Structure of Atoms
23.2K Views

7.14 : The Aufbau Principle and Hund's Rule
Electronic Structure of Atoms
39.4K Views

7.15 : Electron Configuration of Multielectron Atoms
Electronic Structure of Atoms
35.6K Views
Copyright © 2025 MyJoVE Corporation. All rights reserved