An In Vitro Adult Mouse Muscle-nerve Preparation for Studying the Firing Properties of Muscle Afferents
In This Article
Summary
Muscle sensory neurons are involved in proprioceptor signaling and also report on metabolic state and injury related events. We describe an adult mouse in vitro muscle-nerve preparation for studies on stretch-activated muscle afferents.
Abstract
Muscle sensory neurons innervating muscle spindles and Golgi tendon organs encode length and force changes essential to proprioception. Additional afferent fibers monitor other characteristics of the muscle environment, including metabolite buildup, temperature, and nociceptive stimuli. Overall, abnormal activation of sensory neurons can lead to movement disorders or chronic pain syndromes. We describe the isolation of the extensor digitorum longus (EDL) muscle and nerve for in vitro study of stretch-evoked afferent responses in the adult mouse. Sensory activity is recorded from the nerve with a suction electrode and individual afferents can be analyzed using spike sorting software. In vitro preparations allow for well controlled studies on sensory afferents without the potential confounds of anesthesia or altered muscle perfusion. Here we describe a protocol to identify and test the response of muscle spindle afferents to stretch. Importantly, this preparation also supports the study of other subtypes of muscle afferents, response properties following drug application and the incorporation of powerful genetic approaches and disease models in mice.
Introduction
Skeletal muscles are innervated by sensory neurons that provide the central nervous system with important information about the muscle environment. Muscle spindles are highly specialized encapsulated structures composed of intrafusal muscle fibers that are located in parallel with extrafusal muscle fibers. Spindles are innervated by Group Ia and II afferents that encode changes in muscle length and intrafusal fiber tone is dynamically regulated by innervating gamma motor neurons1. Group Ib afferents are positioned between muscle fibers and their tendon insertions and are sensitive to changes in muscle force, for instance during muscle contraction2. The Group Ia, Ib and II afferents provide proprioceptive feedback that aids in appropriate motor control. Additional populations of muscle afferents located throughout the muscle (Group III and IV) serve to signal metabolite buildup, the presence of nociceptive stimuli, and muscle temperature3. This muscle sensory information is critical to maintaining homeostasis, preventing muscle damage and modulating movement. Muscle afferents can alter their firing patterns based on prior experience4. Activation of nociceptors can lead to the induction of chronic pain states5 and aberrant signaling from the muscle proprioceptors can lead to problems with balance or movement6. We use an isolated muscle-nerve in vitro preparation to study the response of muscle sensory neuron receptor endings from adult mice of both sexes (shown are responses from 2-4 month old C57Bl/6 mice). The mouse is the model genetic species for studies in mammals. This preparation requires the isolation of the deep peroneal branch of the sciatic nerve and the extensor digitorum longus (EDL) muscle, a fast twitch muscle of the peroneal group found in the lateral part of the lower leg. The EDL is often used to study muscle contractile properties7-9, has tendons that are easy to isolate and is small enough to allow adequate diffusive oxygen supply at rest and with reasonable contraction duty cycles10. Other muscles in this area (for instance soleus and tibialis anterior) are of similar size and this preparation could be easily modified to record from afferents from these muscles. A suction electrode is placed onto the cut end of the nerve to record muscle sensory afferent firing. Individual neurons can be identified and analyzed based on their spike shape using spike sorting software. Stimulating electrodes in the bath or on the nerve can be used to evoke muscle contraction. Muscle length and force can be controlled and measured using commercially available systems. Similar in vitro preparations have been used in rodents to study Group III and IV muscle afferents in the rat EDL11, Group Ia and II spindle afferents in the rat fourth lumbrical toe muscle12 and Group III and IV muscle afferents in the mouse plantar muscle13.
An in vitro system has the advantages of pharmacological accessibility and direct control of perfusate and physiochemical variables like temperature and pH. An in vitro approach eliminates the potential in vivo confounds of anesthesia and muscle perfusion status. While the muscle-nerve preparation allows for the study of the direct response of a perturbation on the afferents, the ability to study gamma efferent modulation of spindle sensitivity and other integrated responses that is possible with in vivo14 or ex vivo3 preparations is sacrificed as there are no neuronal cell bodies in this preparation.
This preparation was previously used to characterize the response of mouse muscle spindle afferents to a battery of ramp and hold stretches and vibrations and it was determined that mouse spindle afferent responses were similar to those reported in other species such as rats, cats, and humans. Mouse spindle afferent responses were found to be similar at both 24 °C and 34 °C bath temperatures, although at 34 °C absolute firing rates were faster and the afferents were more able to respond to faster length changes15. We describe below how this preparation can be used to identify and study the muscle spindle afferents. Moreover, this preparation can easily be modified to study the response of other muscle afferent subtypes13, to compare the properties of sensory afferents in response to a drug or disease state or assorted other variables (e.g., age, sex, gene knockout).
Protocol
Appropriate national and institutional ethics should be obtained before performing animal experiments.
1. Removal of EDL Muscle and Nerve
- Weigh and deeply anesthetize an adult mouse with inhaled isofluorane using a vaporizer with 5% isofluorane plus a 1.5 L/min oxygen flow rate or a bell jar with isofluorane soaked cotton on the bottom.
- Ensure that the mouse is deeply anesthetized and does not respond to a toe pinch. Quickly decapitate using large, sharpened scissors or a guillotine.
- Make a ventral midline cut through the ribs using scissors and remove the internal organs.
- Skin the animal by grasping the skin from the neck area and pulling it past the feet.
- Remove the legs by cutting above the hips and place the skinned legs into a dish with chilled (4 °C), carbogenated (95% O2, 5% CO2) low calcium, high magnesium bicarbonate buffered saline solution containing in mM: 128 NaCl, 1.9 KCl, 1.2 KH2PO4, 26 NaHCO3, 0.85 CaCl2, 6.5 MgSO4, and 10 glucose (pH of 7.4±0.05)16. The low calcium, high magnesium solution inhibits synaptic transmission during the dissection.
- Place the legs dorsal side up in the dish and pin the legs and hips down using needles or insect pins so that the knee and ankle joints are at a 90° angle. Place a pin on each end of the feet and one pin on each of the anterior and posterior thighs to hold the tissue in place.
- Using Castroviejo spring scissors, lift up the top layer of muscle on the thighs and make a midline cut to expose the sciatic nerve directly below. The sciatic nerve is located just below the top muscle layer and runs above the femur from its exit near the hips until it branches into the common peroneal and tibial nerve just before the knee joint.
- Remove the muscle above the sciatic nerve until the point at which the deep peroneal nerve branch dives into the flexor hallucis longus (FHL) muscle is visible.
- Using #55 forceps, dissect the connective tissue around the peroneal branch to free it from the gastrocnemius and soleus muscles, which are on the medial side of the tibia. Cut the tendons of the gastrocnemius and soleus muscles and carefully remove them from under the nerve.
- Remove the superficial muscle on the lateral side of the tibia to expose three distinct tendon bundles at the ankle joint—from medial to lateral: FHL, EDL, and tibialis anterior (TA), with the EDL hidden underneath the TA.
- Cut the TA tendon at the ankle joint, lift the TA up and away from the EDL, and cut the muscle near the knee to remove it.
- Cut the FHL tendon at the ankle and lift it back to reach the area where the nerve enters the FHL. Cut just below that point and remove ~2/3 of the FHL muscle.
- Cut the sciatic nerve as close to the hip joint as possible and gently strip away all nerve branches except for the deep peroneal branch.
- Cut the EDL tendons at both the ankle and knee joints using large spring scissors.
- Using sharp scissors, remove the EDL, the remaining FHL and nerve from the surrounding tissue by cutting through the tibia bone at the knee and midway through the thigh. Cut away the remaining tibia bone so that just the EDL, part of the FHL and nerve remain.
2. Mounting of the EDL Muscle and Nerve into the Tissue Bath (Figure 1A)
- Before the start of the experiment, prepare the tissue bath. Constantly perfuse the bath with oxygenated (100% O2) synthetic interstitial fluid (SIF) containing (in mM) 123 NaCl, 3.5 KCl, 0.7 MgSO4, 1.7 NaH2PO4, 2.0 CaCl2, 9.5 NaC6H11O (sodium gluconate), 5.5 glucose, 7.5 sucrose, and 10 N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES); pH 7.4±0.0517. A flow rate of 15-30 ml/min is recommended. Shown is a commercially available bath of 25 ml capacity with two stimulating electrodes fixed to the bottom of the bath, a mounted tissue post and a mount for a force and length controller (approximate bath dimensions 8.5 cm x 3 cm x 1 cm; for specifications see Table of Materials/Equipment).
- Use the remaining FHL tissue to handle the isolated muscle-nerve and place it into the tissue bath. Place a small piece of sylgard on the bottom of the dish and use an insect pin through the remaining FHL tissue to stabilize the muscle.
- Use 6-0 silk sutures to tie both tendons and affix one end to the tissue post and the other to the lever arm of the force and length controller (see Table of Materials/Equipment for specifications). Use the smallest suture length that is practical. Remove the insect pin and sylgard after you have tied the tendons. NOTE: To facilitate easy connection to the lever arm a small piece of wire can be bent into a “j” shaped hook and fixed into the lever arm with epoxy. The suture can then be tied to the wire instead of threaded into the small hole on the lever arm.
- Make suction electrodes from SA 16 glass by first using a glass micropipette puller (Heat = 286, Pull = 0, Velocity = 150, Time = 200); break the tip back and manually grind it on a sharpening stone until there is about a 3 mm taper. Melt the tip using a microforge to the desired tip inner diameter of between 10 - 100 µm depending on the area of nerve one wishes to sample from (see Figures 1B-1C for electrode schematic and Table of Materials/Equipment for product information).
- Fill a premade glass suction electrode to the inner silver wire with SIF.
- Suction the cut end of the nerve into the electrode and connect to the positive port of a differential amplifier. Wrap the electrode with a chlorided silver wire that connects to the negative port of the headstage. Ground the SIF bath by running a second chlorided silver wire from the bath to the headstage’s ground port. Also ground the perfusion tubing to the Faraday cage at multiple points to mitigate electrical noise introduced via the perfusion pumps.
- Stimulate the muscle via the electrodes mounted on either side of the muscle in the tissue bath to induce a twitch contraction. Alternatively place a stimulating electrode on the cut end of the nerve. Increase the stimulating voltage until a peak contractile force is observed and then increase the voltage by an additional 15% to reach supramaximal voltage (0.5 msec pulse width). Continue the twitch contractions at the supramaximal voltage, with a 10 sec rest in-between, but vary the length of the muscle until a peak contractile force is reached to find the optimal length (Lo) of the muscle. All length ramps and vibrations will start with the muscle at this length.
- Allow the muscle-nerve preparation to remain in the bath for at least 1 hr before subsequent data collection to allow the tissue to reach the bath temperature and for normal synaptic transmission to recover following dissection in a low calcium solution.
- To collect data at a temperature other than room temperature, place a temperature probe into the tissue bath near the muscle. Slowly bring the bath up to temperature by pumping heated water through the tissue bath base plate. Wrap clay microwavable heating pads around the SIF reservoir to help maintain a steady temperature.
3. Data Collection
- To identify an afferent as a spindle afferent, record the neuronal activity during repeated twitch contractions produced by a 0.5 msec supramaximal voltage stimulus delivered once every second. NOTE: Muscle spindle afferents should pause during the twitch contraction18,19 (Figure 2).
- Use data acquisition software to apply length changes at different speeds and to different lengths. Use a custom script to automate this task (see supplemental information for a screenshot of the script and directions on how to customize the stretches given). Apply ramp-and-hold stretches of 4 sec at stretch lengths of 2.5%, 5%, and 7.5% Lo and stretch speeds of 20, 40, or 60% Lo/sec 15. NOTE: See user manual for specific force and length controller for necessary voltage-to-millimeter conversion factor.
- At the end of the experiment, determine muscle health at 24 °C using maximal isometric tetanic contractions (500 msec train, 120 Hz train frequency, 0.5 msec pulse width, supramaximal voltage). Compare the peak contractile force to previously reported values (~24 N/cm2 9,20).
Representative Results
The response of muscle afferents can be recorded following a variety of perturbations, depending on which afferent subtype is being studied. Representative responses of muscle spindle afferents to muscle contraction and ramp and hold stretch are shown here. To identify an afferent as a spindle afferent, twitch contractions are given once every second (0.5 msec pulse width) to see if there is a pause in firing during contraction18,19. Figure 2 shows a representative trace of neuronal activity and muscle tension during these twitch contractions. No neuronal activity is observed during twitch contractions, as expected for spindle afferents. If the recorded afferent was a Group Ib Golgi tendon organ afferent, an increase in firing rate during the contraction would be expected.
Under control conditions most afferents with a regular firing pattern (~12 impulses/sec at 24 °C and ~32 impulses/sec at 34 °C) are muscle spindle afferents. A subset of spindle afferents will only fire during stretch (in our hands ~11% of spindle afferents)15. Figure 3A shows a representative raw trace of two muscle spindle afferents responding to a ramp and hold stretch produced by the force and length controller. The Spike Histogram feature of LabChart is used to identify and analyze the instantaneous firing frequency of the two individual neurons separately (Figures 3B-3C).
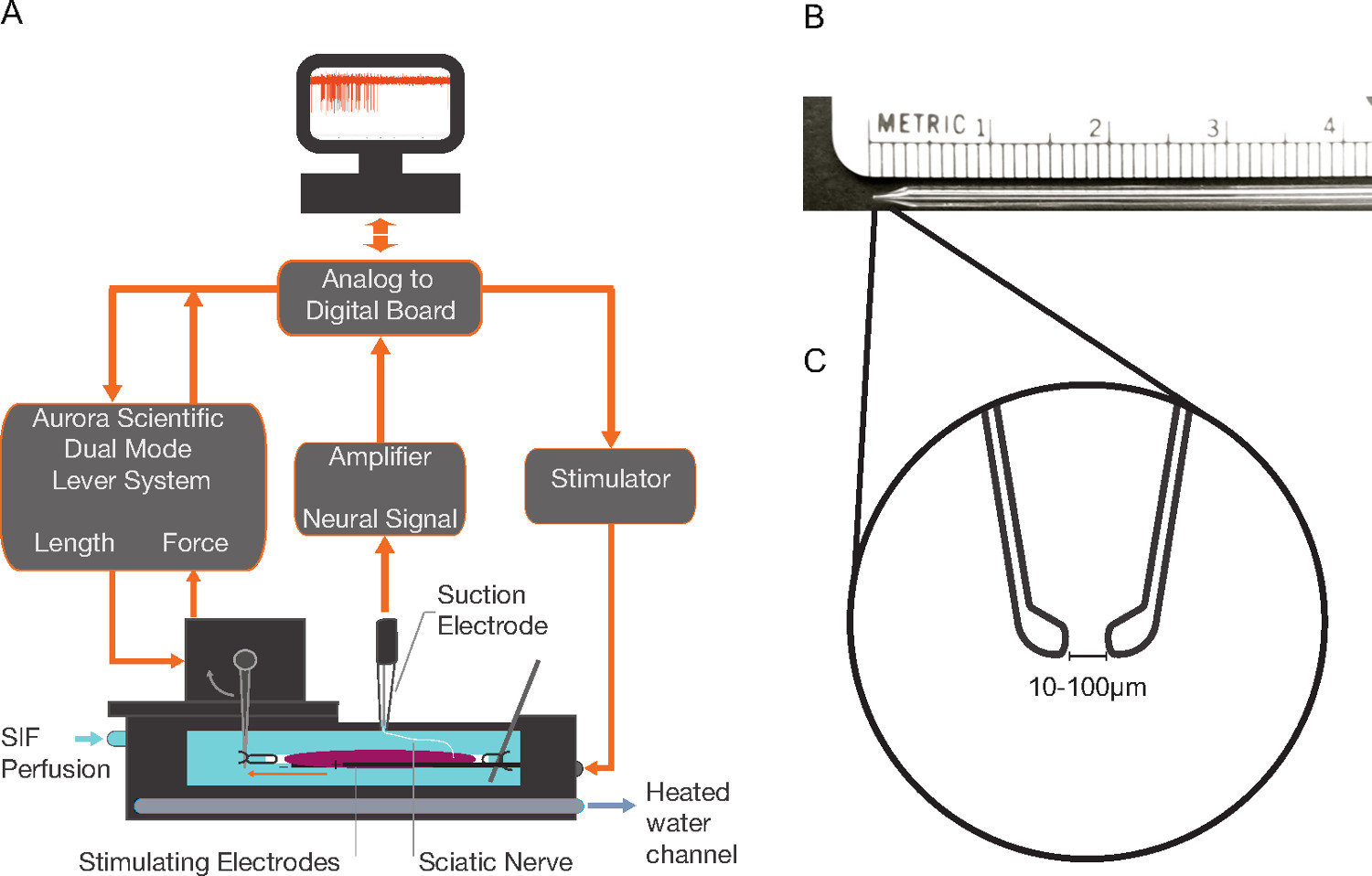
Figure 1. Isolated Muscle Preparation A) The extensor digitorum longus (EDL) and innervating sciatic nerve are mounted in an isolated tissue bath perfused with oxygenated synthetic interstitial fluid (SIF). An extracellular amplifier connected to a suction electrode records neural activity. A dual force and length controller—with appropriate software—controls and measures muscle force and length. The tissue bath electrodes deliver stimuli to produce twitch or tetanic contractions. Pumping heated water through internal bath plate channels controls the tissue bath temperature. B) Pulled glass suction electrode with ~3 mm tapered tip. C) Magnified depiction of the ideal tip shape for the suction electrode that is produced using a microforge. Please click here to view a larger version of this figure.
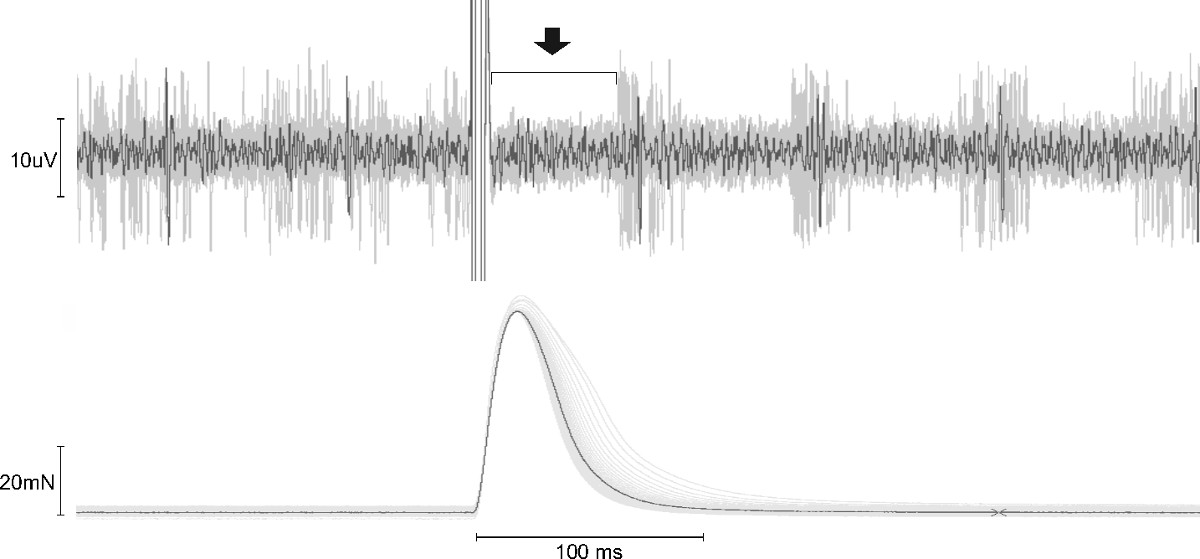
Figure 2. Spindle Afferent Response to Twitch Contraction. Neuronal activity (top trace) and muscle tension (bottom trace) from 30 twitch contractions are superimposed with top overlapping trace in black. Afferent activity pauses during contraction-induced tension increase, which is a characteristic response of muscle spindle afferents. Bracket and arrow above the top trace denote the time during contraction when activity is paused.
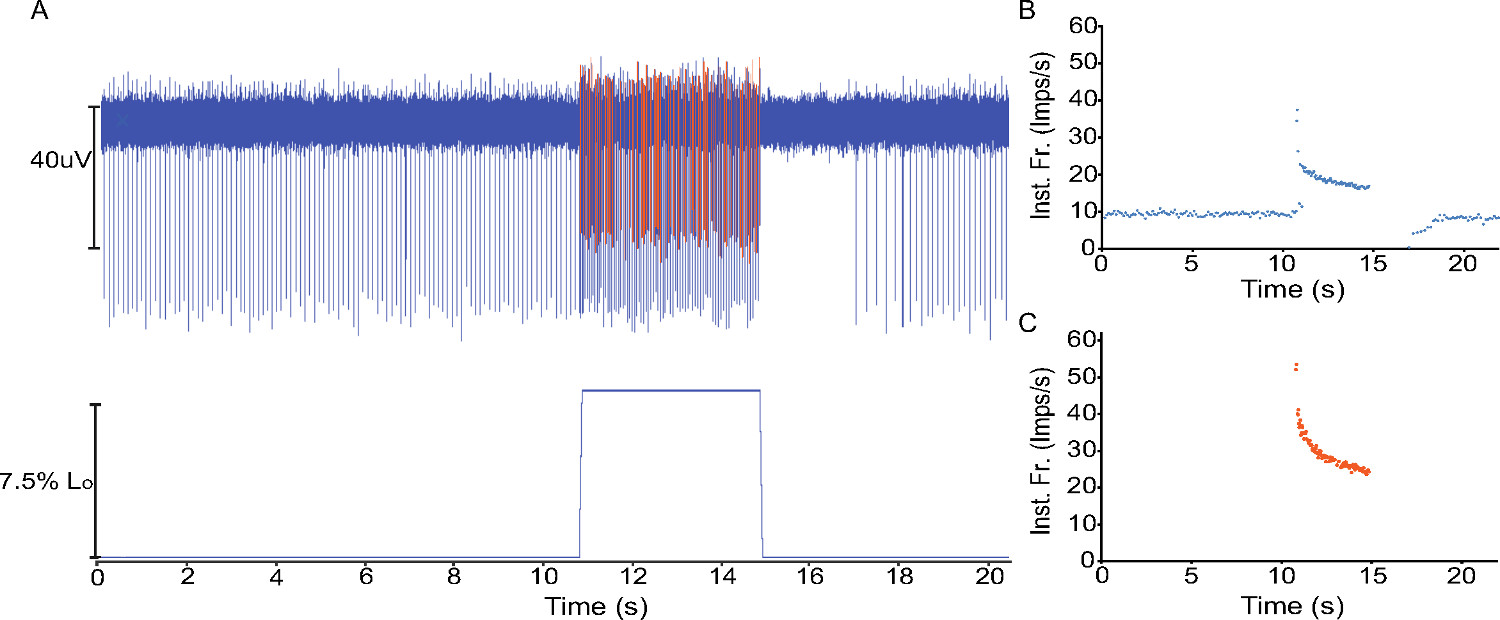
Figure 3. Spindle Afferent Response to Ramp and Hold Stretch A) Raw neural activity of two afferents (top trace) during a ramp and hold stretch applied to the EDL (muscle length shown in bottom trace). B) Instantaneous firing frequency (Inst. Fr., impulses per sec) of the unit exhibiting activity at Lo from A (shown in blue). C) Instantaneous firing frequency of the smaller unit that only fires during the stretch (shown in orange). Both units exhibit the spike frequency adaptation during stretch that is characteristic of muscle spindle afferents1.
Discussion
The goal of this article was to describe a method for recording from muscle spindle afferents in an isolated mouse muscle-nerve preparation. We have found that mouse spindle afferents respond similarly to stretch as those from rats, cats and humans15, and other laboratories have used mice as model organisms to study sensory neurons in both the muscle and the skin in vitro (for example3,13) .
Muscle sensory afferents can be recorded for at least 6-8 hr at both 24 °C and 34 °C. To minimize handling the EDL and nerve, use the remaining portion of the FHL to handle the tissue whenever possible. Following the dissection, wait at least 1 hour to start data collection to allow the tissue to equilibrate to bath temperature and for normal synaptic transmission to recover. Previously, muscle health was verified on a subset of muscles used in this preparation by determining that the maximal tetanic contractile force generated by the muscles is similar to that reported by others at the beginning and end of the experiment15.
Muscle spindle afferents were identified functionally in this preparation by looking for a characteristic pause in firing in response to twitch contraction (Figure 2) as well as the expected instantaneous frequency increases in response to length changes (Figure 3). In our experience, most afferents that fire in control conditions are muscle spindle afferents. The length of the nerve retained in this preparation (~7 mm maximum) is not long enough to use afferent conduction velocity differences to identify muscle afferent subtypes13. Additionally, unlike in cats and humans, measures of dynamic sensitivity were not able to clearly differentiate Group Ia and II afferents in mice (for further discussion see Wilkinson et al.15). Other subtypes of afferents (i.e., Group III and IV) can be identified using additional functional tests in this preparation, for instance by adding substances like capsaicin, ATP, or bradykinin, decreasing bath pH, exposing the muscle to ischemia, etc.3,13,21-23 Using suction electrodes allows the activity from multiple sensory neurons to be recorded at once, which increases the amount of data that can be collected from a single muscle. This preparation can be used to gauge the overall effect of a perturbation on sensory neuron population responses or the responses of identified afferents. If the spike shapes are unique enough, up to 4 sensory neurons can be discriminated by software (both Spike2 (Cambridge Electronic Design) and LabChart Pro (AD Instruments) have performed similarly). In cases where neurons cannot be discriminated, changes in electrode placement or tip diameter can easily be implemented.
In summary, the mouse muscle-nerve in vitro preparation is a simple experimental approach that can be used to investigate the response properties of muscle sensory afferents to various physicochemical perturbations, injury and disease models. Additionally, this preparation is ideally suited to take advantage of the powerful genetic tools available in mice, including transgenic animals and optogenetic tools.
Disclosures
The open access charges for this manuscript have been paid by Aurora Scientific, Inc., the company that makes the force and length controller and in vitro bath plate used in this manuscript.
Acknowledgements
This work was supported by a California State University Program for Education and Research in Biotechnology (CSUPERB) New Investigator Grant (KAW), a grant from Pfizer (WS753098, SH), and an NIH MARC fellowship (#2T34GM008253-21, JAF). The authors would like to thank Michael Sawchuk, Anusha Allawala, Nina Bubalo, Remie Mandawe and Peter Nguyen for their technical support.
Materials
| Name | Company | Catalog Number | Comments |
| Force and Length Controller & Tissue Bath | Aurora Scientific, Inc. | 300C-LR & 809B-IV | |
| Masterflex L/S Digital Drive Perfusion Pump & Easy Load II Pump Head | Cole Parmer | EW-07523-80 & EW-77200-60 | |
| 2 Channel Microelectrode AC Differential Amplifier & Headstage Amplifier | A-M Systems | Model 1800; 700000 & 700500 | |
| Simulator | Grass OR Aurora Scientific, Inc. | S88 OR 701 C | |
| Dissecting Microscope | Swift | 892070 | |
| Fiber Optic Light Source | Fiberlite | Model 190 | |
| Analog to Digital Board | AD Instruments | PL3508/P (PowerLab 8/35) | |
| Data Acquisition & Analysis Software | AD Instruments | LabChart Pro 7 | |
| Electrode Holder | A-M Systems | 672445 | |
| Electrode Glass | Dagan | SA16 | |
| Electrode Puller | Sutter Instrument Co. | P-80/PC | |
| Microforge | Narishige | MF-9 | |
| Isoflourane | MWI Veterinary Supply | 18627 | |
| Surgical Tools | |||
| Large Dissecting Scissors | Fine Science Tools | 14014-17 | |
| Large Forceps | Fine Science Tools | 11000-12 | |
| Large Spring Scissors | Fine Science Tools | 15006-09 | |
| #5 Forceps | Fine Science Tools | 11252-20 | |
| #55 Forceps | Fine Science Tools | 11255-20 | |
| Sharp Scissors | Fine Science Tools | 14059-11 | |
| 6-0 Silk Suture | Fine Science Tools | 18020-50 | |
| Chemicals for Low Calcium- High Magnesium aCSF and SIF (Low Calcium solution is equilibriated with 95% 02/5% C02 gas and pH of 7.4 ± 0.05; SIF is equilibriated with 100% 02 gas and pH of 7.4 ± 0.05) | |||
| Sodium Chloride | Sigma | S9888-1KG | |
| Magnesium Sulfate Heptahydrate | Sigma | 230391-500G | |
| Calcium Chloride Dihydrate | Sigma | 223506-500G | |
| Sodium Gluconate | Sigma | S2054-500G | |
| Sodium Phosphate Monobasic | Sigma | S8282-500G | |
| Glucose | Sigma | G5767-500G | |
| Sucrose | Sigma | S5016-500G | |
| HEPES | Sigma | H3375-250G | |
| Potassium Phosphate Monobasic | Sigma | P0662-500G | |
| Potassium Chloride | Sigma | P3911-500G | |
| Sodium Bicarbonate | Sigma | S6014-500G | |
References
- Matthews, P. B. C., Brookes, V. B. Section 1, The Nervous System, Motor Control. Handbook of Physiology. , 189-228 (1981).
- Houk, J., Simon, W. Responses of Golgi tendon organs to forces applied to muscle tendon. J Neurophysiol. 30, 1466-1481 (1967).
- Jankowski, M. P., Rau, K. K., Ekmann, K. M., Anderson, C. E., Koerber, H. R. Comprehensive phenotyping of group III and IV muscle afferents in mouse. J Neurophysiol. 109, 2374-2381 (2013).
- Nichols, T. R., Cope, T. C. Cross-bridge mechanisms underlying the history-dependent properties of muscle spindles and stretch reflexes. Can J Physiol Pharmacol. 82, 569-576 (2004).
- Mense, S. Nociception from skeletal muscle in relation to clinical muscle pain. Pain. 54, 241-289 (1993).
- Proske, U., Gandevia, S. C. The Proprioceptive Senses: Their Roles in Signaling Body Shape, Body Position and Movement, and Muscle Force. Physiol Rev. 92, 1651-1697 (2012).
- Close, R. Force: velocity properties of mouse muscles. Nature. 206, 718-719 (1965).
- McCully, K. K., Faulkner, J. A. Injury to skeletal muscle fibers of mice following lengthening contractions. J Appl Physiol. 59, 119-126 (1985).
- Brooks, S. V., Faulkner, J. A. Contractile properties of skeletal muscles from young, adult and aged mice. J Physiol. 404, 71-82 (1988).
- Barclay, C. J. Modelling diffusive O(2) supply to isolated preparations of mammalian skeletal and cardiac muscle. Journal of Muscle Research and Cell Motility. 26 (2), 225-235 (2005).
- Taguchi, T., Mizumura, K. Augmented mechanical response of muscular thin-fiber receptors in aged rats recorded in vitro. European Journal of Pain. 15, 351-358 (2011).
- Simon, A., Shenton, F., Hunter, I., Banks, R. W., Bewick, G. S. Amiloride-sensitive channels are a major contributor to mechanotransduction in mammalian muscle spindles. J Physiol. 588, 171-185 (2010).
- Wenk, H. N., McCleskey, E. W. A novel mouse skeletal muscle-nerve preparation and in vitro model of ischemia. J Neurosci Methods. 159, 244-251 (2007).
- Bullinger, K. L., Nardelli, P., Wang, Q., Rich, M. M., Cope, T. C. Oxaliplatin neurotoxicity of sensory transduction in rat proprioceptors. J Neurophysiol. 106, 704-709 (2011).
- Wilkinson, K. A., Kloefkorn, H. E., Hochman, S. Characterization of muscle spindle afferents in the adult mouse using an in vitro muscle-nerve preparation. PLoS One. 7, e39140 (2012).
- Shreckengost, J., Calvo, J., Quevedo, J., Hochman, S. Bicuculline-sensitive primary afferent depolarization remains after greatly restricting synaptic transmission in the mammalian spinal cord. J Neurosci. 30, 5283-5288 (2010).
- Koltzenburg, M., Stucky, C. L., Lewin, G. R. Receptive properties of mouse sensory neurons innervating hairy skin. J Neurophysiol. 78, 1841-1850 (1997).
- Matthews, B. H. C. Nerve endings in mammalian muscle. J Physiol. 78, 1-53 (1933).
- Hunt, C. C., Kuffler, S. W. Stretch receptor discharges during muscle contraction. J Physiol. 113, 298-315 (1951).
- Oishi, P. E., Cholsiripunlert, S., Gong, W., Baker, A. J., Bernstein, H. S. Myo-mechanical analysis of isolated skeletal muscle. J Vis Exp. (48), (2011).
- Hoheisel, U., Reinöhl, J., Unger, T., Mense, S. Acidic pH and capsaicin activate mechanosensitive group IV muscle receptors in the rat. Pain. 110, 149-157 (2004).
- Taguchi, T., Sato, J., Mizumura, K. Augmented mechanical response of muscle thin-fiber sensory receptors recorded from rat muscle-nerve preparations in vitro after eccentric contraction. J Neurophysiol. 94, 2822-2831 (2005).
- Xu, J., Gu, H., Brennan, T. J. Increased sensitivity of group III and group IV afferents from incised muscle in vitro. Pain. 151, 744-755 (2010).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
ABOUT JoVE
Copyright © 2025 MyJoVE Corporation. All rights reserved