A Protocol for Safe Lithiation Reactions Using Organolithium Reagents
In This Article
Summary
The safe and proper use of organolithium reagents is described.
Abstract
Organolithium reagents are powerful tools in the synthetic chemist's toolbox. However, the extreme pyrophoric nature of the most reactive reagents warrants proper technique, thorough training, and proper personal protective equipment. To aid in the training of researchers using organolithium reagents, a thorough, step-by-step protocol for the safe and effective use of tert-butyllithium on an inert gas line or within a glovebox is described. As a model reaction, preparation of lithium tert-butyl amide by the reaction of tert-butyl amine with one equivalent of tert-butyl lithium is presented.
Introduction
Organolithium reagents (RLi) are powerful bases that exploit the non-polar, strong bonds of hydrocarbons to generate conjugate bases that can deprotonate almost any compound of even moderate acidity. They serve as more aggressive alternatives to lithium amides (e.g., LDA) and Grignard reagents. Their incredibly strong basicity makes them of immense utility in organic and inorganic syntheses, and their wide applicability has been thoroughly described in several recent reviews1-3. Organolithium reagents can easily deprotonate extremely weak acids such as alcohols, amines, and both benzylic and aliphatic hydrocarbons. The reaction is driven by the formation of a stable, strong, alkyl C-H bond.
Li+R- + HX → LiX + RH (1)
General concepts surrounding organolithium reagents have been reviewed4-7, but we highlight here the utility of these reagents to exploit the differing pKa values of several different hydrocarbons in order to select a conjugate base with appropriate deprotonating power. For instance, since the acidity of aliphatic hydrocarbons decreases with increasing levels of substitution (i.e., 1° >2° >3°), tert-butyllithium is the most aggressive alkyllithium reagent, while methyllithium is the most mild. Phenyllithium is considerably milder than methyllithium due to the ability of the phenyl ring to delocalize the charge of the deprotonated phenyl anion. Thus, the most commonly used organolithium reagents are, in order of increasing basicity: PhLi <MeLi <BuLi <s-BuLi <tBuLi. While precise pKa values of the protonated alkanes are difficult to measure due to their lack of acidity, approximate pKa values are provided in Table 17-10, along with other common protic reagents commonly deprotonated by organolithium reagents in synthetic chemistry. Table 1 provides, in a glance, a visual tool to predict which bases may be used to deprotonate which acids.
Beyond acid-base chemistry, alkyllithium reagents have been exploited in inorganic and organometallic chemistry as a means to provide carbon-based ligands11,12, transmetallate reagents in catalysis13-15, or facilitate organometallic reactivity by photolytic M-Me bond homolysis16,17. While alkyllithium reagents are thermodynamically very strong bases, their reactivity can be sluggish in some reactions, requiring optimization of reaction conditions18. Generally, their kinetic behavior can be improved by replacement of the Lewis acidic lithium ion with a weaker Lewis acid such as potassium, as is seen in the generation of "Schlosser's base" from BuLi and potassium tert-butoxide19.
While the utility of organolithium reagents in synthesis is undeniable, the use of these reagents requires appropriate precautions. The reagents are pyrophoric, reacting violently in air or with water and with a vigorous exotherm. They generate volatile organics which frequently ignite due to the high temperatures of decomposition. Thus, fires can occur during lithiations, particularly when careful standard operating procedures are not followed. Most infamous is the case of a recently-graduated undergraduate alumna of The University of California, Los Angeles (UCLA) working as a research assistant. As a result of a tragic accident during a lithiation reaction with the most reactive organolithium reagent, tert-butyl lithium, the student received fatal burns when a syringe full of the solution came apart and ignited her clothes20. Among the mistakes that were made were the use of an inappropriately-sized syringe and needle, a lack of appropriate personal protective equipment (PPE), and a failure to use the available safety shower20. The sensitive nature of common carbanion reagents has inspired the development of safer alternatives in high polarity solvents21, such as eutectic solvent mixtures22-24, and for Grignard reagents, even water25-27. Nevertheless, the versatility of organolithium reagents makes them of continued utility for the foreseeable future.
The objective of this protocol and visualized experiment is to demonstrate a thorough and careful approach to lithiation, accessible to any well-trained chemistry student who has a need for organolithium reagents. It is our hope that this open access protocol will illustrate what to do (and what not to do) to achieve a successful and safe lithiation, that other laboratories may use this publication as a training resource, and that through this thorough, visual demonstration, future accidents may be avoided. Here, a safe protocol for lithiation using the most reactive tert-butyl lithium is described, which can be adapted for use with any of the less reactive organolithium reagents.
Protocol
NOTE: tBuLi solutions (1.7 M in pentane) and anhydrous tert-butylamine were purchased and used immediately, without purification. In our experience, this protocol works best with freshly-purchased reagents. Standardization of the organolithium reagent may be employed via titration with dibromoethane28, diphenylacetic acid29, or N-pivaloylanilines30, since concentrations of commercial organolithium reagents may vary and the quality of reagents degrades over time. Pentane was purified using a solvent purification system. Solvents were degassed and stored over activated molecular sieves for 24 hr before use.
1. Preparation of Hood Space
NOTE: See Figure 1.
- Clear a hood of all clutter.
- Fill a small beaker with a volume of toluene approximately equal to the volume of organolithium reagent to be used (here, 10 ml for the small-scale protocol and 50 ml for the large-scale protocol) and cover with an appropriately-sized watch glass.
- Prepare a beaker of isopropanol with a volume approximately 5 times the amount of organolithium reagent to be used (here, 50 ml for the small-scale and 250 ml for the large-scale) and cover with an appropriately-sized watch glass.
- Prepare a beaker containing dry ice pellets filled up to the volume line approximately 10 times the volume of organolithium reagent to be used (here, 100 ml for the small scale and 500 ml for the large-scale).
- Before proceeding further, inspect the seal/cap of the lithiating agent for corrosive buildup. If the seal is compromised, dispose of the reagent by slowly adding it to 8-10x its volume of dry ice in a beaker.
2. Procedure for Small-scale Lithiation in a Hood
NOTE: See Figure 1.
- Charge a 25 ml Schlenk flask with a stir bar and neat tBuNH2 (1.8 ml, 17.1 mmol) and fit it with a rubber septum.
- Degas the neat tBuNH2 by opening the Schlenk flask stopcock and turning the Schlenk line to vacuum briefly (~1 sec; tBuNH2 is volatile and will evaporate if held under vacuum). Immediately backfill with inert gas by turning the Schlenk stopcock to inert gas. Repeat twice more. Close the flask stopcock to isolate the flask.
- Prepare an inert gas blanket by attaching three tubes to a glass "T" adapter. Attach one tube to an inert gas source, a second to an oil bubbler, and a third to a Luer-lock needle adapter.
- Purge the blanket apparatus with inert gas for 5 min.
- Slow the flow rate so that a few bubbles per second pass through the oil bubbler.
- Insert the inert gas blanket needle into the septum of the reaction flask and immerse the flask in a dry ice/acetone bath with a magnetic stirrer. Stir gently until the flask has cooled.
- Clamp the tBuLi bottle (25 ml, 1.7 M in pentane) to a ring stand and remove the outer cap. If present, remove any Parafilm and wipe away any grease.
- Select a 20 ml glass syringe and an appropriately-sized plunger. The plunger should slide in easily and should not be able to wiggle or rattle. If a thumb is placed over the syringe tip to seal it, the plunger should not be easily pulled out.
- Fit the 20 ml glass syringe with a long (12 inches), flexible syringe needle. Always be certain to select a syringe with a volume at least double the volume of the reagent to be drawn, and always be sure to attach the needle securely to the syringe.
- Remove the needle adapter for the inert gas blanket from the reaction flask and move it to the tert-butyllithium bottle, piercing the bottle septum to put the reagent bottle under ambient inert gas pressure.
- Alternatively, use a septum-inlet transfer adapter from the reagent vendor as an inert gas blanket. Attach the septum-inlet transfer adapter to the reagent bottle and open the side and top caps. Attach a Schlenk hose to the side arm and purge with inert gas. While purging, replace the top cap with a septum. Leave the Schlenk line stopcock open to the septum-inlet transfer adapter to keep it under positive pressure.
NOTE: The commercial vendor suggests the use of a pressurized inert gas source instead of a blanket. This permits the reagent to be "pushed" into the syringe instead of drawn in by pulling the syringe. If the back-pressure setting is not right, over-pressurization can cause the plunger to be pushed out, exposing the reagent to air. Further, back pressure requires the experimenter to apply an equal and opposite pressure on the plunger with the thumb once the desired volume is reached so that the volume in the syringe is held constant. This can cause the reagent to squirt when the needle is pulled from the septum. Thus, the authors prefer the use of an ambient pressure inert gas blanket.
- Alternatively, use a septum-inlet transfer adapter from the reagent vendor as an inert gas blanket. Attach the septum-inlet transfer adapter to the reagent bottle and open the side and top caps. Attach a Schlenk hose to the side arm and purge with inert gas. While purging, replace the top cap with a septum. Leave the Schlenk line stopcock open to the septum-inlet transfer adapter to keep it under positive pressure.
- Purge the syringe with inert gas. Open an unoccupied Schlenk hose to inert gas so that there is a gentle flow of inert gas out of the Schlenk hose. Place the needle of the syringe loosely into the end of the hose and draw the plunger in and out several times to purge the interior of the syringe with inert gas.
- With the syringe plunger fully depressed, pierce the bottle septum and immerse the needle in the reagent.
- Gently draw back the plunger until an excess (~11 ml) of reagent has been drawn into the syringe (never invert the reagent bottle). Expel the headspace gas and excess reagent from the syringe by flexing the needle so that the syringe points up and then depressing the plunger until there is no headspace and there is 10.0 ml of reagent in the syringe. At this point, relax the flexion of the syringe needle, turning the syringe right side up.
- With the syringe needle still in the bottle septum, move the inert gas blanket needle adapter back to the reagent flask septum and pierce it.
- Remove the syringe needle from the bottle septum using a free hand (never pull on the syringe to remove the needle, as the needle may pop off). Some flames may be observed upon removal of the needle from the septum. Pierce the rubber septum of the reaction flask with the long syringe needle and suspend it above the stirred tBuNH2.
- Depress the plunger slowly to add all the tBuLi solution dropwise to the stirred tBuNH2.
- Remove the long syringe needle from the septum, leaving the inert gas blanket needle in the reaction flask septum.
- Remove the watch glass from the beaker of toluene and draw a volume of toluene approximately equal to the volume of tBuLi used (~10 ml) into the syringe to dilute the residual tBuLi.
- Remove the watch glass from the isopropanol beaker, place the long needle into the isopropanol, and empty the dilute solution in the syringe into the isopropanol.
- Rinse the syringe several more times with isopropanol to remove residual reagent, after which the syringe is clean.
- Seal the tBuLi reagent bottle septum with some grease to avoid leaks at the puncture sites and place a piece of Parafilm over the greased septum. Replace the outer cap.
- Remove the flask from the ice bath and stir under an ambient inert gas atmosphere until it comes to room temperature.
- Remove the inert gas blanket needle.
- Store the flask at -30 °C overnight. After this time, white powdery solid of [LiNHtBu]8 will be observed.
- Filter the solution, rinse the solid with cold pentane under an inert atmosphere, and dry in vacuo.
3. Procedure for Large-scale Lithiation in a Hood
- Charge a 100 ml Schlenk flask with a stir bar and neat tBuNH2 (9 ml, 85.5 mmol) and fit it with an addition funnel that holds at least 50 ml. Clip the addition funnel to the flask using a keck clamp. Cap the top of the addition funnel with a rubber septum. Close the addition funnel's stopcock.
- Degas the neat tBuNH2 by opening the Schlenk flask stopcock and turning the Schlenk line to vacuum briefly (~1 sec; tBuNH2 is volatile and will evaporate if held under vacuum). Immediately backfill with inert gas by turning the Schlenk stopcock to inert gas. Repeat twice more. Close the flask stopcock to isolate the flask and the addition funnel.
- Prepare an inert gas blanket by attaching three tubes to a glass "T" adapter. Attach one tube to an inert gas source, a second to an oil bubbler, and a third to a Luer-lock needle adapter.
- Purge the blanket apparatus with inert gas for 5 min.
- Slow the flow rate so that a few bubbles per sec pass through the oil bubbler.
- Clamp the tBuLi bottle to a ring stand and remove the outer cap. Remove any Parafilm and wipe away any grease.
- Transfer the inert gas blanket to the septum of the addition funnel. Lower the flask into a dry ice bath to cool.
- Using another inert gas line, apply a gentle flow of inert gas to the tBuLi bottle.
- Insert one end of a cannula into the tBuLi bottle and suspend it above the solution.
- Insert the other end into the addition funnel so that the tip is below the pressure equalizing side-arm.
- Lower the end of the cannula above the tBuLi into the liquid and control the speed of addition via the inert gas line. Fill the addition funnel to the 50 ml line.
- When the addition is complete, remove the cannula end from the lithiating reagent solution and leave it suspended above the tBuLi reagent.
- Remove the opposite end of the cannula from the addition funnel.
- Remove the end of the cannula in the tBuLi bottle. Then, remove the inert gas line from the tBuLi bottle.
- Turn the stopcock on the addition funnel to add the tBuLi dropwise to the stirring tBuNH2.
- Seal the tBuLi reagent bottle septum with some grease to avoid leaks at the puncture sites and place a piece of Parafilm over the greased septum. Replace the outer cap.
- Remove the addition funnel from the Schlenk flask using the following steps:
- Place the Schlenk flask under positive pressure of inert gas by opening the Schlenk flask stop cock and the Schlenk line stop cock. Remove the keck clamp and the addition funnel from the Schlenk flask. The flask will be protected by a flow of inert gas out of the flask, but the addition funnel may smoke or flame briefly upon exposure to air.
- Wipe away the grease on the inner neck of the Schlenk flask using a paper towel wetted with hexanes and repeat until the ground glass of the flask appears dry. Stopper the flask with a rubber septum.
- Place the inert gas blanket needle into the Schlenk flask septum.
- Remove the flask from the ice bath and stir under an ambient inert gas atmosphere until it comes to room temperature.
- Remove the inert gas blanket needle.
- Store the flask at -30 °C overnight. After this time, white powdery solid of [LiNHtBu]8 will be observed.
- Filter the solution, rinse the solid with cold pentane under an inert atmosphere, and dry in vacuo.
4. Procedure for Lithiation in a Glovebox
- Bring all reagents, a reaction flask, a stir bar, a stopper, and a greased desiccator (or another sealable container to be used for waste) into the glovebox via the antechamber.
- Charge a flask with a stir bar and degassed neat tBuNH2 (1.8 ml, 17.1 mmol). Cover the flask with a glass stopper or septum to prevent evaporation of volatile tert-butyl amine.
- Clamp the tBuLi bottle (25 ml, 1.7 M in pentane) to a ring stand and remove the outer cap. Optional: Remove the septum cap using a bottle opener, with the bottle firmly clamped in place. Once the bottle cap is removed, do not remove the bottle from the glovebox until empty. If removed, carefully quench the remaining tBuLi in a hood with an appropriate quenching reagent such as dry ice or isopropanol.
- Prepare a small vial of ~10 ml toluene to wash the syringe after the addition.
- Fit a 20 ml syringe with a needle. Always be certain to select a syringe with a volume at least double the volume of the reagent to be drawn, and always be sure to attach the needle securely to the syringe.
- Insert the needle into the tBuLi reagent and gently draw the plunger back until an excess (~11 ml) of reagent has been drawn into the syringe. Then, invert the syringe, pointing the needle up.
- Hold a paper towel near the needle and gently depress the plunger to remove the headspace gas until a microdroplet of reagent emerges from the end of the needle. Remove excess reagent from the syringe by placing the needle into the reagent bottle and depressing the plunger until 10.0 ml of reagent remains in the syringe. If any reagent solution spills, wipe it up with a paper towel or Kimwipe and place the waste into the waste desiccator.
- Remove the stopper or septum from the reaction flask and slowly add the tBuLi to the stirred tBuNH2. Since the reaction is performed without a cold bath, take care to avoid adding the reagent too quickly, as the exotherm can cause boiling. Stopper the reaction flask.
- Draw toluene from the toluene vial into the syringe to dilute the residual reagent, and place the syringe, needle, and any paper towel waste into the desiccator. Seal the desiccator.
- Re-cap and store the tBuLi reagent bottle, preferably in a glovebox freezer to improve longevity.
- Remove the sealed desiccator containing the used glassware, the syringe with toluene, and any paper towels from the glovebox, and immediately place it into a hood.
- Open the desiccator and empty the syringe containing dilute tBuLi into a beaker of isopropanol to quench the reagent. Rinse the syringe several more times with isopropanol.
- Store the reaction flask at -30 °C overnight, after which time white powdery solid of [LiNHtBu]8 are observed.
- Filter the solution, rinse the solid with cold pentane under an inert atmosphere, and dry in vacuo.
5. How to Abort the Reaction or in Case of Fire
NOTE: See Figure 1.
- If at any point the reaction needs to be aborted, slowly empty any unused organolithium reagent in the syringe into the dry ice. Flames may occur as the reagent is emptied, but the dry ice should quench them.
- If at any point the toluene or isopropanol catches fire, simply place the watch glass onto the beaker so that the flames will be smothered.
- If a circumstance ever occurs where a fire cannot be quenched by this method, immediately use the fire extinguisher.
- In the unlikely event that hair or clothing catches fire, immediately use the safety shower.
Representative Results
The typical yield of this reaction is ~670 mg (8.5 mmol, ~50%). Additional crops of crystals may be obtained by concentrating the filtrate and chilling the solution. However, purity is often compromised by additional crops. When this protocol is followed carefully by a prepared and practiced researcher, it generally proceeds without incident. In our experience, in the rare cases when the reaction must be aborted or a fire occurs, the availability of watch glass covers, dry ice, and isopropanol quench beakers, and the localization of the operation in a hood provide sufficient contingency.
Confirmation of the product by NMR (Figure 4) or X-ray diffraction is necessary, as the use of impure or water-contaminated reagents frequently leads to failure to obtain the desired product. The 1H NMR spectrum shows two peaks, as expected, in a ratio of 1:9 (representing, respectively, the single amide proton and the nine tert-butyl protons). Indexing of a crystal grown from pentane or hexane is consistent with the reported crystal structure of the product31. NMR (400 MHz, benzene-d6) δ -1.53 (s, 1H, NH), 1.37 (s, 9H, But). Unit cell: P2/n, a = 12.05(2), b = 12.62(2), c = 18.24(3) Å, β = 105.52(5)°, V = 2672(14) Å3.
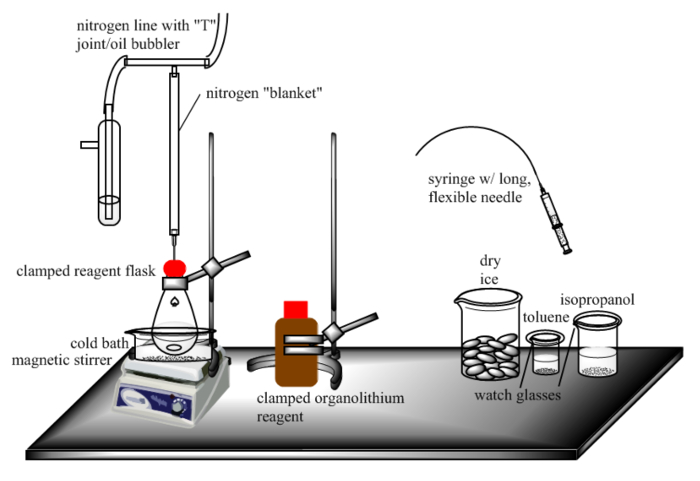
Figure 1: Apparatus Diagram. The appearance of the interior of a hood for reaction outside the glovebox is shown. Please click here to view a larger version of this figure.

Figure 2: The Syringe with Needle. A 10 ml syringe with a needle attached using a Luer-lock tip is shown. Please click here to view a larger version of this figure.

Figure 3: Septum-sealed Bottle Cap. The reagent is sold by the vendor with a sealed metal bottle cap with a rubber septum that may be pierced with a needle. Please click here to view a larger version of this figure.
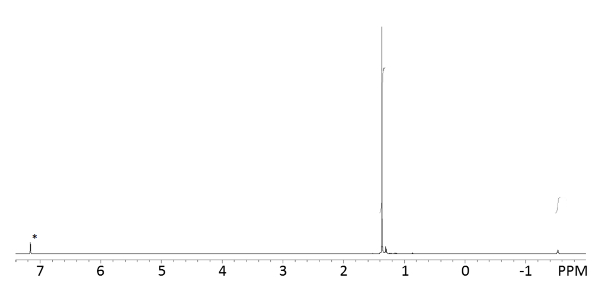
Figure 4: 400 MHz 1H NMR Spectrum of LiNHtBu in C6D6. The NMR spectrum of the product shows the expected two signals for the amide and tert-butyl protons, with an integral ratio of 1:9, respectively. Residual protiosolvent signal is labelled with *. Please click here to view a larger version of this figure.
| Acid | pKa | Base |
| i-butane7 | >51 | tBuLi |
| n-butane (2o carbon) 7 | ~50 | s-BuLi |
| n-butane (1o carbon) 7 | ~50 | BuLi |
| methane7 | 48 | MeLi |
| benzene7 | 43 | PhLi |
| toluene7 | 40 | TolLi |
| R2NH8 | 36 | RNHLi |
| ArNH29 | 31 | ArNHLi |
| ROH9 | 15 | ROLi |
| ArOH8,9 | 10 | ArOLi |
Table 1: pKa values of Hydrocarbons and Their Corresponding Lithiated Conjugate Bases.
Discussion
For this lithiation experiment, tert-butyl lithium amide (LiNHtBu) is synthesized via lithiation of tert-butyl amine (tBuNH2) using tert-butyl lithium (tBuLi), forming isobutane as a side product. The described protocol is a modification of a previously-reported protocol31 and proceeds according to the following reaction:
tBuNH2 + tBuLi → tBuH + 1/8 [LiNHtBu]8. (2)
The original report for the synthesis of LiNHtBu differs from this protocol in that it employed the use of less reactive n-butyl lithium as the organolithium reagent. In general, one should always choose the less reactive organolithium reagent whenever possible. However, for the purpose of this paper, the authors have elected to demonstrate the safe usage of the more reactive tert-butyl lithium solution so that viewers can observe the proper handling of the most challenging reagent. This protocol may be easily applied to the use of the less reactive organolithium reagents.
Critical Steps
Due to the highly pyrophoric nature of organolithium reagents, all operations must be carried out under inert atmosphere conditions, necessitating the use of a Schlenk or inert gas line, or an inert atmosphere glovebox. While operation in a glovebox is a far simpler approach, it is associated with its own risks, different from those of performing lithiations on an inert gas line. Either of these approaches therefore requires great care and adherence to protocol. Described here are two protocols for lithiation: one on an inert gas (Schlenk) line, and one within a glovebox. When performing a lithiation on an inert gas line, a familiarity with the operation of air-free glassware and protocols is invaluable. However, since different laboratories may adopt slightly different practices, a step-by-step protocol for each method is thoroughly described. The chemical vendor offers its own recommended glassware apparatus and protocol for proper use of air-sensitive reagents32. The Protocol section outlines a procedure similar to the vendor's, but which has been modified to maximize safety and ease, specifically for alkyllithium protocols. The detailed procedure is available in the Protocol section, but here, some important points are highlighted to maximize safety and success.
NOTE: Never work in the laboratory alone.
PPE
An extremely important consideration is the use of proper personal protective equipment (PPE), which for lithiation includes a proper-fitting lab coat, safety glasses, long pants (preferably made of non-flammable material), closed-toed shoes, and a hair tie (if applicable). While best practices can ensure that no fires occur in most cases, tert-butyl lithium is extremely pyrophoric, and accidents can happen. When they do, the safety of the researcher is better secured if they are shielded by the proper PPE. The UCLA alumna's most significant mistakes were that she performed a lithiation with no laboratory coat and that she was wearing clothing made of flammable material20.
Ventilation
Lithiations outside the glovebox should always be performed in a hood. If a clear hood is not available, do not perform a lithiation until a clear, uncluttered hood space free of other flammable chemicals is secured. The sash should be lowered as much and as often as possible. An additional mistake of the UCLA alumna was that there were other flammables in the hood (hexanes), which spilled and caught fire, igniting her clothes20.
Inert Gas
A lithiation requires the use of inert gas. A Schlenk line (double manifold switchable between inert gas and vacuum) is ideal, though any inert gas source with good flow control will work.
Syringe
Glass syringes are preferable to plastic syringes due to their chemical inertness and smoother plunger motion. A long (1-2 ft)32, flexible needle must always be attached securely to the delivery syringe. Another of the UCLA alumna's mistakes was the use of a too-short (1.5 inches)20 needle, which may have necessitated inverting the reagent bottle to draw the reagent into the syringe, which can lead to spills and fire. Thus, a long needle should always be used so that the bottle does not need to be inverted. The needle should be attached securely so that it does not pop off during reagent delivery. Luer-lock style syringes (Figure 2) are best. If using a push-on "slip-tip" syringe needle system, ensure that the needle is extremely well attached before proceeding. A syringe should always be selected that is at least twice the volume of the desired quantity of organolithium reagent32. This is due to the fact that head space always occupies some volume of the syringe while drawing a reagent. Another of the UCLA alumna's mistakes was the use of a syringe that was too small. When the syringe reached capacity, it likely popped open, splashing tBuLi onto her unprotected arm20.
Quenching Agents
A small beaker containing toluene (volume approximately equal to the volume of organolithium reagent to be delivered) should be located in the hood within reach of – but not right next to – the reaction vessel. A watch glass appropriately sized to cover this beaker in case of fire should also be placed over the beaker. This beaker will be used to dilute the residual reagent contaminating the syringe after the reagent addition (Figure 1).
A second beaker containing isopropanol (volume approximately five times the volume of organolithium reagent to be delivered) should also be located in the hood within reach of – but not right next to – the reaction vessel. A second watch glass appropriately sized to cover this beaker in case of fire should also be placed on top of the beaker. This vessel is used to quench the residue left in the syringe after the addition (Figure 1).
Third, a beaker of dry ice (approximately ten times the volume of organolithium reagent to be delivered) should be located in reach of the reaction vessel. In the event of the syringe needle coming loose, or something else going wrong, this dry ice may be used to quench the remaining organolithium reagent in the syringe (Figure 1).
Finally, a fire extinguisher should be located nearby in case of emergency, and the location and proper operation of the safety shower should be noted.
The Reagent Bottle
Outside the glovebox, use only organolithium reagent bottles with septum-sealed bottle caps (Figure 3). The purchase of small bottles is recommended since 1) organolithium reagents degrade over time, and long-term storage is not recommended, 2) septa can degrade over time, exposing the reagent to air, and 3) small volumes of pyrophorics are less dangerous than large volumes. The organolithium reagent bottle should be set on the bench and clamped to a ring stand before use (Figure 1).
The Reaction Vessel
The reaction vessel should be oven- or flame-dried and cooled to room temperature under an inert atmosphere to ensure that no traces of water exist on the sides of the glass. The vessel containing the reagent to which the organolithium solution will be added should be clamped above a stir plate and degassed to remove air. This may be done either by purging the vessel with inert gas or by performing several evacuation-inert gas fill cycles on a Schlenk line. Alternatively, the flask can be charged with reagents and solvent in an inert atmosphere glovebox and sealed before removal from the glovebox. The degassed flask should be fitted with a septum and protected by an inert gas blanket (see Protocol and Figure 1). If the synthetic protocol permits, the flask should also be immersed into a cold bath such as dry ice/acetone to control the exotherm that will result when the organolithium reagent is added.
Notes on Lithiation in an Inert-atmosphere Glovebox
The use of air-free gloveboxes makes the handling of air-sensitive reagents vastly simpler, but it comes with its own risks. Since organolithium reagents are shielded from air in the glovebox, it is easier to become complacent and careless. While handling the reagents is simpler, a spill within the glovebox creates a dilemma: the spilled reagent must be wiped up with paper towels, but then the pyrophoric reagent and flammable cloth must be removed from the box and placed back into air, at which point, they will immediately catch fire. To avoid these hazards, reagents and reaction flasks should always be clamped securely within the glovebox, and open bottles and flasks should never be moved or handled by hand. Any materials containing residual reagent should be removed from the glovebox in a sealed desiccator (or similar container) and moved to a hood before being opened and exposed to air.
Know the Location and Operation of Emergency Equipment
Know the location and operation of the lab's fire extinguisher, so that in the event of a fire that cannot be put out by smothering with a watch glass, one can react quickly and decisively. Know also the location and operation of the laboratory's safety shower. In the unlikely event that a piece of clothing catches fire, immediately use the safety shower. If someone else's clothes catch fire, immediately direct them to the safety shower. If the laboratory does not have both a safety shower and a fire extinguisher, do not attempt a lithiation reaction. What may have been the final opportunity to save the life of the UCLA alumna was missed when neither she nor the postdoc working with her used the safety shower or an extinguisher to extinguish the flames. Rather, her postdoctoral coworker attempted to pat out the flames with a lab coat, which also caught fire. Ultimately, she sat on the floor while her postdoctoral coworker attempted to put out the flames by pouring beakers of water, filled from the sink, on the flames20.
Organolithium reagents are excellent for the deprotonation of weakly-acidic hydrogens or for acting as a source of alkyl groups, and they are more aggressive and reactive than the more standard Grignard reagents. Limitations of this technique can include kinetically sluggish reactions, in which case modification of the protocol can aid the chemical transformation19. Additionally, the high reactivity of organolithiums can interfere with desired chemistry. For instance, carbanions are generally excellent nucleophiles. Attempted deprotonation of an electrophilic substrate (such as a carboxylic acid) is likely to lead to nucleophilic attack instead of deprotonation. Thus, chemical knowledge and intuition is required when selecting reagents of this (or any) sort. Lithiation reactions will continue to play a role in synthetic organic and inorganic chemistry for the foreseeable future, and thus, an understanding of safe use is essential. Lithiation reactions are accomplished safely every day, and there is no cause to fear performing this reaction chemistry. However, the reagents deserve a measure of respect and care. It is essential that the multiple required fail-safes be followed to avoid the possibility of injury. In this protocol, a step-by-step procedure for a safe lithiation reaction is demonstrated and published as an open access article so that any researcher in the world can use it as training, free of charge. As such, the authors hope that this report may make the lithiation protocol accessible to a wide array of groups and prevent future tragedies.
Disclosures
The authors have nothing to disclose.
Acknowledgements
Support of this research by the National Science Foundation through grants 1254545 and 1437814 is gratefully acknowledged.
Materials
| Name | Company | Catalog Number | Comments |
| Schlenk Flask, 25 mL | Chemglass | AF-0520-02 | 25mL Flask, Reaction, 14/20 outer joint, 2mm glass stpk, Airfree, Schlenk |
| Rubber Septum | Chemglass | CG-3024-01 | Septum stopper, suba-seal, For 14/20-14/35 outer joints and 12.5mm ID tubing |
| Stir Bar | Fisher Scientific | 14-512-130 | Various sized stir bars |
| tert-butyllithium | Sigma-Aldrich | 186198-4X25ML | 1.7M t-butyllithium in pentane, 4 x 25mL |
| tert-butylamine | Sigma-Aldrich | 391433-100ML | tert-butylamine, purified by redistillation, >99.5% |
| hexanes | Fisher Scientific | H292-4 | 4L, certified ACS, hexanes, >98.5% |
| isopropanol | Fisher Scientific | A416-4 | 4L, 2-propanol, certified ACS plus, >99.5% |
| Dry ice | Airgas | ||
| Pure Solv Solvent Purification System | Inert Technology | MD-5 | Alumina collumns through which fresh, degassed solvents are passed to remove water. |
| Aldrich Sure/Seal septum-inlet transfer adapter | Sigma-Aldrich | Z407186 | Adapter for removal of air-sensitive reagents under nitrogen blanket |
| Keck Standard Taper Clips | Chemglass | CG-145-03 | clamp for securing glassware connections |
| Addition Funnel | Kontes | K634000-0060 | Funnel for dropwise addition of reagent to flask |
References
- Reich, H. J. What's Going on with These Lithium Reagents?. J Org Chem. 77 (13), 5471-5491 (2012).
- Reich, H. J. Role of Organolithium Aggregates and Mixed Aggregates in Organolithium Mechanisms. Chem Rev. 113 (9), 7130-7178 (2013).
- Capriati, V., Perna, F. M., Salomone, A. 34;The Great Beauty" of organolithium chemistry: a land still worth exploring. Dalton Trans. 43 (38), 14204-14210 (2014).
- Degennaro, L., Giovine, A., Carroccia, L., Luisi, R. . Lithium Compounds in Organic Synthesis. , 513-538 (2014).
- Carey, F. A., Sundberg, R. J. . Advanced Organic Chemistry: Part A: Structure and Mechanisms. , 579-628 (2007).
- Smith, M. B. . Organic Synthesis, 3rd Ed. , (2011).
- Smith, M. B., March, J. Ch. 5. March's Advanced ORganic Chemistry. , (2007).
- Renaud, P., Fox, M. A. Electrochemical behavior of lithium dialkylamides: the effect of aggregation. J Am Chem Soc. 110 (17), 5702-5705 (1988).
- Bordwell, F. G., Cheng, J., Ji, G. Z., Satish, A. V., Zhang, X. Bond dissociation energies in DMSO related to the gas phase values. J Am Chem Soc. 113 (26), 9790-9795 (1991).
- Jencks, W. P., Regenstein, J., Lundblad, R. L., Macdonald, F. M. . Handbook of Biochemistry and Molecular Biology. 4, (2010).
- Yelamos, C., Heeg, M. J., Winter, C. H. Imido complexes of titanium bearing eta(2)-pyrazolato ancillary ligand sets. Organometallics. 18 (7), 1168-1176 (1999).
- Campora, J., et al. Synthesis of dialkyl, diaryl and metallacyclic complexes of Ni and Pd containing pyridine, alpha-diimines and other nitrogen ligands crystal structures of the complexes cis-NiR(2)py(2) (R = benzyl, mesityl). J Organomet Chem. 683 (1), 220-239 (2003).
- Guijarro, D., Pastor, I. M., Yus, M. Non-Deprotonating Methodologies for Organolithium Reagents Starting from Non-Halogenated Materials. Part 2: Transmetallation and Addition to Multiple Bonds. Curr Org Chem. 15 (14), 2362-2389 (2011).
- Ortiz, R., Yus, M. Tandem intramolecular carbolithiation-transmetallation: from lithium to copper or boron chemistry. Tetrahedron. 61 (7), 1699-1707 (2005).
- Coldham, I., Hufton, R. Synthesis of 3-alkylpyrrolidines by anionic cyclization. Tetrahedron. 52 (38), 12541-12552 (1996).
- Leiva, C., et al. Synthesis and X-ray structure of the rhenium methyl complex trans-Cp*Re(CO)(2)(Me)I and a study of the products of photolysis of the rhenium alkyl methyl and dimethyl complexes Cp*Re(CO)(2)(Me)R (R = Ph, p-tolyl, Me) under CO. Organometallics. 18 (2), 339-347 (1999).
- Goldberg, K. I., Bergman, R. G. Synthesis of dialkyl- and alkyl(acyl)rhenium complexes by alkylation of anionic rhenium complexes at the metal center. Mechanism of a double carbonylation reaction that proceeds via the formation of free methyl radicals in solution. J Am Chem Soc. 111 (4), 1285-1299 (1989).
- Rathman, T., Bailey, W. F. Optimization of Organolithium Reactions. Org Process Res Dev. 13 (2), 144-151 (2009).
- Schlosser, M. Superbases for organic synthesis. Pure Appl Chem. 60 (11), 1627-1634 (2009).
- Kemsley, J. N. Learning From UCLA. Chem Eng News. 87 (31), 29-34 (2009).
- Garcìa-Álvarez, J., Hevia, E., Capriati, V. Reactivity of Polar Organometallic Compounds in Unconventional Reaction Media: Challenges and Opportunities. Eur J Org Chem. 2015 (31), 6779-6799 (2015).
- Mallardo, V., et al. Regioselective desymmetrization of diaryltetrahydrofurans via directed ortho-lithiation: an unexpected help from green chemistry. Chem Comm. 50 (63), 8655-8658 (2014).
- Vidal, C., Garcìa-Álvarez, J., Hernán-Gòmez, A., Kennedy, A. R., Hevia, E. Introducing Deep Eutectic Solvents to Polar Organometallic Chemistry: Chemoselective Addition of Organolithium and Grignard Reagents to Ketones in Air. Angew Chem Int Ed. 53 (23), 5969-5973 (2014).
- Sassone, F. C., Perna, F. M., Salomone, A., Florio, S., Capriati, V. Unexpected lateral-lithiation-induced alkylative ring opening of tetrahydrofurans in deep eutectic solvents: synthesis of functionalised primary alcohols. Chem Comm. 51 (46), 9459-9462 (2015).
- Cicco, L., et al. Water opens the door to organolithiums and Grignard reagents: exploring and comparing the reactivity of highly polar organometallic compounds in unconventional reaction media towards the synthesis of tetrahydrofurans. Chem Sci. 7 (2), 1192-1199 (2016).
- Li, C. J., Zhang, W. C. Unexpected Barbier−Grignard Allylation of Aldehydes with Magnesium in Water. J Am Chem Soc. 120 (35), 9102-9103 (1998).
- Li, C. J., Meng, Y. Grignard-Type Carbonyl Phenylation in Water and under an Air Atmosphere. J Am Chem Soc. 122 (39), 9538-9539 (2000).
- Gilman, H., Cartledge, F. K. The analysis of organolithium compounds. Journal of Organometallic Chemistry. 2 (6), 447-454 (1964).
- Kofron, W. G., Baclawski, L. M. A convenient method for estimation of alkyllithium concentrations. J Org Chem. 41 (10), 1879-1880 (1976).
- Suffert, J. Simple direct titration of organolithium reagents using N-pivaloyl-o-toluidine and/or N-pivaloyl-o-benzylaniline. J Org Chem. 54 (2), 509-510 (1989).
- Barnett, N. D. R., et al. Novel octameric structure of the lithium primary amide [{ButN(H)Li}8] and its implication for the directed synthesis of heterometallic imide cages. Chem Comm. 32 (20), 2321-2322 (1996).
- Sigma-Aldrich. . Technical Bulletin AL-134: Handling Air-Sensitive Reagents. , (2012).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved