Optimization and Utilization of Agrobacterium-mediated Transient Protein Production in Nicotiana
In This Article
Summary
Transient protein production in Nicotiana plants based on vacuum infiltration with Agrobacteria carrying launch vectors (Tobacco mosaic virus-based) is a rapid and economic approach to produce vaccine antigens and therapeutic proteins. We simplified the procedure and improved target accumulation by optimizing conditions of bacteria cultivation, selecting host species, and co-introducing RNA silencing suppressors.
Abstract
Agrobacterium-mediated transient protein production in plants is a promising approach to produce vaccine antigens and therapeutic proteins within a short period of time. However, this technology is only just beginning to be applied to large-scale production as many technological obstacles to scale up are now being overcome. Here, we demonstrate a simple and reproducible method for industrial-scale transient protein production based on vacuum infiltration of Nicotiana plants with Agrobacteria carrying launch vectors. Optimization of Agrobacterium cultivation in AB medium allows direct dilution of the bacterial culture in Milli-Q water, simplifying the infiltration process. Among three tested species of Nicotiana, N. excelsiana (N. benthamiana × N. excelsior) was selected as the most promising host due to the ease of infiltration, high level of reporter protein production, and about two-fold higher biomass production under controlled environmental conditions. Induction of Agrobacterium harboring pBID4-GFP (Tobacco mosaic virus-based) using chemicals such as acetosyringone and monosaccharide had no effect on the protein production level. Infiltrating plant under 50 to 100 mbar for 30 or 60 sec resulted in about 95% infiltration of plant leaf tissues. Infiltration with Agrobacterium laboratory strain GV3101 showed the highest protein production compared to Agrobacteria laboratory strains LBA4404 and C58C1 and wild-type Agrobacteria strains at6, at10, at77 and A4. Co-expression of a viral RNA silencing suppressor, p23 or p19, in N. benthamiana resulted in earlier accumulation and increased production (15-25%) of target protein (influenza virus hemagglutinin).
Introduction
Plants are now recognized as a safe, reliable, scalable and inexpensive platform for producing heterologous recombinant biopharmaceuticals and industrial proteins1-3 and have important advantages over microbial and animal cell expression systems4. Plants are able to express correctly folded proteins with post-translational modifications, including assembled multimeric antibodies5-7. Several plant-derived recombinant pharmaceutical proteins are undergoing clinical evaluation8. These include patient-specific recombinant idiotype vaccines (scFv) for the treatment of non-Hodgkin’s lymphoma9, hemagglutinin-based pandemic and seasonal influenza vaccine candidates10,11 (Cummings et al., submitted to Vaccine), anti-Streptococcus surface antigen I/II antibody for the treatment of dental caries12, and human insulin for the treatment of diabetes13. Furthermore, human recombinant glucocerebrosidase for enzyme replacement therapy in patients with Gaucher disease has been approved in Israel and the US and is provided under the Expanded Access Program outside of the US14,15.
Heterologous proteins can be produced in stably transformed (transgenic or transplastomic) or transiently transformed plants. Transient protein production offers several advantages over production in transgenic plants, including short timeframe to achieve expression and accumulation16, and can be achieved by introducing bacterial binary vectors or recombinant plant viral vectors into plant tissues4. The most advanced transient expression system is based on the use of ‘launch vectors’ that combine components of plant viruses and binary plasmids, and are delivered by agroinfiltration17,18. Agroinfiltration of a launch vector based on Tobacco mosaic virus (TMV) has been successfully applied at lab scale to produce vaccine antigens against pathogens such as human papilloma virus19, Yersinia pestis20, influenza viruses A21,22, Bacillus anthracis23, and smallpox virus24 in N. benthamiana leaves. Agrobacterium-mediated transient expression is also a promising method for the simultaneous production of multiple proteins2,25-27. For example, plant transient expression systems have been used to produce tumor-specific recombinant antibodies28,29, a glycosylated recombinant antibody against the epidermal growth factor receptor30, and a monoclonal antibody specific for anthrax protective antigen31,32. Co-infiltration of Nicotiana benthamiana plants with a target gene and a suppressor of gene silencing results in enhanced target protein expression33,34.
Agroinfiltration is a common method for uniformly introducing bacteria harboring a gene of interest into plant tissues35-37. Vacuum infiltration of Agrobacterium for transient gene expression in intact plant leaves is a rapid, scalable, and useful method for production of foreign proteins without the need to generate transgenic plants38-41. During vacuum agroinfiltration, plants are flipped upside down and aerial parts submerged in Agrobacterium suspension. Then vacuum is applied causing gases to evacuate from leaf intercellular spaces through stomata. Rapid re-pressurization following release of the vacuum results in the infusion of the Agrobacterium suspension into the leaf. Following vacuum infiltration of Agrobacteria, plants are further cultivated and target expression is monitored. The highest levels of target expression are typically observed 2-3 days post infiltration (dpi) with a binary vector and 4-7 dpi with a launch vector, after which the expression level typically decreases17,18,42-45. Agrobacterium tumefaciens is the most widely used vehicle for delivering a gene of interest into a plant for protein production. Agroinfiltration works exceptionally well in N. benthamiana but relatively poorly in most other plants, including Arabidopsis thaliana46.
In this study, we developed a simple, efficient, and economical method for transient protein production in 5–6 week-old N. benthamiana using A. tumefaciens infiltration. The major drawback of industrial scaling of the agroinfiltration technique is centrifugation of harvested bacteria and resuspension of the bacterial pellet in medium containing 4'-hydroxy-3',5'-dimethoxyacetophenone (acetosyringone), monosaccharides, and 2-(N-morpholino)-ethanesulfonic acid (MES) buffer for induction of the vir genes. We have been able to overcome these problems by optimizing the Agrobacterium growth in AB medium (minimal medium) followed by directly diluting in Milli-Q water and by controlling the infiltration duration and conditions. We have also compared target protein production in the wild-type tobacco host species N. benthamiana and N. excelsior, as well as in hybrid N. excelsiana.
Protocol
1. Plant Growing
For subsequent agroinfiltration we evaluated two wild-type Nicotiana species (N. benthamiana and N. excelsior) and a hybrid (N. excelsiana) grown hydroponically on rockwool in indoor facilities.
- Soak rockwool slabs in a plant fertilizer solution.
- Sow seeds of wild-type N. benthamiana, N. excelsior and N. excelsiana (hybrid of N. benthamiana × N. excelsior) on the nutrients soaked rockwool surface.
- Grow plants from the seeds under controlled conditions (24 °C and 40-65% relative humidity) and a long-day photoperiod (14 hr light and 10 hr dark, with illumination of 130-150 μE m-2 sec-1) for 4-5 weeks for N. benthamiana and N. excelsiana, and 5-6 weeks for N. excelsior.
2. Construction of Vectors for Agroinfiltration
- Insert a synthetic reporter gene (green fluorescent protein [GFP]), full-length hemagglutinin (HA) from the A/California/04/2009 strain of influenza virus (HAC1), and re-engineered lichenase enzyme (LicKM)18 separately into the launch vector pBID418 (TMV-based vector) to obtain pBID4-GFP, pBID4-HAC1 and pBID4-LicKM, respectively18,32,41,47.
- Introduce 10-50 ng of pBID4 carrying GFP or HAC1 into electrocompetent cells of A. tumefaciens strain GV3101 and LicKM into electrocompetent cells of A. tumefaciens strains GV3101, C58C1, GLA4404, At06, At10, At77 and A4 with the gene MicroPulser electroporator.
- Use the transformed Agrobacteria for infiltration experiments unless otherwise noted.
3. Vacuum Infiltration of Agrobacterium into Nicotiana Plants
- Grow A. tumefaciens strains overnight (O/N) in LB medium, YEB medium or AB medium supplemented with 50 mg/L of Kanamycin at 28 °C with shaking at 200-250 rpm.
- Dilute Agrobacteria in Milli-Q water to an optical density at 600 nm (A600) of 0.5 or centrifuge Agrobacterium cells grown in LB or YEB or AB at 4,000 × g for 10 min at 4 °C, re-suspend in induction medium (1x MS salt, 10 mM MES, 200 µM acetosyringone, 2% sucrose [MMA]) to A600 of 0.5, and stir at room temperature for 1-3 hr, unless otherwise noted.
- Infiltrate plants in a vacuum chamber by submerging Nicotiana plant aerial tissues in Agrobacterium suspension and applying a 50-400 mbar vacuum for 30 or 60 sec. The optimal infiltration is routinely applied at 50-100 mbar for 60 sec.
- Once the vacuum is broken, remove plants from the vacuum chamber, rinse in water, and grow for 5-7 days under the same growth conditions used for pre-infiltration growth.
- To test the efficacy of chemicals inducing Agrobacterium vir gene, different concentrations of acetosyringone (0, 100, 200 or 400 µM) were added to the Agrobacteria suspended in infiltration buffer (1x MS, 10 mM MES, 2% glucose). For the effect of monosaccharide on induction of vir gene, different percentages of glucose (0, 1, 2 or 4%) were added to Agrobacteria suspended in the infiltration buffer (1x MS, 10 mM MES, 200 µM acetosyringone). N. benthamiana plants were infiltrated as mentioned above in steps 3.3 and 3.4).
- Agrobacterium laboratory strains GV3101, C58C1 and LBA4404 and wild-type strains A4, At06, At10, and At77 harboring the pBID4-LicKM vector were diluted in Milli-Q water to A600 of 0.5. N. benthamiana plants were infiltrated with each particular strain as mentioned above in steps 3.3 and 3.4.
4. Co-agroinfiltration Procedure for the Viral Silencing Suppressor
- Mix the Milli-Q water-diluted Agrobacterium GV3101 cultures carrying the GFP gene and the viral silencing suppressor p19 of Tomato bushy stunt virus (TBSV) at 1:1, 2:1, 3:1 and 4:1 ratios. Infiltrate N. benthamiana plants as described above.
- Infiltrate N. benthamiana plants with a mixture of two Milli-Q water-diluted Agrobacterium GV3101 cultures: the first carrying the pBID4-HAC1 plasmid and the second carrying one of the silencing suppressors – p19 of TBSV or p23 of Citrus tristeza virus, in the pCassp plasmid (pCassp19) and in the pGR binary plasmid under the 35S promoter (pGR-P23), respectively, at the ratio 4:1.
5. Western Blot Analysis
- Collect random leaf samples from N. benthamiana, N. excelsior or N. excelsiana plants at 4-7 dpi and pulverize in liquid nitrogen to a fine powder.
- Add three volumes of 1x PBS buffer containing 0.5% Triton X-100 to each sample.
- Gently shake the extracted samples for 15 min at 4 °C.
- Spin the extract for 5 min and collect total soluble protein into a clean Eppendorf tube.
- Dilute the extracts to an appropriate dilution (1:50 1:100) in 1x PBS extraction buffer, and add 5× sample buffer (250 mM Tris-HCl [pH 6.8], 10% SDS, 0.5% bromophenol blue, 50% glycerol v/v, and 500 mM DTT) to a final 1× concentration.
- Boil samples for 5 min.
- Separate proteins by 10% SDS-PAGE, transfer onto Immobilon-P transfer membrane, and block with 0.5% I-block.
- Detect GFP using rabbit polyclonal anti-GFP antiserum at 1:5,000 and HAC1 using mouse anti-poly-histidine monoclonal antibody at 1:1,000 in blocking solution for 1 hr.
- After primary antibody labeling, wash the membranes three times for 10 min each with 1× PBST-20 and incubate with a horseradish peroxidase (HRP)-conjugated anti-rabbit antibody at 1:5,000 or a HRP-conjugated anti-mouse antibody at 1:10,000 for 1 hr, for GFP and HAC1 detection, respectively.
- Process Western blots using the SuperSignal West Pico Chemiluminescent substrate.
- Use the GeneTools Software to analyze band intensity of the protein and obtain the band calibrated quantity.
Protein production: (Calibrated quantity x dilution of sample)/amount of sample loaded) x 4 = mg/kg.
Equation: Protein production (P), Calibrated quantity (C), Dilution of the sample (D) and amount of Sample loaded (S).
6. Zymogram Assay
- Collect pBID4-LicKM-infiltrated N. benthamiana random tissue samples.
- Extract proteins using the same methods described above for Western blot analysis and then analyze by 10% SDS-PAGE with 0.1% lichenan included in the gels.
- After electrophoresis, wash the gels two times for 10 min each in wash buffer (100 mM Tris-HCl [pH 8.0] and 0.1% Triton X-100) and then incubate in wash buffer at 65 °C for 1 hr.
- After incubation, discard the wash buffer and stain the gels with 0.5% Congo Red for 5 min at room temperature.
- Rinse the gels in Milli-Q water three times for 10 min each, and add 1 M NaCl to visualize lichenase activity. The purified bacterial lichenase protein was used as a positive control for enzyme activity.
7. GFP Imaging
- Perform visual detection of GFP fluorescence in whole transiently transformed plants using a hand-held long-wavelength UV lamp.
- Photograph transiently transformed plants with a digital camera through a Yellow 8, ES 52 filter (exposure time, 15 sec).
- Obtain images from Western blot analyses using the GeneSnap software on a GeneGnome and quantify the results using the GeneTools software, with a calibration curve based on purified GFP standard.
- Quantify HAC1 protein using a calibration curve based on purified HAC protein standard from the A/Indonesia/05/05 strain of influenza virus.
- Calculate mean values from 3-4 replicates for all experiments.
Results
Nutrient requirements for plant growth. The use of hydroponic plant growth medium (Rockwool) and nutrient solution ensures uniformity of N. benthamiana growth and eliminates complexities (mechanical, regulatory and efficiency) associated with using soil for plant cultivation. We grew N. benthamiana on rockwool slabs soaked in commercially available fertilizers to determine the optimal conditions for plant growth and biomass accumulation. We observed 95-100% seed germination. One should note that including phosphorus is critical to achieve germination, because we found that nutrient solution lacking phosphorus failed to support germination and growth of N. benthamiana seeds (Figure 1A).
Effects of Agrobacterium growth and infiltration media on plant health and protein production. We have tested several media conditions to optimize the efficiency of the agroinfiltration technique for large-scale production. Bacteria (A. tumefaciens GV3101 strain) harboring the pBID4-GFP construct were cultivated O/N in different media conditions (YEB, LB or AB), and either centrifuged and re-suspended in induction medium (MMA) (containing 1× Murashige & Skoog [MS] Basal Salt Mixture, 10 mM MES pH 5.6, 20 g/L sucrose and 200 µM acetosyringone) or diluted in Milli-Q water to A600 of 0.5 before using for plant infiltration. We observed that vacuum infiltration of plants with bacteria diluted in water resulted in protein production comparable to those achieved with any infiltration media in previous reports42,48. In contrast, infiltration with undiluted Agrobacteria grown in YEB or LB media resulted in complete wilting of N. benthamiana leaves in less than 24 hr post infiltration, while undiluted Agrobacteria grown in AB medium had no effect on the health of infiltrated plants (data not shown). As illustrated in Figure 1B, plants infiltrated with Agrobacterium cultures grown in YEB, LB or AB media and diluted with Milli-Q water (1:5, A600 of 0.6-0.8 or 1:10, A600 of 0.3-0.4) showed no symptoms and exhibited an average GFP production of 1645, 1520 and 1839, respectively. Agrobacteria centrifuged and re-suspended in induction medium (MMA) showed no symptoms and no significant difference in protein production compared to Agrobacteria directly diluted in Milli-Q water (1671 ± 102 and 1667 ± 131 mg/kg, respectively). Therefore, Milli-Q water is recommended for diluting Agrobacterium cultures for plant infiltration and was routinely used in our subsequent experiments to achieve an A600 of 0.5.
Effects of Agrobacterium suspension cell density and time course on target expression. We next examined if bacterial cell density affects the efficiency of infiltration and levels of target expression. For this purpose, we assessed four different cell suspension densities of Agrobacterium carrying pBID4-GFP, A600 of 1.0, 0.5, 0.1 and 0.05. Following infiltration, N. benthamiana plants were monitored for visible symptom development and time course of target expression by collecting samples at 4, 7 and 10 dpi. At 4 dpi, we observed noticeable differences in GFP fluorescence among plants infiltrated with different cell suspension densities of Agrobacterium (no GFP expression was observed at A600 of 0.05). At 7 dpi, GFP fluorescence was similar in plants infiltrated at cell suspension densities of A600 1.0, 0.5 and 0.1, but was lower in plants infiltrated at an A600 of 0.05. As shown in Figure 1C, these data were confirmed by Western blot analyses of samples collected at 4 dpi, showing very low protein production at A600 of 0.05 (5 mg/kg) and highest at A600 of 1.0 (1739 mg/kg). At 7 dpi, plants showed no significant differences in estimated GFP production at A600 of 1.0, 0.5 and 0.1 (1,662, 1,870 and 1,890, respectively), while A600 of 0.05 showed lower GFP production (1,199 mg/kg). In contrast, at 10 dpi no differences in GFP production were observed among plants infiltrated with either of the four cell suspension densities (1,218, 1,181, 1,197 and 1,304).
Infiltration with alternative strains of Agrobacterium. To increase the diversity of Agrobacterium strains available for transient protein production, we tested wild-type isolates. These strains, isolated from the crown-gall of natural hosts, were kindly provided by Dr. Gelvin (Purdue University, West Lafayette, Indiana). To examine their utility in transient protein production, we infiltrated N. benthamiana with the following strains carrying pBID4-LicKM18: A. rhizogenes (A4) and A. tumefaciens wild-type Nester strains A348, A208, and A281 (named At6, At10, and At77, respectively), as well as engineered laboratory strains of A. tumefaciens GV3101, C58C1, and LBA4404. The infiltrated leaves were collected at 7 dpi and the level of LicKM expression was estimated by Western blot assay. As shown in Figure 2A, the highest level of LicKM production can be achieved with the strains GV3101, A4 and LBA4404 (~1,750 ± 163, 1,650 ± 26 and 1,450 ± 117 mg/kg, respectively), with slight differences; the lowest level of expression (~900 ± 102 mg/kg) with C58C1; and intermediate production with At6, At10 and At77 (~1,250 ± 19, 1,100 ± 42 and 1,200 ± 111 mg/kg, respectively). The lichenase enzymatic activity was demonstrated using Zymogram assay. Figure 2B shows that lichenase produced in infiltrated plant tissues using any of the Agrobacterium strains was enzymatically active. One should also note that N. benthamiana plants infiltrated with A4 and At77 strains showed pathological symptoms (stunting, petiole elongation and curling, and leaf curling), while with At10 strain the symptoms were mild. No symptoms were observed in N. benthamiana plants infiltrated with laboratory strain GV3101 (Figure 2C).
Infiltration of alternative Nicotiana species. We compared the rates of biomass generation and protein production in two wild-type species of the Nicotiana genus (N. benthamiana and N. excelsior) and in a hybrid species, N. excelsiana (N. benthamiana × N. excelsior). Of the tested species, N. benthamiana, a widely used host for transient protein production using Agrobacterium-based or viral-based expression systems2,34,49, reaches infiltration readiness within 4-5 weeks of germination. The necessary growth period to generate the optimal level of biomass is also 4-5 weeks for N. excelsiana but is longer (6-7 weeks) for N. excelsior. In addition, the plant internodes are relatively short for N. excelsior compared to other Nicotiana species.
Furthermore, we observed that vacuum infiltration of N. benthamiana and N. excelsiana at 50-250 mbar for 60 sec is highly efficient for agroinfiltration of entire leaves, while N. excelsior is difficult to infiltrate due to their lower canopy and leathery leaves, even when a vacuum was applied three times for 1 min each in the presence of non-ionic surfactants such as Sillwet-77 or S240. Also, the germination rate of N. excelsiana and N. excelsior seeds was ~40-50%; in order to increase the germination rate to 90-100%, seeds must be treated with 10% bleach for 1 hr before seeding. Under the same growth conditions, the highest leaf biomass that can be generated from N. excelsiana is approximately two-fold higher compared with N. benthamiana (Table 1).
Protein production was examined in N. benthamiana, N. excelsior and N. excelsiana infiltrated with the Agrobacterium strain GV3101 harboring pBID4-GFP. GFP accumulation was assessed at 7 dpi in whole infiltrated leaves using UV light followed by Western blot analysis. Figure 3A shows even distribution of GFP in N. benthamiana and N. excelsiana and uneven distribution in N. excelsior (due to a difficulty of infiltrating an entire leaf area of N. excelsior). Figure 3B shows the level of GFP production estimated by UV light illumination in infiltrated leaves collected from the three Nicotiana species at 7 dpi. The GFP accumulation level was higher in N. benthamiana (~2.23 g/kg) than in N. excelsiana and N. excelsior (~1.89 and 1.54 g/kg, respectively). The low level of protein production in N. excelsior is due to uneven infiltration and distribution of accumulated GFP in the collected leaf.
We observed that upper leaves directly exposed to light often exhibit the earliest and highest levels of transient GFP accumulation (at 2-4 dpi) than leaves under the canopy. However, in our studies, GFP accumulation was the highest at 7 dpi and was distributed evenly across most leaves, except in uninfiltrated newly growing leaves which show no GFP accumulation.
Effects of vacuum pressure and duration on transient protein production. Vacuum infiltration significantly increases transient expression levels comparing to pressure applied by hand injection with a needleless syringe42. The application of a vacuum causes gases to evacuate from submerged plant leaves through stomata. When the vacuum is broken and pressure rapidly increases, the suspension of Agrobacterium is driven into leaves to replace the evacuated gases50.
To test the effect of vacuum pressure on the leaves of N. benthamiana, we infiltrated plants with the Agrobacterium strain GV3101 harboring pBID4-GFP under various vacuum pressures (50-400 mbar) for 30 or 60 sec. It was demonstrated that the stronger vacuum (below 50 mbar) applied for 30 or 60 sec results in mechanical damage of infiltrated leaves, leading to tissue wilting and plant death shortly after infiltration (24-48 hr). On the other hand, application of the milder vacuum (400 mbar) results in infiltration of only 50% of the leaf area and a decreased level of GFP production (303 ± 90 mg/kg) (Figure 4A). Importantly, we observed no differences in GFP production under 50, 100 and 200 mbar (1,651 ± 107, 1,688 ± 40, 1,594 ± 26 mg/kg, respectively) (Figure 4A) and mild to no, detrimental impacts on plant health when vacuum pressures from 50-200 mbar were applied for 30 or 60 sec. Therefore, 50-100 mbar of vacuum pressure is recommended for infiltration experiments.
The effect of duration of the vacuum on target expression was assessed by infiltrating one flat of N. benthamiana plants every hour with an A600 of 0.5 of GV3101 harboring pBID4-GFP for 8 hr in the same Agrobacterium culture. Figure 4B shows that the level of GFP production was similar at all-time points up to 8 hr, suggesting that over this period of time the Agrobacterium maintains its ability to launch a single-stranded DNA.
Effect of chemical induction on protein production. Certain plant phenolic metabolites and sugars can induce virulence genes of A. tumefaciens1,52. As a consequence, many chemicals and monosaccharaides have been reported to enhance transient protein production in various plant species. Acetosyringone is most commonly added to cultures of A. tumefaciens to induce the vir operon before agroinfiltration40,53-57.
We have assessed the effect of different concentrations of acetosyringone (0, 100, 200 and 400 µM) and glucose (0-4%) on transient GFP protein production in N. benthamiana infiltrated with the Agrobacterium strain GV3101 harboring pBID4-GFP. For this purpose, we re-suspended Agrobacterium cells in MMA induction media containing different concentration of acetosyringone and glucose for 1-3 hr before infiltration. According to the results of both visual observation (data not shown) and Western blot analysis (Figure 4C), none of the tested concentration of these compounds induced a significant increase in GFP fluorescence or protein production compared with control where induction media contained no acetosyringone or glucose.
Effect of co-infiltration of a silencing suppressor on transient production of GFP and HAC1 genes in N. benthamiana leaves. It has been previously demonstrated that co-expression of a silencing suppressor (p19 of Tomato bushy stunt virus [TBSV]) interferes with post-transcriptional gene silencing (PTGS), resulting in enhanced production of reporter proteins34.
We have evaluated the effect of co-infiltration of N. benthamiana with the launch vector carrying the GFP reporter gene (pBID4-GFP) and p19. Prior to infiltration, an A600 of 0.5 dilutions of A. tumefaciens GV3101 cultures harboring pBID4-GFP and p19 were respectively mixed at ratios of 1:1, 2:1, 3:1 and 4:1. Expression of the silencing suppressor was controlled by the Cauliflower mosaic virus 35S promoter. As indicated by the results of Western blot analysis at 7 dpi (Figure 5A), the presence of p19 did not increase or decrease GFP production in N. benthamiana, at any ratio of the two Agrobacterium suspensions.
We have also compared the effects of two viral gene silencing suppressors – p23and p19 – on the prevention of PTGS for HAC1. Cultures of Agrobacterium carrying the launch vector pBID4-HAC1 (H1N1 A/California/04/2009) and one of the two viral silencing suppressor plasmids were diluted to an A600 of 0.5, mixed at a ratio of 4:1, respectively, and co-infiltrated into 4-5-week old N. benthamiana. A suspension of A. tumefaciens carrying pBID4-HAC1 alone was infiltrated as a control. The infiltrated leaf samples were collected from 3 to 8 dpi. The experiment was repeated three times and average levels of the HAC1 expression determined by Western blot analysis.
As demonstrated in Figure 5B, co-infiltration of N. benthamiana with p23 or p19 resulted in (642 ± 157 and 764 ± 108 mg/kg, respectively) an increase in HAC1 production compared with using no silencing suppressor (approximately 15-25%, respectively) at 6 dpi. This suggests that p23 and p19 are efficient in our system. However, it should be noted that accumulation of HAC1 occurred a day earlier when pBID4-HAC1 was co-infiltrated with p19. Therefore, our results demonstrate that the effects of the silencing suppressor p19 on HAC1 and GFP accumulation are different, suggesting selective enhancement of transient expression and/or stability of some proteins in N. benthamiana.
We also observed that both in the presence and in the absence of a silencing suppressor the level of the HAC1 protein production started declining at 7 dpi. This indicates that the timing of the decline in the transient protein production in N. benthamiana infiltrated with the launch vector is target-specific.
The cell bank of Agrobacterium harboring the launch vector was evaluated every year for target gene stability, Agrobacterium viability and the level of protein accumulation. The glycerol stock of the cell bank of GV3101 strain transformed with pBID4-HAC1 that was stored at -80 °C has been shown to be very stable for more than three years without changes in the level of transient protein production in infiltrated N. benthamiana plants. Figure 5C demonstrates that the HAC1 protein production estimated by Western blotting in the years of 2010, 2011, 2012 and 2013 was 670, 685, 566 and 683 mg/kg, respectively. The average HAC1 production in N. benthamiana plants was 651 ± 49.4 mg/kg.
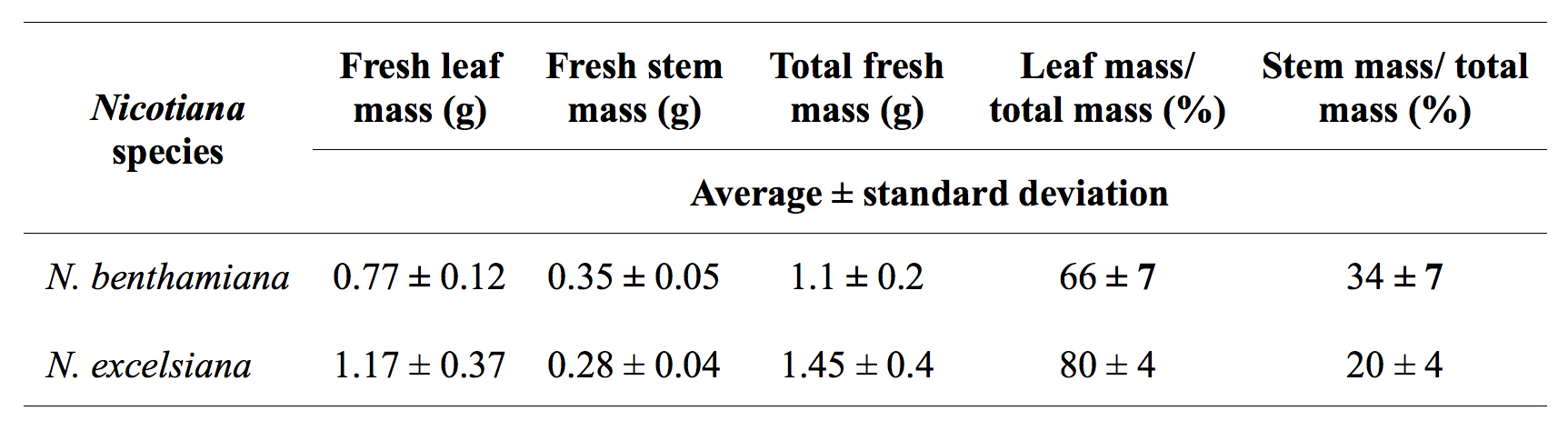
Table 1. Comparison of N. benthamiana and N. excelsiana plant biomass production.
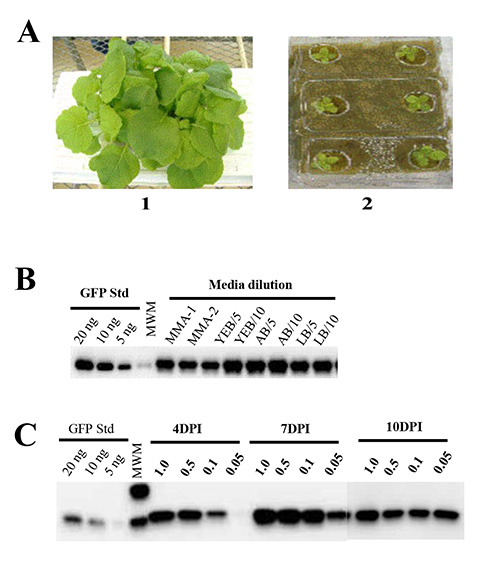
Figure 1. Western blot analysis of transient gene expression in N. benthamiana. (A) Six-week-old N. benthamiana 1) plants growing in a fertilizer solution containing 4.8% phosphorus and 2) plants growing in a fertilizer solution containing 0% phosphorus. Twenty five µg of fresh leaf weight equivalent was loaded per lane. (B) Comparison of GFP production in plants vacuum infiltrated with pBID4-GFP–harboring Agrobacterium GV3101 cultures grown in three different media: YEB, AB and LB. GV3101 cultures grown O/N in YEB or LB media were centrifuged at low speed and re-suspended in induction medium (MMA) (lanes: MMA-1 and MMA-2, respectively), or grown O/N in YEB, LB or AB media and directly diluted to 1:5 or 1:10 with Milli-Q water (lanes: YEB/5 and YEB/10; AB/5 and AB/10; LB/5 and LB/10). (C) Comparison of GFP expression at 4, 7 and 10 dpi following vacuum infiltration with different concentrations (A600 of 1.0, 0.5, 0.1 and 0.05) of A. tumefaciens GV3101 strain carrying pBID4-GFP.
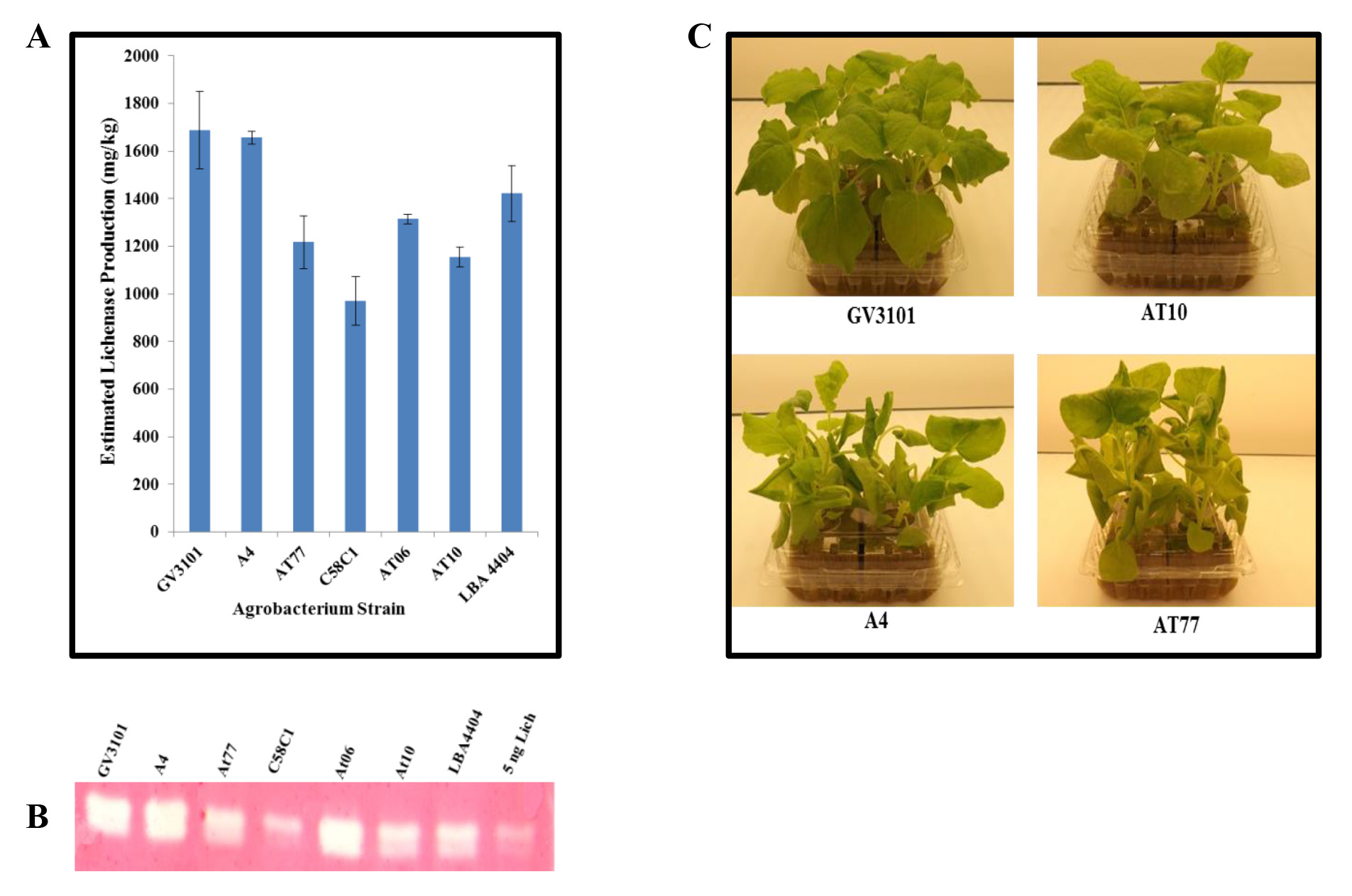
Figure 2. Comparison of transient lichenase production and activity following vacuum infiltration of N. benthamiana plants with different strains of Agrobacteria. Cultures of Agrobacteria strains (GV3101, A4, At77, C58C1, At6, At10 and LBA4404) harboring the launch vector pBID4-LicKM were infiltrated individually into leaves of N. benthamiana. Infiltrated leaves were collected at 7 dpi. (A) Lichenase production quantified by Western blotting. (B) Zymogram assay demonstrating lichenase production through enzymatic activity. (C) Effect of Agrobacterium (wild-type A4, At10, At77 and laboratory strain GV3101) infiltration on N. benthamiana plant health at 7 dpi. Twenty five µg of fresh leaf weight equivalent was loaded per lane.
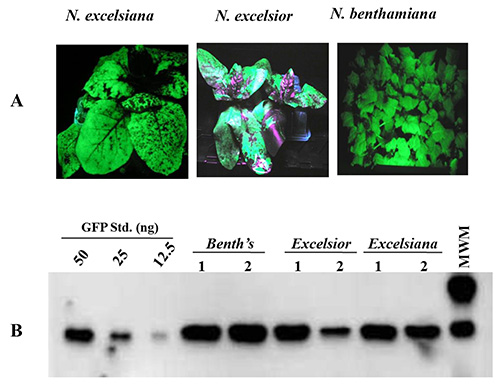
Figure 3. Transient GFP expression in leaves of N. benthamiana, N. excelsiana and N. excelsior at 7 dpi after vacuum infiltration with A. tumefaciens harboring the launch vector pBID4-GFP. (A) Visual examination of GFP expression under UV light. (B) Western blot analysis of GFP accumulation.
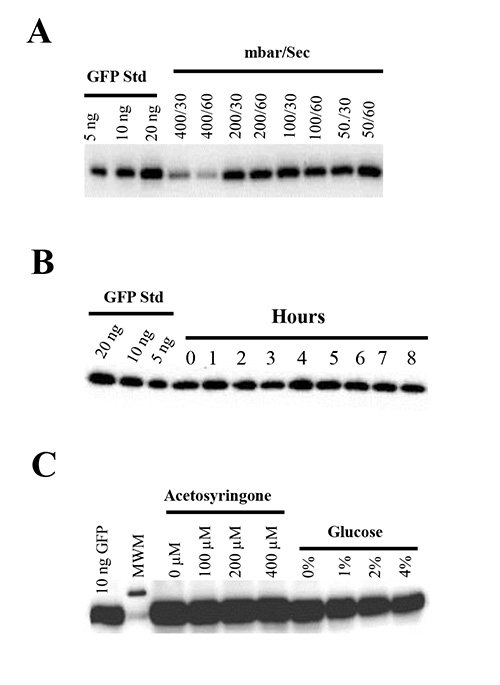
Figure 4. (A) Effects of vacuum pressure on transient GFP expression and plant health. N. benthamiana plants were infiltrated with pBID4-GFP under vacuum pressures of 400, 200, 100 or 50 mbar, at vacuum holding time of 30 or 60 sec. (B) Stability and infectivity of A. tumefaciens in N. benthamiana infiltrated with Agrobacterium GV3101 harboring pBID4-GFP grown in AB medium and diluted to an A600 of 0.5. Agroinfiltration was performed by infiltrating one flat of N. benthamiana plants every hour in the same diluted Agrobacterium culture (lanes 0-8). (C) Effect of different concentrations of acetosyringone and glucose on transient expression of GFP. The Agrobacterium strain GV3101 harboring pBID4-GFP was grown O/N in YEB media, centrifuged and resuspended to an A600 of 0.5 either in MMA containing 2% glucose with acetosyringone at 0, 100, 200 or 400 µM, or in MMA containing 200 µM acetosyringone with glucose at 0, 1, 2 or 4%. The Agrobacterium suspensions were kept for 3 hr at room temperature before infiltration.
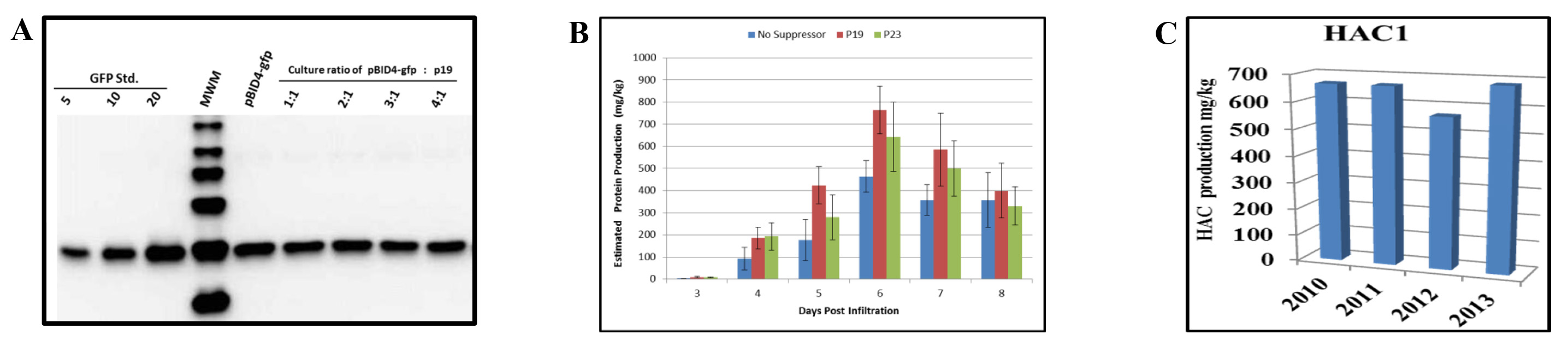
Figure 5. Effects of silencing suppressors on transient protein production in N. benthamiana leaves. (A) Western blot analysis of GFP protein following co-infiltration of pBID4-GFP and p19 at different ratios. Samples collected at 7 dpi (25 µg of fresh leaf weight equivalent was loaded per lane). (B) A culture of Agrobacterium carrying pBID4-HAC1 was individually mixed at a ratio of 4:1 with a culture carrying the p19 or p23 silencing suppressor plasmids. The resulting combinations of Agrobacterium cultures were vacuum infiltrated into plants. Infiltrated tissues of HAC1 were collected daily up to 8 dpi for recombinant protein quantification. (C) Stability of Agrobacterium cell bank. Plants were infiltrated with the same batch of the Agrobacterium cell bank every year to evaluate protein accumulation. Fifty µg of fresh leaf weight equivalent was loaded per lane. Please click here to view a larger version of this figure.
Discussion
In this study, we have developed a simple agroinfiltration protocol for routine transient protein production in selected Nicotiana species using Agrobacterium strains carrying the launch vector. In addition, we have identified the optimum conditions to achieve the highest recombinant protein production level in our transient plant expression system.
Vacuum infiltration of the diluted A. tumefaciens strain GV3101 harboring the launch vector pBID4 into N. benthamiana, N. excelsiana and N. excelsior resulted in higher levels of target protein production within 7 dpi compared to other plant species, such as Pisum sativum infiltrated with GV3101 harboring Alfalfa mosaic virus- or Cucumber mosaic virus-based vectors expressing the GFP reporter gene under the 35S promoter41, or Lactuca sativa, Solanum lycopersicum and Arabidopsis thaliana infiltrated with the C58C1 strain of A. tumefaciens carrying the beta-glucuronidase reporter gene57. N. benthamiana and N. excelsiana were easy to vacuum infiltrate at 50 mbar for 30-60 sec, with 90-95% infiltration efficiency. The remaining 5-10% of leaf area was not infiltrated because of some floating of leaves on the surface of the Agrobacterium suspension during application of the vacuum. Since the launch vector has ability for cell-to-cell movement18, transient protein accumulation occurs in entire leaves as well as petioles at 7 dpi. At 10 dpi, the estimated GFP production was slightly lower because the pBID4 expression vector is able to move from cell to cell but not to move systemically18; therefore, newly grown leaves do not contain the vector and do not contribute to production of target. In addition, degradation of the recombinant protein over time may contribute to reduced protein level at 10 dpi. Our results showed that infiltration of the A. tumefaciens strain GV3101 mediated high levels of transient protein production in N. benthamiana. Furthermore, target protein can be engineered as N-terminal, C-terminal or internal fusions to lichenase (LicKM), β-1,3-1,4-glucanase, which is a thermostable enzyme from Clostridium thermocellum and confers thermostability to many target protein fusion18. Infiltration of N. benthamiana with A. tumefaciens wild-type strains (at6, at10 and at77) harboring the gene of interest elicited mild or severe symptoms: leaf curling, petiole elongation, and curling. No pathological symptoms were observed in N. benthamiana infiltrated with the laboratory strain GV3101 harboring empty pBID4 vector, while some genes inserted into pBID4 and transformed into laboratory strains GV3101, C58C1 or LBA4404 elicited mild necrotic responses and leaf chlorosis/yellowing symptoms in infiltrated regions of leaves. Necrotic symptoms caused by wild-type Agrobacterium or disarmed strains in solanaceous plants have been reported previously56,57. The necrotic response could result from virulence factors of the Type III secretion system, bacterial proteins transferred to the plant cell by the Type IV secretion system, and/or sensitivity to flagellin58-60. We have found that transient production of heterologous proteins may also elicit pathogenicity and a hypersensitive response in infiltrated plant leaves. Many researchers reported that agroinfiltration of different plant species with plant binary vectors produced up to 5-20 times higher levels of transient protein production compared to stably transformed plants28,57. Our data shows that N. benthamiana infiltrated with GV3101 harboring pBID4-GFP transiently expressed high levels of GFP, which is similar to the GFP yield reported for N. benthamiana infiltrated with Agrobacteria carrying the pICH-GFPSYS viral vector (up to 80% of total soluble protein)44. Agroinfiltration using our launch vector resulted in high production of the thermostable protein LicKM, 50-fold higher than that observed using a standard binary plasmid18.
To test the infectivity of A. tumefaciens and the stability of the launch vector, we infiltrated one flat of N. benthamiana every hour for up to 8 hr using the same Milli-Q water-diluted culture of GV3101 harboring the pBID4-GFP plasmid. Our data showed that the GV3101 strain is efficiently infective for at least 8 hr and pBID4 (the launch vector) is very stable during the 8 hr-long infiltration.
Glycerol stock of GV3101 strain harboring the launch vector pBID4-HAC1 (cell bank) stored at -80 °C has been shown to be very stable for three years without changes in transient protein production in infiltrated plants.
N. benthamiana plants grown under optimal conditions and between 35 and 42 days post sowing were optimal for vacuum infiltration-mediated transient gene expression40. Younger plants (3-4 weeks old) cannot be infiltrated entirely because of leaves floating on the cell suspension surface and tissue damage from the mechanical effects of applying a vacuum. In plants older than 45 days, N. benthamiana bolting stage, under the optimal light conditions used, the level of transient expression is low.
Low molecular weight phenolic compounds (acetosyringone) and monosaccharaides (glucose) are known to induce vir genes in A. tumefaciens55,61. Moreover, infiltration of N. benthamiana with the binary vector pCAMBIA(gfp) in the presence of acetosyringone at the concentrations of 50-600 µM were shown to slightly increase transient gene expression40. We studied the effect of different concentrations of acetosyringone and glucose in our system by adding these compounds to GV3101 cultures harboring pBID4-GFP in MMA for 3 hr, and found no difference in GFP expression. Interestingly, GV3101 cultures harboring pBID4-GFP and diluted in Milli-Q water to an A600 of 0.5 and infiltrated without the vir gene induction expressed the same amounts of GFP as those infiltrated with induced cultures. The A. tumefaciens vir gene(s) could potentially be induced by plant phenolic compounds (acetosyringone and sinapinic acid) and plant monosaccharaides (glucose and fructose) present in leaf tissue. Therefore, we speculate that similar levels of GFP expression in the presence or absence of exogenous vir gene inducers may be a result of the effect of cytoplasmic vir gene inducers present during replication of the GFP-expressing launch vector in plant cells.
Wild-type N. benthamiana has been used as a model host for transient protein production49. However, N. benthamiana’s relatively low biomass yield hinders its application for large-scale production of recombinant proteins. The optimal host should combine a high level of transient expression, easy growth in a greenhouse, and be susceptible to Agrobacterium infiltration2. To select an alternative host, we infiltrated two different wild-type species of Nicotiana (N. benthamiana and N. excelsior) and a hybrid N. excelsiana (N. benthamiana × N. excelsior) with the A. tumefaciens strain GV3101 harboring the pBID4-GFP plasmid. Among these three species, the level of GFP expression was slightly higher in N. benthamiana. N. excelsior plants showed difficulty in vacuum agroinfiltration due to their leathery leaves, and N. excelsiana produced approximately two-fold more biomass under the same growth conditions. The transient production of GFP at 7 dpi is relatively similar in N. benthamiana and N. excelsiana. Therefore, N. excelsiana may be a more suitable host for recombinant protein production.
Agrobacterium-mediated transient protein production is limited by PTGS26, which can be overcome by co-expression of gene silencing suppressors of plant virus origin62. Transient protein production has been previously shown to be enhanced 50-fold in the presence of the p19 protein of TBSV, which inhibits PTGS in infiltrated tissues34. In our study, we assessed the effect of two viral silencing suppressors (p19 and p23) separately co-infiltrated with the launch vector pBID4-HAC1. The co-infiltration of these silencing suppressors seemed to have little influence on transient expression of HAC1, with only a slight increase in HAC1 protein accumulation (15-25%) in the presence of co-infiltrated p23 or p19. To positively affect protein production, silencing suppressors may need to be specifically selected to be effective for the targeted plant species and viral vector63. TMV helicase has a suppressor of RNA silencing activity64,65. Our data confirm this observation as co-infiltration of p23 or p19 with pBID4-HAC1 resulted in no increase in GFP or a slight increase in the transient HAC1 protein production.
In conclusion, we have modified and optimized plant and Agrobacterium growth conditions and improved the efficiency of vacuum infiltration. This technology enabled us to grow and infiltrate hundreds of kilograms of plant material in a few hours. We successfully automated the plant transient expression technique for high-throughput vaccine production at industrial scales under current Good Manufacturing Practices (cGMP) conditions. For more information about automation and utilization of the plant transient protein production system for the production of recombinant proteins, including subunit vaccine candidates, under cGMP conditions, readers are referred to the website www.fhcmb.org/.
Disclosures
We have nothing to disclose.
Acknowledgements
This work was supported by Fraunhofer USA Center for Molecular Biotechnology, iBio, Inc. and the Defense Advanced Research Projects Agency (grant # HDTRA1-07-C-0054). The authors acknowledge the generous gifts by Drs. Stanton Gelvin of Biological Science Dept., Purdue University (Agrobacterium tumefaciens strains) and Wayne Fitzmaurice of Large Scale Biology Corp. (N. excelsiana seeds), as well as Jennifer Nicholson of US Nicotiana Collection, North Carolina State University (N. excelsior seeds). The authors thank Margaret Shillingford and Christopher Hull for providing plants and excellent technical assistance. The authors also thank Drs. Stephen Streatfield and Natasha Kushnir for editorial assistance.
Materials
| Name | Company | Catalog Number | Comments |
| Nicotiana benthamiana | Tobacco Germplasm Collection, Crop Science Dept., North Carolina State University | PI 555478 TW16 | Infiltration |
| Nicotiana excelsior | Tobacco Germplasm Collection, Crop Science Dept., North Carolina State University | PI 555685 TW47 | Infiltration |
| Nicotiana excelsiana | Dr. Wayne Fitzmaurice, Large Scale Biology Corporation, Vacaville, CA | LSBC EBA 042304.02 | Infiltration |
| Vacuum skid | Abbas, Ashland, MA | Custom made | Plant infiltration |
| Rockwool | Grodan Inc., Ontario, Canada | AO 50/40 | Hydroponic media for growing plant |
| 2-(N-Morpholino) ethanesulfonic acid | Acros Organics, Thermo Fisher Scientific NJ | 172595000 | Buffer |
| Murashige & Skoog salt (MS salt) | Phyto Technology Lab | M524 | Tissue culture media |
| Acetosyringone | Sigma-Aldrich, St. Louis, MO | D134406-5G | Agrobacterium induction |
| Immobilon-P transfer membrane | Millipore, Billerica, MA | IPVH00010 | Western blotting |
| I-block | Applied Biosystems, Foster City, CA | T2015 | Western blotting |
| Rabbit polyclonal anti-GFP antiserum | Washington Biotechnology, Baltimore, MD | Western blotting | |
| Mouse anti–poly-histidine monoclonal antibody | Qiagen GmbH, Hilden | 34670 | Western blotting |
| Horseradish peroxidase-conjugated anti-rabbit antibody | Jackson ImmunoResearch, West Grove, PA | 111-035-003 | Western blotting |
| Horseradish peroxidase-conjugated anti-mouse antibody | Jackson ImmunoResearch, West Grove, PA | 115-035-003 | Western blotting |
| SuperSignal® West Pico chemiluminescent substrate | Thermo Scientific Pierce, Rockford, IL | 34078 | Western blotting |
| Lichenan (1-3: 1-4-beta-D-glucan) | Megazyme, Bray, Co. Wicklow, Ireland | P-LICHN | Lichenase Activity |
| Congo Red | Sigma-Aldrich, St. Louis, MO | C6277 | Gel staining |
| Digital camera | Olympus, Center Valley, PA | C-8080 | Chemiluminescence imaging |
| GeneGnome | Syngene, Frederick, MD | Chemiluminescence imaging | |
| GFP standard | Made in house | Chemiluminescence imaging | |
| Plant fertilizer solution | Griffin Greenhouse & Nursery Supplies, Newark, DE | 67-23-20 | Plant growing |
| Lichenase Standard | Purified in house | Western blotting | |
| MicroPulser Electroporator | BioRad, Hercules, CA | 165-2100 | Agrobacterium transformation |
References
- Ma, J. K., Drake, P. M., Christou, P. The production of recombinant pharmaceutical proteins in plants. Nature Reviews Genetics. 4, 794-805 (2003).
- Sheludko, Y. V., Sindarovska, Y. R., et al. Comparison of several Nicotiana species as hosts for high-scale agrobacterium-mediated transient expression. Biotechnology and Bioengineering. 96, 608-614 (2007).
- Mett, V., Farrance, C. E., Green, B. J., Yusibov, V. Plants as biofactories. Biologicals. 36, 354-358 (2008).
- Yusibov, V., Rabindran, S. Recent progress in the development of plant derived vaccines. Expert Review of Vaccines. 7, 1173-1183 (2008).
- Stoger, E., Sack, M., Fischer, R., Christou, P. Plantibodies: Applications, advantages and bottlenecks. Current Opinion in Biotechnology. 13, 161-166 (2002).
- Mahmoud, K. Recombinant Protein Production: Strategic Technology and a Vital Research Tool. Research Journal of Cell and Molecular Biology. 1, 9-22 (2007).
- Rai, M. P. H. Expression systems for production of heterologous proteins. Current Science. 80, 1121-1128 (2001).
- Yusibov, V., Streatfield, S. J., Kushnir, N. Clinical development of plant-produced recombinant pharmaceuticals: vaccines, antibodies and beyond. Human Vaccines. 7, 313-321 (2011).
- McCormick, A. A., Reddy, S., et al. Plant-produced idiotype vaccines for the treatment of non-Hodgkin's lymphoma: Safety and immunogenicity in a phase I clinical study. Proceedings of the National Academy of Sciences of the United States of America. 105, 10131-10136 (2008).
- Medicago Inc, . . , (2013).
- Medicago Inc, . . , (2013).
- Planet Biotechnology Inc, . . , (2013).
- Sys Genetics, S. e. m. B. i. o. . , (2013).
- Protalix, . , (2013).
- Protalix, . , (2013).
- Plesha, M. A., Huang, T. -. K., et al. Optimization of the bioprocessing conditions for scale-up of transient production of a heterologous protein in plants using a chemically inducible viral amplicon expression system). Biotechnology Progress. 25, 722-734 (2009).
- Gleba, Y., Klimyuk, V., Marillonnet, S. Magnification - A new platform for expressing recombinant vaccines in plants. Vaccine. 23, 2042-2048 (2005).
- Musiychuk, K., Stephenson, N., et al. A launch vector for the production of vaccine antigens in plants. Influenza and Other Respiratory Viruses. 1, 19-25 (2007).
- Massa, S., Franconi, R., et al. Anticancer activity of plant-produced HPV16 E7 vaccine. Vaccine. 25, 3018-3021 (2007).
- Mett, V., Lyons, J., et al. A plant-produced plague vaccine candidate confers protection to monkeys. Vaccine. 25, 3014-3017 (2007).
- Mett, V., Musiychuck, K., et al. A plant-produced influenza subunit vaccine protects ferrets against virus challenge. Influenza and Other Respiratory Viruses. 2, 33-40 (2008).
- Shoji, Y., Chichester, J. A., et al. Plant expressed HA as a seasonal influenza vaccine candidate. Vaccine. 26, 2930-2934 (2008).
- Chichester, J. A., Musiychuk, K., et al. Immunogenicity of a subunit vaccine against Bacillus anthracis. Vaccine. 25, 3111-3114 (2007).
- Golovkin, M., Spitsin, S., et al. Smallpox subunit vaccine produced in Planta confers protection in mice. Proceedings of the National Academy of Sciences of the United States of America. 104, 6864-6869 (2007).
- Porta, C., Lomonossoff, G. Use of viral replicons for the expression of genes in plants. Molecular Biotechnology. 5, 209-221 (1996).
- Johansen, L. K., Carrington, J. C. Silencing on the spot: induction and suppression of RNA silencing in the Agrobacterium-mediated transient expression system. Plant Physiology. 126, 930-938 (2001).
- Vézina, L. P., Faye, L., et al. Transient co-expression for fast and high-yield production of antibodies with human-like N-glycans in plants. Plant Biotechnology Journal. 7, 442-455 (2009).
- Vaquero, C., Sack, M., et al. Transient expression of a tumor-specific single-chain fragment and a chimeric antibody in tobacco leaves. Proceedings of the National Academy of Sciences of the United States of America. 96, 11128-11133 (1999).
- Galeffi, P., Lombardi, A., et al. Expression of single-chain antibodies in transgenic plants. Vaccine. 23, 1823-1827 (2005).
- Rodríguez, M., Ramírez, N. I., et al. Transient expression in tobacco leaves of an aglycosylated recombinant antibody against the epidermal growth factor receptor. Biotechnology and Bioengineering. 89, 188-194 (2004).
- Hull, A., Criscuolo, C. J., et al. plant-produced monoclonal antibody for the treatment of anthrax. Vaccine. 23, 2082-2086 (2005).
- Roy, G., Weisburg, S., Rabindran, S., Yusibov, V. A novel two-component Tobacco mosaic virus-based vector system for high-level expression of multiple therapeutic proteins including a human monoclonal antibody in plants. Virology. 405, 93-99 (2010).
- Silhavy, D., Molnar, A., et al. A viral protein suppresses RNA silencing and binds silencing-generated, 21-to 25-nucleotide double-stranded RNAs. EMBO Journal. 21, 3070-3080 (2002).
- Voinnet, O., Rivas, S., Mestre, P., Baulcombe, D. An enhanced transient expression system in plants based on suppression of gene silencing by the p19 protein of tomato bushy stunt virus. Plant Journal. 33, 949-956 (2003).
- Bechtold, N., Pelletier, G. In planta Agrobacterium-mediated transformation of adult Arabidopsis thaliana plants by vacuum infiltration. Methods in Molecular Biology. 82, 259-266 (1998).
- Bechtold, N., Ellis, J., Pelletier, G. In planta Agrobacterium mediated gene transfer by infiltration of adult Arabidopsis thaliana plants. C. R. Academy of Science Paris, Life Sciences. 316, 1194-1199 (1993).
- Tague, B., Mantis, J. In planta Agrobacterium-mediated transformation by vacuum infiltration. Methods in Molecular Biology. 323, 223-215 (2006).
- Fischer, R., Vaquero-Martin, C., et al. Towards molecular farming in the future: transient protein expression in plants. Biotechnology and Applied Biochemistry. 30, 113-116 (1999).
- Horn, M. E., Woodard, S. L., Howard, J. A. Plant molecular farming: systems and products. Plant Cell Reports. 22, 711-720 (2004).
- Wydro, M., Kozubek, E., Lehmann, P. Optimization of transient Agrobacterium-mediated gene expression system in leaves of Nicotiana benthamiana. Acta Biochimica Polonica. 35, 289-298 (2006).
- Green, B. J., Fujiki, M., et al. Transient protein expression in three Pisum sativum (green pea) varieties. Biotechnology Journal. 4, 1-8 (2009).
- Kapila, J., DeRycke, R., Van Montagu, M., Angenon, G. An Agrobacterium-mediated transient gene expression system for intact leaves. Plant Science. 122, 108-101 (1997).
- Yang, Y., Li, R., Qi, M. In vivo analysis of plant promoters and transcription factors by agroinfiltration of tobacco leaves. Plant Journal. 22, 543-551 (2000).
- Marillonnet, S., Thoeringer, C., Kandzia, R., Klimyuk, V., Gleba, Y. Systemic Agrobacterium tumefaciens-mediated transfection of viral replicons for efficient transient expression in plants. Nature Biotechnology. 23, 718-723 (2005).
- Gleba, Y., Klimyuk, V., Marillonnet, S. Viral vectors for the expression of proteins in plants. Current Opinion in Biotechnology. 18, 134-141 (2007).
- Goodin, M. M., Zaitlin, D., Naidu, R. A., Lommel, S. A. Nicotiana benthamiana: Its history and future as a model for plant-pathogen interactions. Molecular Plant-Microbe Interactions. 21, 1015-1026 (2008).
- Shoji, Y., Bi, H., et al. Plant-derived hemagglutinin protects ferrets against challenge infection with the A/Indonesia/05/05 strain of avian influenza. Vaccine. 27, 1087-1092 (2009).
- Llave, C., Kasshau, K. D., Carrington, J. C. Virus-encoded suppressor of posttranscriptional gene silencing targets a maintenance step in the silencing pathway. Proceedings of the National Academy of Sciences of the United States of America. 97, 13401-13406 (2000).
- McCormick, A. A., Kumagai, M. H., et al. Rapid production of specific vaccines for lymphoma by expression of the tumor-derived single-chain Fv epitopes in tobacco plants. Proceedings of the National Academy of Sciences of the United States of America. 96, 703-708 (1999).
- Simmons, C. W., VanderGheynst, J. S., Upadhyaya, S. K. A model of agrobacterium tumefaciens vacuum infiltration into harvested leaf tissue and subsequent in planta transgene transient expression. Biotechnology and Bioengineering. 102, 965-970 (2009).
- Ankenbauer, R. G., Nester, E. W. Sugar-mediated induction of Agrobacterium tumefaciens virulence genes: structural specificity and activities of monosaccharides. Journal of Bacteriology. 172, 6442-6446 (1990).
- Cangelosi, G. A., Ankenbauer, R. G., Nester, E. W. Sugars induce the Agrobacterium virulence genes through a periplasmic binding protein and a transmembrane signal protein. Proceedings of the National Academy of Sciences of the United States of America. 87, 6708-6712 (1990).
- Stachel, S. E., Nester, E. W., Zambryski, P. C. A plant cell factor induces Agrobacterium tumefaciens vir gene expression. Proceedings of the National Academy of Sciences of the United States of America. 83, 379-383 (1986).
- Rogowsky, P. M., Close, T. J., Chimera, J. A., Shaw, J. J., Kado, C. I. Regulation of the vir genes of Agrobacterium tumefaciens plasmid pTiC58. Journal of Bacteriology. 169, 5101-5112 (1987).
- Hiei, Y., Ohta, S., Komari, T., Kumashiro, T. Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. Plant Journal. 6, 271-282 (1994).
- Vander Hoorn, J. A. L., Laurent, F., Roth, R., De Wit, P. J. G. M. Agroinfiltration is a versatile tool that facilitates comparative analyses of Avr9/cf-9-induced and Avr4/Cf-4-induced necrosis. Molecular Plant-Microbe Interactions. 13, 439-446 (2000).
- Wroblewski, T., Tomczak, A., Michelmore, R. Optimization of Agrobacterium-mediated transient assay of gene expression in lettuce, tomato and Arabidopsis. Plant Biotechnology Journal. 3, 259-273 (2005).
- Salmond, G. P. C. Secretion of extracellular virulence factors by plant pathogenic bacteria. Annual Review of Phytopathology. 32, 181-200 (1994).
- Felix, G., Duran, J. D., Volko, S., Boller, T. Plants have a sensitive perception system for the most conserved domain of bacterial flagellin. Plant Journal. 18, 265-276 (1999).
- Goodner, B., Hinkle, G., et al. Genome sequence of the plant pathogen and biotechnology agent Agrobacterium tumefaciens C58. Science. 294, 2323-2328 (2001).
- Lee, Y. -. W., Jin, S., Sims, W. -. S., Nester, E. W. Genetic evidence for direct sensing of phenolic compounds by the vir A protein of Agrobacterium tumefaciens. Proceedings of the National Academy of Sciences of the United States of America. 92, 12245-12249 (1995).
- Voinnet, O., Pinto, Y. M., Baulcombe, D. C. Suppression of gene silencing: a general strategy used by diverse DNA and RNA viruses. Proceedings of the National Academy of Sciences of the United States of America. 96, 14147-14152 (1999).
- Voinnet, O. RNA silencing as a plant immune system against viruses. Trends in Genetics. 17, 449-459 (2001).
- Ding, X. S., Liu, J., et al. The Tobacco mosaic virus 126-kDa protein associated with virus replication and movement suppresses RNA silencing. Molecular Plant-Microbe Interactions. 17, 583-592 (2004).
- Harries, P. A., Palanichelvam, K., Bhat, S., Nelson, R. S. Tobacco mosaic virus 126-kDa protein increases the susceptibility of Nicotiana tabacum to other viruses and its dosage affects virus-induced gene silencing. Molecular Plant-Microbe Interactions. 21, 1539-1548 (2008).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved