Culturing and Electrophysiology of Cells on NRCC Patch-clamp Chips
Summary
We show how planar patch-clamp chips fabricated at the National Research Council of Canada are sterilized, primed, loaded with medium, plated with cells, and used for electrophysiological recordings.
Abstract
Due to its exquisite sensitivity and the ability to monitor and control individual cells at the level of ion channels, patch-clamping is the gold standard of electrophysiology applied to disease models and pharmaceutical screens alike 1. The method traditionally involves gently contacting a cell with a glass pipette filled by a physiological solution in order to isolate a patch of the membrane under its apex 2. An electrode inserted in the pipette captures ion-channel activity within the membrane patch or, when ruptured, for the whole cell. In the last decade, patch-clamp chips have been proposed as an alternative 3, 4: a suspended film separates the physiological medium from the culture medium, and an aperture microfabricated in the film replaces the apex of the pipette. Patch-clamp chips have been integrated in automated systems and commercialized for high-throughput screening 5. To increase throughput, they include the fluidic delivery of cells from suspension, their positioning on the aperture by suction, and automated routines to detect cell-to-probe seals and enter into whole cell mode. We have reported on the fabrication of a silicon patch-clamp chip with optimized impedance and orifice shape that permits the high-quality recording of action potentials in cultured snail neurons 6; recently, we have also reported progress towards interrogating mammalian neurons 7. Our patch-clamp chips are fabricated at the Canadian Photonics Fabrication Centre 8, a commercial foundry, and are available in large series. We are eager to engage in collaborations with electrophysiologists to validate the use of the NRCC technology in different models. The chips are used according to the general scheme represented in Figure 1: the silicon chip is at the bottom of a Plexiglas culture vial and the back of the aperture is connected to a subterranean channel fitted with tubes at either end of the package. Cells are cultured in the vial and the cell on top of the probe is monitored by a measuring electrode inserted in the channel.The two outside fluidic ports facilitate solution exchange with minimal disturbance to the cell; this is an advantage compared to glass pipettes for intracellular perfusion.
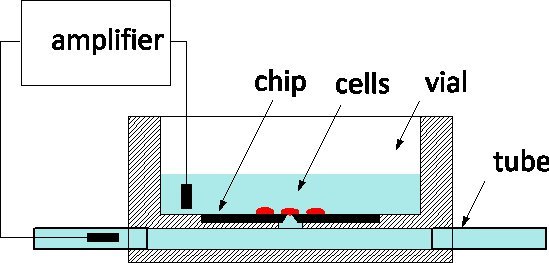
Figure 1. Principle of measurement using the NRCC patch-clamp chip
We detail here the protocols to sterilize and prime the chips, load them with medium, plate them with cells, and finally use them for electrophysiological recordings.
Protocol
1. Chip fabrication
The process described in 6 results in a 3 μm thick self-standing film, a low 1 Mohm access resistance and a smooth-surfaced funnel shaped aperture that facilitates an intimate seal with the cell 9, see Figure 2. The chips are singulated and glued in Plexiglas packages with the aperture facing a hole connecting the chip to a subterranean channel. The gluing is dispensed in a way that minimizes the shunt capacitance to a nominal 17 pF. Packages are fitted with two 1.5 mm diameter and 6 mm long glass tubes as fluidic ports (Figure 3).
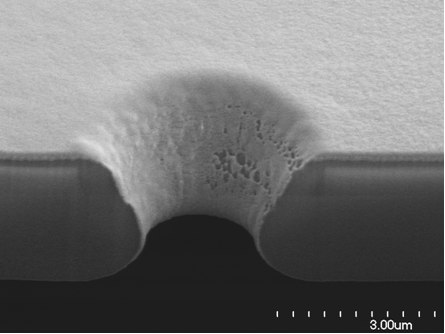
Figure 2. Scanning electron micrograph of a focused ion beam section of an NRCC patch-clamp chip shows a smooth silicon dioxide surface and funnel shape that favors intimate cell contacts, and a shallow orifice that accounts for a low access resistance.

Figure 3. A chip is glued at the bottom of the culture vial in a Plexiglas package fitted with subterranean fluidics and glass tubes.
2. Sterilization, priming and testing
The following steps should be performed in a biology safety cabinet to avoid contamination or plugging of the aperture.
- Sterilize chips in a Harrick basic plasma cleaner (www.harrickplasma.com) with a 0.1-0.3 mbar residual air pressure for 15 min at the maximum power of 18 W. Other air plasma systems may be used, with comparable power density (24 mW/cm3) and keeping the product of power density and time constant. The plasma treatment also renders the chips hydrophilic, which facilitates priming.
- Fit glass tubes with sterile Silastic Laboratory silicone tubing, 1mm I.D. x 2mm O.D. (Cole Parmer Cat. # 96115-08), 3inch on one side, 1 inch on the other side.
- Fill fluidics through the tubes with sterile filtered standard Phosphate Buffer Solution (PBS), making sure no bubble is trapped.
- Clip the long silicone tube while pressurizing the PBS from the short side at 1 atm. A pool of PBS seeping through the aperture might be visible in the vial. Clip the pressurized supply of the PBS and detach the short silicone tube from that supply.
- Fill vial with filtered PBS using a syringe.
- Immerse an Ag/Ag:Cl electrode in the short tube and a counter-electrode in the vial. An impedance meter is used to confirm that the access resistance is between 300kohm and 3Mohm and the shunt capacitance is between 10pF and 25pF. A chip with a lower resistance is likely to have a leak, and a higher capacitance is considered improper for use as it may not capture the fastest dynamics of the cell′s electrophysiology 6. A chip with a lower capacitance or higher resistance is considered plugged, though it could be simply that a bubble is trapped in the orifice. Some chips are re-tested after 1H and found to have the correct electrochemical impedance.
- Flush the fluidic channel with sterile deionized water; empty the top vial and rinse with the same, twice.
- Immerse the packaged chip with tubes in fresh sterile deionized water for 30min then load with other chips in a sterile container filled with sterile deionized water. This ensures that chips remain hydrophilic and protected from contamination.
3. Chips preparation in cell biology lab
The following steps are performed in a HEPA filtered laminar flow hood using aseptic techniques, all solutions used in this procedure must be filtered sterilized prior to use using a 0.22μm filter.
- Open a chip container under a HEPA filtered laminar flow hood and remove chips from container face down to avoid scooping possible floating contaminants into top vial.
- Rinse top of vial of chip 2x with freshly filtered sterile deionized water to remove any residual debris from the chip surface.
- Aspirate sterile deionized water off the top vial.
- Connect one end of the silastic tubing attached to the fluidic channel to a pressurized syringe containing sterile filtered deionized water. In our system, syringes filled with solution are pressurized by connection to a compressed air tank set to 20psi. To ensure sterility, the air is filtered (0.22 μm filter) prior to entry into the syringe and our system also has an on/off valve that allows us to stop or apply pressure when needed.
- Using a hemostat, clamp the output end of the tubing.
- Open the on/off valves to pressurize the solution.
- To rinse the subterranen fluidic of the chip with fresh sterile deionized water, unclamp the output end of the tubing.
- To force the water up through the aperture, clamp the output end of the tubing with a hemostat.
- To avoid water drainage, clamp the input and output ends of the tubing with an hemostats and close the on/off valves.
- Detach the input end of the tubing from the syringe.
- Insert glass plugs into both ends of the tubing and remove the hemostats.
- Fill top vial with filter steriled deionized water.
- Place the lid of a 35 mm sterile dish into the base of a 100 mm sterile dish
- Place chip on top of the 35 mm lid and cover with 100 mm dish lid.
- Image chips to ensure that the membrane and aperture of the chips are free of debris and/or air bubbles. In our lab we use an upright microscope and a 20x long working distance objective.
- If debris and/or air bubbles are present, repeatedly flush fluidic channel and top vial. If the debris and/or air bubbles cannot be removed the chip is discarded.
- Once the chips are imaged, return to laminar flow hood and unplug both ends of the tubing.
- Attach one end of the tubing to a pressurized syringe containing physiological media.
- Clamp the output end of the tubing with a hemostat
- Open pressure valve, remove the hemostat from the output end of the fluidics and flush the fluidic channel with physiological media
- To rinse the subterranean fluidic with physiological media, open the on/off valves and remove the hemostat from the output end of the tubing.
- To force the physiological media up through the aperture, clamp the output end of the tubing with a hemostat.
- Clamp input end of the tubing with a hemostat and close the on/off valves.
- Remove the input end of the tubing from the syringe and plug both ends of the tubing with glas plugs.
- Remove the hemostats.
- Remove the water from the top vial.
- Fill the top vial with filter sterilized physiological media.
- Place the chip back into the 100 mm Petri dish.
- Place the base of a 35 mm dish into the 100 mm dish and fill it with filter sterilized deionized water to enrich humidity.
- Cover the dish until it is time for plating
4. Cell plating of snail neurons
The patch-clamp chips may be suitable for a variety of preparations. We are currently testing our chips with mammalian primary cortical neurons and have obtained preliminary results with cells cultured for 14 days 7, which indicates that our sterilization protocol is adequate and that the chips are not cytotoxic in long-term cultures. For the purpose of this protocol, snail neurons were chosen because they represent a simple but well-established model to study neuronal electrophysiology 11, and it is with those cells that we have obtained the most significant results to date 10. Detailed cell isolation and culture procedures have been described previously 12, 13.
-
Remove the outer shell from 2-3 month old Lymnaea stagnalis with blunt forceps and anesthetize animals in Lymnaea saline containing 10% Listerine.
All subsequent steps performed aseptically within a laminar airflow hood with sterilized dissection equipment and solutions at room temperature. - Replace physiological media in the fluidics with the appropriate recording solutionusing an Eppendorf pipette. In the case of Lymnaea neurons the solution contains (in mM): 50 KCl, 5 MgCl2, 5 ethylenebis(oxyethylenenitrilo)tetraacetic acid (EGTA) and 5 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES; pH 7.4; 130 mOsm)14
- Pin the snails into a Sylgard dish filled with Lymnaea saline and remove entire brain as previously described 13
- Treat the isolated brains in defined media (DM) with trypsin enzyme (0.2% Solution, Sigma, Catalog # T-9201) for 18 minutes followed by treatment in DM and trypsin inhibitor (Sigma, Soybean, Catalog # T-9003) for 15 minutes.
- Pin the brains into a small Sylgard dish filled with high osmolarity defined media (HODM - DM with glucose added; 750 μL of 1M glucose solution to 20 mL DM).
- Using fine forceps and dissection scissors, remove the outer and inner sheath of the ganglia of interest, exposing the neurons.
- Using a fire polished glass pipette filled with HODM and attached to a microsyringe, apply gentle suction near the cell of interest (Left pedal dorsal 1) until the neuron detaches from the brain and is suspended in the pipette.
- The glass pipette is then moved and dipped into individual neurochips where gentle expulsion from the microsyringe allows the neurons to be gently pushed out of the pipette and placed on top of the individual patch holes on the chips.
- Allow cells to sit undisturbed for a minimum of 2h at room temperature to promote their attachment to the chip surface surrounding the aperture.
5. Electrophysiological recordings
To connect the chips to the amplifier (in our case a Multiclamp 700B amplifier, Molecular Devices, Foster City, CA, USA)
- Replace the solution in the subterranean fluidic and in the chip vial with the appropriate recording solutions. In the case of Lymnaea neurons , the upper culture chambers are filled with Lymnaea saline (in mM: 51.3 NaCl, 1.7 KCl, 4 CaCl2, and 1.5 MgCl2) buffered to pH 7.9 with HEPES.14
- For stability, glue the chip onto a glass slide and place it under a microscope.
- To connect the chip to the amplifier (in our case a Multiclamp 700B amplifier, Molecular Devices, Foster City, CA, USA), cut one end of the tubing and insert a silver wire which is connected to the head-stage.
- Place the reference electrode in the top vial of the chip.
- The chip is now ready for recording.
- Apply a 5 mV step with the amplifier to measure total resistance (Rt) and determine the configuration of the neurons: cell-attached (Rt > 1 GΩ); whole-cell (Rt = 50-100 MΩ plus capacitive transients, indicative of membrane rupture over the aperture); or no seal (Rt < 5 MΩ). In our last set of experiments 10, 58% percent of cells had high-resistance seals and, of those, 80% of cells showed excitable responses.
6. Representative Results
- Apply depolarizing current pulses (20 pA increment) to the neuron (in this case LPeD1).
- Hold the neuron at Vm close to its resting potential (~ - 60 mV).
- Observe the responses to these depolarizing pulses. Overshooting action potentials should be observed if the neuron is viable.
- Figure 4 shows a representative result which is discussed in details in 14, 15.
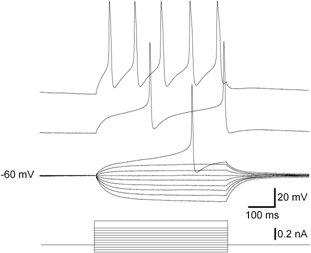
Figure 4. Voltage responses (top) of a LPeD1 neuron to graded series of intracellular current pulses (below). The current pulses were applied at Vm = - 60mV.
Troubleshooting
- Before plating, image chips to determine if membrane and aperture are free of debris and suitable for plating. We use an upright microscope and a 20x long working distance objective. In our last set of experiments 1, 67% percent of chips were successfully primed in that manner.
- If debris and/or air bubbles are present discard chip and try another one.
- Various surface treatments (plasma) or coatings (PDL, PEI etc.) may be tried, but success may be highly dependent upon cell type used.
- The composition of the fluidic ("pipette") solution in critical for the formation of the giga-seal and the whole-cell. Adjust solution, paying attention to osmolarity, pH, and ionic makeup.
- For longer term culture, begin with media in microfluidic channel, then switch to pipette solution prior to recording via gentle gravity fed perfusion (~0.5 ml/min).
Discussion
NRCC's patch-clamp chip interrogation platform is a potentially powerful tool for high information content pharmaceutical assays and to investigate in vitro models of disease. Its advantages compared to glass pipettes are a low access resistance, which is an advantage to probe large cells, and despite a somewhat larger capacitance will result in comparable dynamics for smaller cells. Spontaneous cell to aperture seals have been routinely obtained, and whole cell entry has been observed to be spontaneous 14. A clear difference between chips and the glass pipette method is the fact that the probe is part of the cell culture dish and is not manually brought in contact with the cell membrane. Culturing cells, possibly part of functional networks, results in more biologically relevant models as disease models, and a different mechanism for securing high cell to probe seals 16. However, by contrast with cell suspensions, aspiration cannot be used to position a cell on the probe. Snail neurons, as other large cells, are amenable to manual positioning on top of the probe. For smaller cells requiring longer culture times, we have obviated the need for any manipulation and keep a high probability of obtaining a seal by patterning adhesion polypeptides on top of the probes to place cells on top of the probes, and demonstrated placement of cells on the probe 17,18.
NRCC is also developing a polyimide microfluidic patch-clamp chip 19 with a capacitance comparable to that of glass pipette. The ultimate goal of that project is a multiple-probes patch-clamp chip which allows the simultaneous monitoring of the electrophysiological activity of several neurons engaged in network behavior at the resolution of individual ion channels 14. This method is a high resolution complementary method to multi-electrode arrays 20.
Acknowledgements
The authors wish to acknowledge Alexei Bogdanov for the fabrication of patch-clamp chips at the CPFC, and Hue Tran, Ping Zhao and Matthew Shiu for assistance with assembly. Naweed Syed was supported by a Canadian Institute of Health Research (CIHR) grant. Collin Luk is the recipient of NSERC and Alberta Heritage Foundation for Medical Research (AHFMR) studentships.
References
- Walz, W. Patch-Clamp Analysis: Advanced Techniques. Neuromethods. , 38 (2007).
- Neher, E., Sakmann, B. Single-channel currents recorded from membrane of denervated frog muscle fibers. Nature. 260, 799-802 (1976).
- Behrends, J. C., Fertig, N. Ch. 14: Planar Patch-clamp. Neuromethods. , (2007).
- Fertig, N., Tilke, A., Blick, R. H. Stable integration of isolated cell membrane patches in a nanomachined aperture. Applied Physics Letters. 77, 1218-1220 (2000).
- Dunlop, J., Bowlby, M., Peri, R., Vasilyev, D., Arias, R. High-throughput electrophysiology: an emerging paradigm for ion-channel screening and hysiology. Nat. Rev. Drug. Discov. 7 (4), 358-368 (2008).
- Py, C., Denhoff, M., Martina, M. A novel silicon patch-clamp chip permits high-fidelity recording of ion channel activity from functionally defined neurons. Biotechnology and Bioengineering. 107 (4), 593-600 (2010).
- Martinez, D., Martina, M., Kremer, L. Development of patch-clamp chips for mammalian cell applications. Micro and Nanosystems. 2 (4), (2010).
- Py, C., Salim, D., Monette, R. Cell to aperture interaction in patch-clamp chips visualized by fluorescence microscopy and focused-ion beam sections. Biotechnology & Bioengineering. 108, 1395-1403 (2011).
- Martina, M., Luk, C., Py, C. Interrogation of Cultured Neurons using Patch-Clamp Chips. Journal of Neural Engineering. 8, 034002 (2011).
- Bell, H. J., Syed, N. I. Hypoxia-induced modulation of the respiratory CPG. Frontiers in bioscience : a journal and virtual library. 14, 3825-3835 (2009).
- Syed, N. I., Bulloch, A. G. M., Lukowiak, K. In vitro reconstruction of the respiratory central pattern generator of the mollusk Lymnaea. Science. 250, 282-285 (1990).
- Syed, N. I., Zaidi, H., Lovell, P., Windhorst, U., Johansson, H. In vitro reconstruction of neuronal circuits: A simple model system approach. Modern techniques in neuroscience research. , (1999).
- Martina, M., Luk, C., Py, C. Interrogation of Cultured Neurons using Patch-Clamp Chips. Journal of Neural Engineering. 8, 034002 (2011).
- Py, C., Denhoff, M., Martina, M., et al. Silicon patch-clamp chip suitable for high-fidelity recording of ion channel activity from cultured neurons. Biotechnology and Bioengineering. 107 (4), (2010).
- Ong, W. -. L., Yobas, L., Ong, W. -. Y. A missing factor in chip-based patch clamp assay: gigaseal. Journal of Physics: Conference Series. 34, 187 (2006).
- Charrier, A., Martinez, D., Monette, R. Cell placement and guidance on substrates for neurochip interfaces. Biotechnology and Bioengineering. 105, 368-373 (2010).
- Diaz-Quijada, D., Maynard, C. C. o. m. a. s., Monette, T., Py, R., A, C. K. r. a. n. t. i. s., Mealing, G. Surface Patterning with Chemisorbed Chemical Cues for Advancing Neurochip Applications. Industrial & Engineering Chemistry Research. 50 (17), 10029-10035 (2011).
- Martinez, D., Py, C., Denhoff, M., et al. High-fidelity patch-clamp recordings from neurons cultured on a polymer microchip. Biomedical Microdevices. 12, 977-97 (2010).
- Taketani, M., Baudry, M. . Advances in Network Electrophysiology: Using Multi-Electrode Arrays. , (2006).
Explore More Articles
This article has been published
Video Coming Soon
ABOUT JoVE
Copyright © 2024 MyJoVE Corporation. All rights reserved