A subscription to JoVE is required to view this content. Sign in or start your free trial.
Kupffer Cell Isolation for Nanoparticle Toxicity Testing
In This Article
Summary
Liver macrophages, named Kupffer cells, are responsible for the capture of circulating nanoparticles. We describe here a method, of high cell purity and yield, for Kupffer cell isolation. The modified LDH assay is used here to measure the toxicity induced by carbon nanotubes in Kupffer cells.
Abstract
The large majority of in vitro nanotoxicological studies have used immortalized cell lines for their practicality. However, results from nanoparticle toxicity testing in immortalized cell lines or primary cells have shown discrepancies, highlighting the need to extend the use of primary cells for in vitro assays. This protocol describes the isolation of mouse liver macrophages, named Kupffer cells, and their use to study nanoparticle toxicity. Kupffer cells are the most abundant macrophage population in the body and constitute part of the reticulo-endothelial system (RES), responsible for the capture of circulating nanoparticles. The Kupffer cell isolation method reported here is based on a 2-step perfusion method followed by purification on density gradient. The method, based on collagenase digestion and density centrifugation, is adapted from the original protocol developed by Smedsrød et al. designed for rat liver cell isolation and provides high yield (up to 14 x 106 cells per mouse) and high purity (>95%) of Kupffer cells. This isolation method does not require sophisticated or expensive equipment and therefore represents an ideal compromise between complexity and cell yield. The use of heavier mice (35-45 g) improves the yield of the isolation method but also facilitates remarkably the procedure of portal vein cannulation. The toxicity of functionalized carbon nanotubes f-CNTs was measured in this model by the modified LDH assay. This method assesses cell viability by measuring the lack of structural integrity of Kupffer cell membrane after incubation with f-CNTs. Toxicity induced by f-CNTs can be measured consistently using this assay, highlighting that isolated Kupffer cells are useful for nanoparticle toxicity testing. The overall understanding of nanotoxicology could benefit from such models, making the nanoparticle selection for clinical translation more efficient.
Introduction
The field of nanotoxicology research aims to characterize the biological effect of nanoparticles. Toxicological studies based on in vivo investigations remain the most accurate methods. However, their use is limited by their cost, labor and time requirements1. As alternatives, in vitro assays have been used because of their simplicity and the possibility of developing high-throughput in vitro testing platforms2 - so extending the number of conditions tested. Most nanotoxicological studies are performed using in vitro assays with immortalized cell lines. However, there are concerns regarding the extrapolation of....
Protocol
All animal experiments were executed in compliance with all relevant guidelines, regulations and regulatory agencies. The protocol being demonstrated was performed under the guidance and approval of the UK Home office regulation
1. Perfusion and Cell Collection (Figure 1)
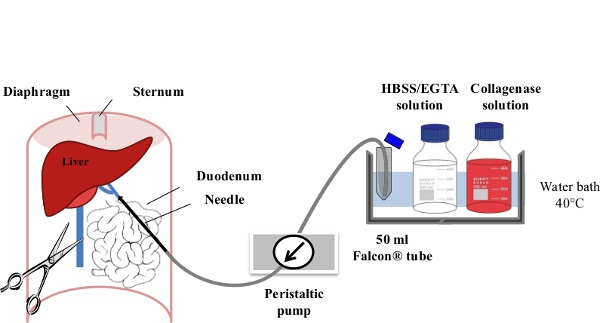
Figure 1: Liver Perfusion. After anaesthesia of the mouse, the digestive tract is laterally moved to the left of the abdomen in order to make the portal vein (PV) accessible. The PV is cannulated using a slow flow rate (....
Results
The number of non-purified parenchymal cells was consistent and ranged between 8 and 14 x 106 cells per mouse (17 isolations were performed). Each mouse isolation was sufficient to plate 16 to 28 wells. Cell viability by trypan blue staining showed cell viability ~95%. Kupffer cells showed a round shape within 30 min of incubation at 37 °C, related to their incomplete adherent morphology (Figure 4A). At 4 hr of incubation and afterwards, cells were spread and cell clusters started to form.......
Discussion
The following steps are critical to achieve high yield and high viability of Kupffer cells. Aseptic conditions should be used to limit the risk of bacterial and fungal contamination. All instruments need to be sterilized before use. Reagents should be freshly prepared before carrying out the isolation procedure.
The choice of collagenase IV, with low tryptic activity, is crucial. Different lots from the same supplier have different enzymatic activity and they may need to be compared initially .......
Disclosures
The authors declare that they have no competing financial interests.
Acknowledgements
This work was supported by EU FP7-ITN Marie-Curie Network program RADDEL. MB and KAJ would like to acknowledge Joe Varguese and Rui Serra Maia for their help and suggestions along the optimization of the Kupffer cell isolation.
....Materials
| Name | Company | Catalog Number | Comments |
| Euthatal (pentobarbital sodium) | Merial | ||
| CD1 mice | Charles River | - | Mouse weight should vary from 35 to 45 g. We advise the use of male CD1 mice as their weight increase rapidly (e.g. a male CD1 mouse of 7 weeks old exceeds 35 g in weight). It is also advised to contact the animal supplier in advance to arrange for the delivery of older animals. Alternatively animals can be in-house to reach the desired weight. |
| HBSS (Ca2+ and Mg2+ free, with bicarbonate) | Life Technologies | 14175-053 | HBSS must be Ca2+ free. |
| Ethylene Glycol Tetraacetic Acid (EGTA) tetrasodium salt | Sigma-Aldrich | E8145 | EDTA can also be used but EGTA has the advantage of chelating Ca2+ selectively. |
| HEPES (1M) | Life Technologies | 15630-056 | 100 ml |
| Collagenase type IV | Worthington | CSL-4 | It is advised to test different batches of collagenase or at least mention to the supplier the product has to be suitable for liver cell isolation |
| Low glucose DMEM | Sigma-Aldrich | D5523 | 500 ml |
| RPMI (with sodium pyruvate and Glutamax®) | Life Technologies | 12633-012 | 500 ml |
| Penicillin/Steptomycin | Life Technologies | 15140-122 | 100 ml |
| Fetal Bovine Serum | First-Link | 60-00-850 | 500 ml |
| Trypan blue solution | Sigma-Aldrich | T8154 | 100 ml |
| Coated silica particle solution (Percoll®) | GE Healtcare | 17-0891-02 | Percoll® is very stable and can be kept for several years |
| 1x Phosphate Buffered Saline | Life Technologies | 10010-023 | 500 ml |
| 10x Phosphate Buffered Saline | Life Technologies | 70011-036 | 500 ml |
| Dimethyl sulfoxide | Fisher | D/4120/PB08 | 500 ml |
| LDH kit (CytoTox 96®) | Promega | G1781 | Keep protected from light |
| DMEM phenol red free | Life Technologies | 31053-028 | 500 ml |
| F4/80 antibody (Alexa 488) | AbD Serotec | MCA497A488 | Do not dilute, used neat for flow cytometry |
| Fluorescent beads | Sigma | L2778 | Latex beads, amine-modified polystyrene, fluorescent red. 1 ml |
| Name of the Material | Company | Catalog number | Comments/Description |
| Butterfly blood collection set (23G/305mm long tubing) | BD | 367288 | |
| Syringe Filters (0.22 μm Blue Rim) | Minisart | 16534-K | |
| Centrifuge Tubes (50 ml Blue Cap) | BD Biosciences, Falcon | 35 2070 | |
| Petri dish (90 x 15 mm) | Thermo Fisher Scientific | BSN 101VR20 | |
| 100 μm cell strainer | BD | 352360 | |
| Peristaltic pump | Watson Marlow | SciQ 300 | Rinse tubing before and after each usage with sterile PBS and 70% ethanol. |
| 24-well plates | Corning | 3526 | |
| 96-well plates | Corning | 3595 | |
| Centrifuge | Eppendorf | 5810R | |
| Plate reader | BMG Labtech | FLUOstar Omega | |
| Serrefine forceps | Hammacher GmbH | Art. Nr. HSE 004-35 / Cat. Nr. 221-0051 | The serrefine forceps allow to clamp the vessel cannulated with the 23G needle without the need of holding the forceps during the perfusion procedure. URL: (http://www.hammacher.de/Laboratory-Products/Clamps-forceps/Serrefines/HSE-004-35-Serrefine::25126.html) |
| Flow cytometry tubes | BD Biosciences, Falcon | 352052 | |
| Microcentrifuge tubes (1.5 ml) | Elkay | 000-MICR-150 | |
| Cell scraper | BD Biosciences, Falcon | 353086 | Cut blade extremities with a pair of scissors to scrape cells in 24-well plates. |
| Name of the Reagent | Company | Catalog number | Comments/Description |
| EGTA (Ethylene Glycol Tetraacetic Acid)/HBSS (Hank's Balanced Salt Solution) Solution | HBSS containing 0.5 mM EGTA and 25 mM HEPES. Adjust pH to 7.4. Prepare 50 ml for each liver to perfuse. | ||
| Collagenase Solution | DMEM low glucose containing collagenase type IV at 100 UI/ml, 15 mM HEPES and 1% Penicllin/Streptamycin (v/v). Adjust pH to 7.4. Prepare 100 ml for each liver to perfuse. After adding the collagenase, it is advised to warm up the solution for 30 min before use. This allows the collagenase activity to be optimum. | ||
| Kupffer Cell Isolation Medium | RPMI containing, 1% Non-Essential Amino-Acids (v/v),1% glutamax® (v/v) and 1% Penicllin/Streptomycin (v/v). Prepare at least 100 ml for 1-3 livers. | ||
| Kupffer Cell Culture Medium | RPMI containing 10% Fetal Bovine Serum, 1% Non-Essential Amino-Acids (v/v),1% Glutamax® (v/v) and 1% Penicllin/Streptamycin (v/v). Prepare at least 100 ml for 1-3 livers. | ||
| SIP (solution of isotonic coated silica particles) | Mix 1.7 ml of 10x Phosphate Buffered Saline with 15.3 ml of Percoll® to obtain 17mL of SIP. | ||
| 25% SIP solution | Mix 5 ml of SIP with 15 ml of 1x Phosphate Buffered Saline | ||
| 50% SIP solution | Mix 10 ml of SIP with 10 ml of 1x Phosphate Buffered Saline | ||
| Lysis Buffer | DMEM media with 0.9% Triton X-100 | ||
| PBS/BSA Solution | Prepare fresh Phosphate Buffered Saline pH 7.4 with 1% Bovine Serum Albumin. | ||
References
- Hoffmann, D., et al. Performance of novel kidney biomarkers in preclinical toxicity studies. Toxicol Sci. 116 (1), 8-22 (2010).
- Nel, A., et al. Nanomaterial T....
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved