Longitudinal Evaluation of Mouse Hind Limb Bone Loss After Spinal Cord Injury using Novel, in vivo, Methodology
In This Article
Summary
A longitudinal examination of bone loss in the femurs and tibiae of adult mice was performed following spinal cord injury using sequential low-dose X-ray scans. Tibia bone loss was detected throughout the study, while bone loss in the femur was not detected until 40 days post injury.
Abstract
Spinal cord injury (SCI) is often accompanied by osteoporosis in the sublesional regions of the pelvis and lower extremities, leading to a higher frequency of fractures 1. As these fractures often occur in regions that have lost normal sensory function, the patient is at a greater risk of fracture-dependent pathologies, including death. SCI-dependent loss in both bone mineral density (BMD, grams/cm2) and bone mineral content (BMC, grams) has been attributed to mechanical disuse 2, aberrant neuronal signaling 3 and hormonal changes 4. The use of rodent models of SCI-induced osteoporosis can provide invaluable information regarding the mechanisms underlying the development of osteoporosis following SCI as well as a test environment for the generation of new therapies 5-7 (and reviewed in 8). Mouse models of SCI are of great interest as they permit a reductionist approach to mechanism-based assessment through the use of null and transgenic mice. While such models have provided important data, there is still a need for minimally-invasive, reliable, reproducible, and quantifiable methods in determining the extent of bone loss following SCI, particularly over time and within the same cohort of experimental animals, to improve diagnosis, treatment methods, and/or prevention of SCI-induced osteoporosis.
An ideal method for measuring bone density in rodents would allow multiple, sequential (over time) exposures to low-levels of X-ray radiation. This study describes the use of a new whole-animal scanner, the IVIS Lumina XR (Caliper Instruments) that can be used to provide low-energy (1-3 milligray (mGy)) high-resolution, high-magnification X-ray images of mouse hind limb bones over time following SCI. Significant bone density loss was seen in the tibiae of mice by 10 days post-spinal transection when compared to uninjured, age-matched control (naïve) mice (13% decrease, p<0.0005). Loss of bone density in the distal femur was also detectable by day 10 post-SCI, while a loss of density in the proximal femur was not detectable until 40 days post injury (7% decrease, p<0.05). SCI-dependent loss of mouse femur density was confirmed post-mortem through the use of Dual-energy X-ray Absorptiometry (DXA), the current "gold standard" for bone density measurements. We detect a 12% loss of BMC in the femurs of mice at 40 days post-SCI using the IVIS Lumina XR. This compares favorably with a previously reported BMC loss of 13.5% by Picard and colleagues who used DXA analysis on mouse femurs post-mortem 30 days post-SCI 9. Our results suggest that the IVIS Lumina XR provides a novel, high-resolution/high-magnification method for performing long-term, longitudinal measurements of hind limb bone density in the mouse following SCI.
Protocol
1. Mouse spinal transection model
- Adult, male, C57BL6 mice (approximately 20-25 g) are anesthetized using a combination of ketamine (200 mg/kg) and xylazine (10 mg/kg). All surgical procedures are performed in an Institutional, IACUC-approved surgical suite under sterile conditions.
- Once deeply anesthetized, the fur of the back is trimmed using electric clippers. The shaved back is first scrubbed with an iodine solution followed by 70% ethanol.
- Prior to the initial incision, the area of the back to be incised is first infiltrated with a local anesthetic (Marcaine) at a concentration of (0.25%, <1ml/kg) to minimize post-surgical pain.
- Using small scissors, a small opening is made in the skin at around the L2 area. This opening is expanded with the same set of scissors, extending longitudinally to the T2 area. The edges of the skin are then held apart via the use of bulldog clamps.
- Micro-scissors are then used to clear muscle tissue from the dorsal spinal lamina at thoracic level 8 (T8). T8 can be identified as described in Kuh and Wrathall (1998) 10. Briefly, T13 can be identified by its dorsal spinous process. Using a pair of #5 Dumont forceps, the last rib can be palpated/identified and counted back to T8. Once cleared, bone Rongeurs are used to perform a dorsal laminectomy at T8.
- Full Spinal Transection Lesion: Once the spinal cord is exposed at T8, an additional set of sterilized micro-scissors are used to sever the spinal cord in the plane perpendicular to the long axis of the cord under a surgical microscope. We use #5 Dumont forceps to gently lift one pole of the spinal cord to confirm the completeness of the lesion.
- Following spinal transection, a small piece of sterile gelfoam, soaked in sterile saline (0.9%) is carefully placed into the lesion cavity to promote hemostasis.
- An additional piece of gelfoam is then placed over the exposed spinal cord. The skin is then closed with sterile, stainless-steel surgical staples. Subjects are then returned to their home cages, placed on a paper towel to prevent aspiration of bedding material and warmed with a heating pad for a period of approximately 12 hours. Subjects are also provided with Hydrogel packs (ClearH2O) and food pellets on the floor of their cages during early recover. Injured subjects are able to access food/water in their home cages once recovered from anesthesia.
- All injured subjects receive twice-daily manual bladder evacuations (at approximately 12 hour intervals) using a modification of the manual method of Crede for the duration of the study (40 days).
- Injured mice are also provided with twice daily intraperitoneal injections of 0.9% saline for three days (0.5 cc) to help maintain hydration, and twice daily injections of the opiate buprenorphine (0.05 mg/kg) to control post-surgical pain for a period of 5 days. If animals exhibit signs of pain after the initial 5 day period, they would receive additional buprenorphine (0.05 mg/kg) daily until signs of pain (reduced mobility, hunched stature, failure to groom, vocalization when handled) have resolved.
2. Longitudinal assessment of bone density using the IVIS Lumina XR on the same cohort of spinally-transected mice
- Beginning on Day 10 post-SCI and continuing at 10 day intervals until day 40, we assessed right and left femurs and tibiae in living, anesthetized subjects (transection SCI and uninjured age-matched controls).
- On the day of scanning, subjects were transferred in their home cages from the Institution's Vivarium area to the room in which the Caliper IVIS Lumina XR is housed. All subjects are then anesthetized using the same Ketamine/xylazine cocktail previously used in the delivery of SCI. This cocktail assures a state of anesthesia for a period of 1 to 1.5 hours; sufficient for the duration of the scanning procedure.
- The investigator initializes the Lumina XR device and allows the internal camera to reach operational temperature (-90°C) (~10 minutes).
- Once anesthetized, the subject (transection or control) is gently placed on the animal platform within the Lumina XR. A high magnification lens is inserted into the device to allow focus on both femur and tibial regions (Field of view 2.4X2.4cm with high-magnification lens). If the subject is improperly positioned outside of the field of view, the door is opened and the subject re-positioned until the left or right hind limb is centered (See Figure 2a for proper placement of the hind limb).
- Once properly positioned, the X-ray function can be executed. Make a selection from the Energy drop-down list suitable for Animal (Living subject, 35 Kv 100uA, filtered X-Rays)
- Once the animal is in the right position, enable the X-ray feature by check-marking X-Ray in the control panel. Acquire the X-ray image. Make sure that the entire femur and tibia are visible (see Figure 2b). Raw image data is automatically saved to the hard drive. Representative .TIFF files are also saved. The mouse is then returned to its home cage and allowed to recover under investigator observation. The process is then repeated with the next mouse.
Note: The Living Image software displays transformed X-ray images by default. To display raw X-ray images, remove the check mark next to X-Ray Absorption in the Corrections/Filtering tools. When the X-ray data has been corrected for absorption, you can evaluate relative bone density by comparing the signal intensities of measurement ROIs. The ROI intensity increases with increasing tissue density.
3. Image analysis of IVIS X-ray scans
- Open the software by double-clicking on the Living Image Icon.
- Load an X- ray image by clicking the Browse button. In the dialog box that appears, select the folder of interest and click OK. The Living Image browser displays the selected data along with the user ID, label information, and camera configuration information. To open data, do one of the following: Double-click the data row, Right-click the data name and select Load on the shortcut menu, Select the data row and click Load, or Double-click the thumbnail. The image and Tool Palette are displayed. Open data is highlighted in green in the browser.
- Click on ROI Tools in the Tool Palette. In the ROI Tools, select Measurement ROI from the Type drop-down list. To load the 3 ROI's used in this experiment, click the Square icon and load 3 squares.
- Using a ruler, measure the length of the femur. Adjust the length of two of the squares to be 1/8th of the total femur length. Adjust the width of these two squares to be 1/24th the total femur length. With your ruler, measure 1/8th of the distance from the proximal end of the femur, and situate the square so that it is centered within the femur. Situate the second square so that it lies 1/4th of the total femur length from the distal end of the femur (Fig. 3). These ROI's can be used to measure both proximal and distal femur regions.
- Using the ruler, measure the length of the tibia. Adjust the length of the third square to be 1/8th of the total tibial length. Adjust the width to 1/30th of the total tibial length. Situate the square so that is centered and 1/8th the total length of the tibia distance from the proximal end of the tibia (see Figure 3).
- Click on the Measure icon (a pencil and ruler). The ROI intensity measurements appear in the X-ray image and the ROI measurements table appears. Export this table to your desired location as a .csv file. This will allow you to open the table using excel.
- Repeat this with all of your saved images.
- Consolidate all of your data onto one excel sheet. Statistical significance was determined via student's t-test using either Microsoft Excel or SigmaPlot 11.0 software (Systat Software).
4. Post-mortem analysis of bone density:
- Following the acquisition of the final longitudinal X-ray scan within the IVIS Lumina XR, mice are subsequently deeply anesthetized with Beuthanasia (75 mg pentobarbital/kg). Once deep anesthesia has been achieved, the mice are transcardially perfused with iced-cold phosphate-buffered saline with heparain (40 mg/liter) to exsanguinate.
- Once exsanguinated, excise both femurs. Pay special care to remove as much soft tissue as possible, for this has been shown to affect density measurements 11. Wrap the femurs with water soaked gauze and store at -20C until you are ready to analyze them.
5. DXA Analysis using a Hologic QDR 4000 Bone Densitometer
- Thaw gauze-soaked femurs and transfer to Bone Densitometer suite.
- Calibrate the apparatus according to the manufacturer's protocol; ensure that the BMC and BMD values fall within the acceptable limits.
- Place a brass collimator in the machine. This allows the user to limit the size and angle of the X-ray beam to focus on a specific target.
- Submerge the thawed femur into a Petri dish filled with water (condyles to the left, with the femur parallel to the bed), and position it just to the right of the laser.
- Enter a Biography (a descriptive title that includes animal identification information, treatment, etc.) for the femur you are about to scan.
- Enter Scan from the Selections Menu, then select regionA hi-res. Indicate your scanning parameters: a scanning region of 2 x 0.7489 inches, with 0.01 inch line spacing and 0.00499 inch point resolution.
Note: Monitor the scan while it is in progress. As the X-ray beam raster scans the sample, monitor to ensure that there is sufficient water in the chamber to completely cover the bone sample. - Enter Analyze under the Analysis Selection menu. Follow the prompts to highlight the entire femur as an ROI. A report page will come up with the calculated BMC (grams) and BMD (gms/cm2).
- Repeat these steps to analyze the remaining femurs.
- Consolidate all of your data onto one excel sheet and perform statistical analysis (t-test).
6. Representative Results:
The relative bone density loss of a mouse tibia and femur after spinal cord injury when compared to naïve mice is detectable using the above method. There is a detectable significant decrease in bone density after just 10 days (12%, p<0.0005), with up to 15% bone density loss at 40 days (p<0.0005, Figure 4). Bone density loss in the femur was observed at 40 days post injury (7% decrease, p<0.05, Figure 5). These results provide evidence for the use of non-invasive x-ray imaging for the longitudinal observation of bone density change after spinal cord injury.
In order to compare the efficacy of this method to what is currently available; we analyzed the excised femurs of these mice 40 days after injury using DXA imaging. A representation of the data output can be seen in Figure 6. We found that there was a significant loss of bone mineral content in the SCI mice when compared to naïve (12% decrease, p<0.05, Figure 7). Bone mineral density did not change significantly, but did follow a similar trend (Figure 8). These results are similar to those found in the literature; Picard et al observed a 13.5% decrease (p<0.001) in BMC, but no significant decrease in BMD (Picard 2008).
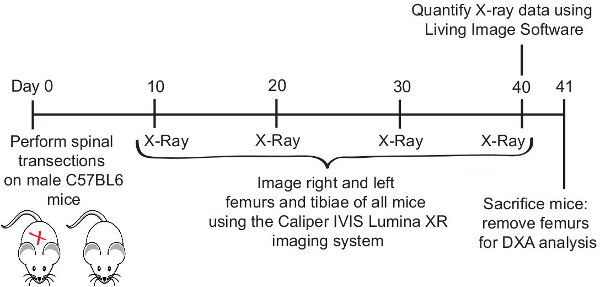
Figure 1. Experimental Timeline.
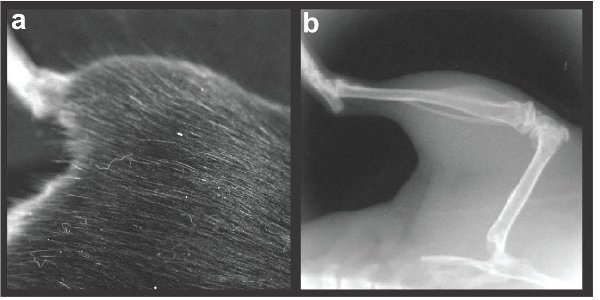
Figure 2. Representative orientation of left hind-limb: a) photograph and b) x-ray.
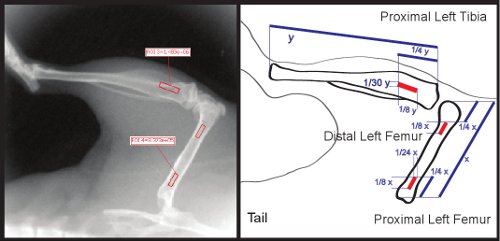
Figure 3. ROI dimensions and orientation within the regions of the proximal femur and tibia.
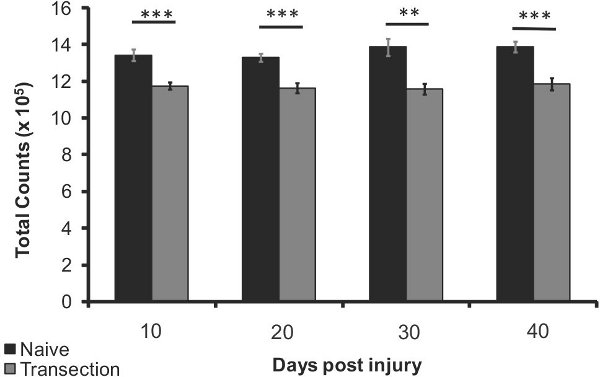
Figure 4. Bone density loss after SCI in the proximal tibia 10, 20, 30 and 40 days after injury (n=5) compared to age-matched naives (n=5). Error bars represent SEM; **p<0.005, *** p<0.0005.
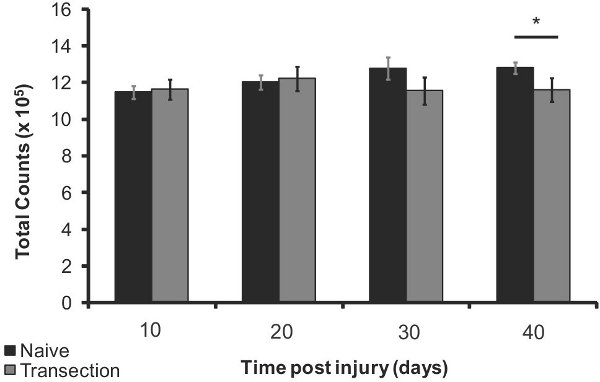
Figure 5. Bone density loss after SCI in the proximal femur 10, 20, 30 and 40 days after injury (n=5) compared to age-matched naives (n=5). Error bars represent SEM; *p<0.05.
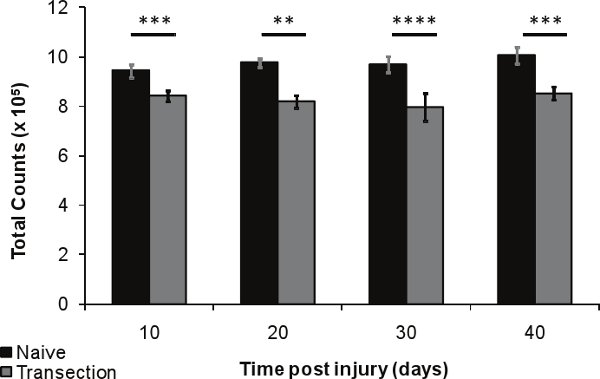
Figure 6. Bone density loss after SCI in the distal femur 10, 20, 30 and 40 days after injury (n=5) compared to age-matched naïve controls (n=5). Error bars represent SEM; *p<0.01-0.05; **p<0.001-0.01; ***p<0.0001-0.001; ****p<0.0001.
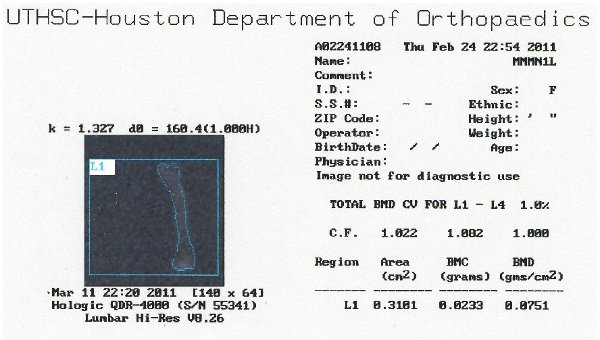
Figure 7. Representative image of DXA data showing BMC and BMD output.
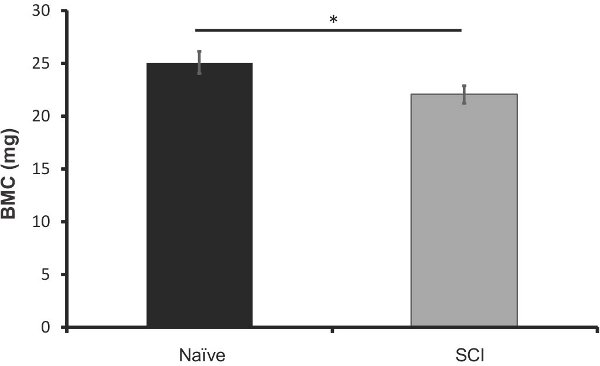
Figure 8. DXA analysis of bone mineral content (grams) in the femurs of SCI mice 40 days post injury (n=5) vs. age-matched naives (n=5). Error bars represent SEM; *p<0.05.
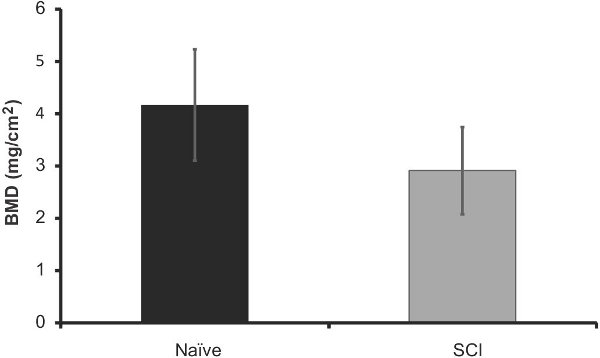
Figure 9. DXA analysis of bone mineral density (mg/cm2) in the femurs of SCI mice 40 days post injury (n=5) vs. age-matched naives (n=5). Error bars represent SEM; no significant difference.
Discussion
This study presents a novel, non-invasive method for assessing density changes within individual bones (tibiae and femurs) longitudinally, within a single cohort of mice, using the Caliper IVIS Lumina XR imaging system. SCI produced a decrease in bone density in both femurs and tibiae when studied within a single cohort of spinally-transected mice over a 40 day assay period compared to age-matched, uninjured, control mice. Our results in the femur were confirmed, post-mortem, through the use of DXA, suggesting that the application of the Caliper Instruments IVIS Lumina XR can serve as a sensitive measuring tool to assess the long-term and progressive effects of SCI on bone loss.
One potential benefit to investigators using this system is the ability to longitudinally follow the development of pathological changes in the hind limb bones of animals following SCI. The ability to assess a single cohort over long time periods provides strong advantages to the investigator in terms of: 1) costs associated with animal purchase and long-term care. Instead of a study requiring large numbers of animals that need to be sacrificed at specific time points in order to assess time-dependent changes, these pathological outcomes can be followed within the same cohort over that same time period, 2) variability: in addition to comparison with control cohorts, the outcomes generated within the single cohort of injured subjects can be assessed and compared between time points for the same animals throughout the extent of the study; once again, reducing both intra-animal variability as well as the need for larger cohorts of experimental subjects.
While the Caliper IVIS Lumina XR provided us with important longitudinal information regarding the development of SCI-induced osteoporosis, there are limitations that should be considered: 1) while this platform has allowed us to generate high-resolution X-ray data in the hind limb bones of mice following SCI, its utility may be limited to small rodents such as mice. The maximum subject height allowed within the Lumina XR is 2.8 cm. Anything above that height cannot be successfully imaged using the X-ray component of the Lumina XR. In our hands, this has precluded our ability to study rats in the 200-250 gram weight. While it may be possible to image rats under this weight range or other rodents such as hamsters or gerbils, this will require additional testing by the PI. 2) Joint orientation. It is critical that the Investigator carefully arrange the limbs in an easily reproducible orientation that will allow successful comparison between imaging sessions. Standardization of limb placement will allow the Investigator to generate and apply standardized ROI's that can be saved and applied across both subjects and time.
Overall, we believe that the IVIS Lumina XR provides an excellent platform with which to model the development of SCI-dependent osteoporosis in mice. The ability to study bone loss longitudinally within the same mouse cohort will allow us to: 1) better understand the temporal nature of bone loss and demineralization after SCI, 2) determine whether these changes stabilize over time, 3) explore, with the availability of both transgenic and null mouse lines, the ability to study the molecular mechanisms underlying these pathological changes in a reductionist manner, and 4) quite possibly the most important advantage, begin to test novel interventions designed to either prevent the development of osteoporosis in the early stages of injury, or, to reverse such changes once osteoporosis has already developed. Finally, the Lumina XR, in addition to providing excellent X-ray imaging potential, can also be used to study both bioluminescent and fluorescent signals in living animals. One could easily imagine incorporating the other modalities offered within this system to look at a multitude of factors that determine bone dynamics mechanistically following SCI or any other disease process that results in osteoporosis.
Disclosures
Free Access to this article is sponsored by Caliper Life Sciences.
Acknowledgements
We would like to thank both Mission Connect and the Gillson-Longenbaugh Foundation for providing the funds for this project. We would also like to thank Dr. Catherine Ambrose for her critical advice and use of her DXA equipment. Finally, we would like to thank Dr. Kaori Ono, Department of Integrative Biology and Pharmacology, for her suggestions in DXA femur bone analysis.
Materials
| Name | Company | Catalog Number | Comments |
| Name of the reagent | Company | Catalogue number | Comments |
|---|---|---|---|
| Beuthanasia | Butler Schein Animal Health | 001848 | |
| Ketathesia (Ketamine Hydrochloride) | Butler Schein Animal Health | 023061 | |
| Xylazine | Butler Schein Animal Health | 037849 | |
| Bupivicaine | Butler Schein Animal Health | 021801 | |
| Gelfoam; 7MM | Fisher Scientific | NC0085178 | To promote hemostasis during surgery |
| IVIS Lumina XR | Caliper Life Sciences | 135400 | |
| ZFOV, 2.5 cm field of view lens | Caliper Life Sciences | 127285 | Absolutely necessary to generate high magnification X-ray images of bone structure |
| QDR Bone Densitometer | Hologic | Model used no longer in production |
References
- Jiang, S. D., Jiang, L. S., Dai, L. Y. Mechanisms of osteoporosis in spinal cord injury. Clin. Endocrinol. (Oxford). 65, 555-565 (2006).
- Uebelhart, D., Demiaux-Domenech, B., Roth, M., Chantraine, A. Bone metabolism in spinal cord injured individuals and in others who have prolonged immobilisation. A review. Paraplegia. 33, 669-673 (1995).
- Elefteriou, F. Neuronal signaling and the regulation of bone remodeling. Cell. Mol. Life. Sci. 62, 2339-2349 (2005).
- Finsen, V., Indredavik, B., Fougner, K. J. Bone mineral and hormone status in paraplegics. Paraplegia. 30, 343-347 (1992).
- Sugawara, H., Linsenmeyer, T. A., Beam, H., Parsons, J. R. Mechanical properties of bone in a paraplegic rat model. J. Spinal. Cord. Med. 21, 302-308 (1998).
- Kiratli, B. J., Smith, A. E., Nauenberg, T., Kallfelz, C. F., Perkash, I. Bone mineral and geometric changes through the femur with immobilization due to spinal cord injury. J. Rehabil. Res. Dev. 37, 225-233 (2000).
- Shen, J., Fan, L., Yang, J., Shen, A. G., Hu, J. M. A longitudinal Raman microspectroscopic study of osteoporosis induced by spinal cord injury. Osteoporos. Int. 21, 81-87 (2010).
- Guertin, P. A. A technological platform to optimize combinatorial treatment design and discovery for chronic spinal cord injury. J. Neurosci. Res. 86, 3039-3051 (2008).
- Picard, S., Lapointe, N. P., Brown, J. P., Guertin, P. A. Histomorphometric and densitometric changes in the femora of spinal cord transected mice. Anat. Rec. (Hoboken). 291, 303-307 (2008).
- Kuhn, P. L., Wrathall, J. R. A mouse model of graded contusive spinal cord injury. J. Neurotrauma. 15, 125-140 (1998).
- Tsujio, M. mineral analysis through dual energy X-ray absorptiometry in laboratory animals. J. Vet. Med. Sci. 71, 1493-1497 (2009).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
ABOUT JoVE
Copyright © 2025 MyJoVE Corporation. All rights reserved