A subscription to JoVE is required to view this content. Sign in or start your free trial.
Chip-based Three-dimensional Cell Culture in Perfused Micro-bioreactors
In This Article
Summary
We describe a chip-based platform for the three-dimensional cultivation of cells in micro-bioreactors. One chip can house up to 10 Mio. cells that can be cultivated under precisely defined conditions with regard to fluid flow, oxygen tension etc. in a sterile, closed circulation loop.
Abstract
We have developed a chip-based cell culture system for the three-dimensional cultivation of cells. The chip is typically manufactured from non-biodegradable polymers, e.g., polycarbonate or polymethyl methacrylate by micro injection molding, micro hot embossing or micro thermoforming. But, it can also be manufactured from bio-degradable polymers. Its overall dimensions are 0.7 1 x 20 x 20 x 0.7 1 mm (h x w x l). The main features of the chips used are either a grid of up to 1156 cubic micro-containers (cf-chip) each the size of 120-300 x 300 x 300 μ (h x w x l) or round recesses with diameters of 300 μ and a depth of 300 μ (r-chip). The scaffold can house 10 Mio. cells in a three-dimensional configuration. For an optimal nutrient and gas supply, the chip is inserted in a bioreactor housing. The bioreactor is part of a closed steril circulation loop that, in the simplest configuration, is additionaly comprised of a roller pump and a medium reservoir with a gas supply. The bioreactor can be run in perfusion, superfusion, or even a mixed operation mode. We have successfully cultivated cell lines as well as primary cells over periods of several weeks. For rat primary liver cells we could show a preservation of organotypic functions for more than 2 weeks. For hepatocellular carcinoma cell lines we could show the induction of liver specific genes not or only slightly expressed in standard monolayer culture. The system might also be useful as a stem cell cultivation system since first differentiation experiments with stem cell lines were promising.
Protocol
This paper describes the use of a chip-based platform (fig. 1) for the three-dimensional cultivation of cell lines as well as primary cells. Since many cells do express organotypic functions only in a 3D-environment, we have developed a polymer chip that provides a scaffold to which the cells can adhere in all spatial directions, and that can be mounted in a bioreactor housing for the control of fluid flow, oxygen tension etc. Depending on the experimental design, the surface of the polymer can be modified by various techniques, e.g., UV-irradiation, PECVD, γ-grafting or conventional wet chemistry.
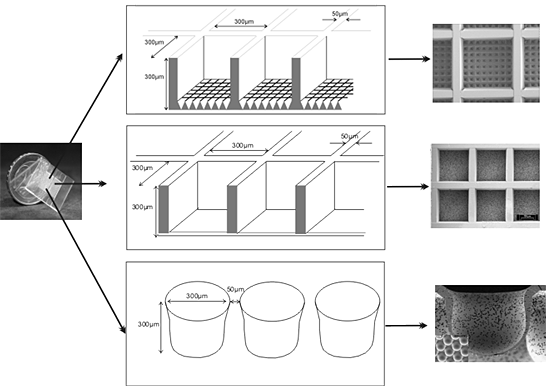
Figure 01
1. De-aeration and hydrophilisation of the chip
Before use, the chip has to be deaerated and hydrophilized. For this, an alcohol series is carried out. Isopropanol solutions consisting of 100%, 70%, 50%, 30% isopropanol in DMPC-treated water are prepared and the chip is dipped in each concentration, beginning with the 100% solution, for up to 30s. The final step of the series consists of pure Dimethyl pyrocarbonate (DMPC)-treated water. From this point on, it is important to keep the chip wet.
2. Collagen I coating
After the alcohol series, the chip is usually coated with a collagen I solution from rat tail. From the collagen stock solution of 2 mg/ml in 0.2% acetic acid an aliquot corresponding to 30 μg collagen protein is diluted with DMPC-treated water to a final volume of 150 μl. This results in a collagen coating of the chip surface with a density of 10 μg collagen I per cm2 surface area.
3. Inoculation of hepatocellular carcinoma cells
Hepatocellular carcinoma cells of line Hep G2 are trypsinized and counted. For short-term experiments (1 to 6 days) 5*106 cells are inoculated in each chip and the corresponding control 6 cm tissue culture petri dishes. To inoculte, the chip 5*106 cells are resuspended in 150 μl culture medium and placed on top of the microstructured area of the chip (fig. 2). Afterwards, it is placed in an incubator for 2-3 hours. During this incubation period the cells sediment into the micro-containers and adhere to the collagen I-coated scaffold.
Figure 2
4. Insertion of the chip into the bioreactor housing
After the incubation period, the chip is removed from the incubator and mounted in the bioreactor housing. For this, under the clean bench, the preassembled bioreactor is removed from the sterile packing and disassembled to a degree that allows for the insertion of the chip. The chip is carefully handled with sterile forceps and placed into the groove that contains the gasket which seals the chip and which results in the generation of an upper and lower compartment in the bioreactor. Then, the bioreactor is assembled again and transferred to the incubator where it is connected to the pump, the gas supply and the oxygen analyser.
5. Filling of the system
As soon as the bioreactor is connected to the medium reservoir, pump and gas supply the closed circulation loop is filled with medium. This is done by positioning the 3-way-connectors in such a way that superfusion, which is defined as the flow of medium over the top of the chip, is achieved. This leads to a discharge of enclosed air from the bioreactor circulation without removing the cells from the scaffold. After the system is completely filled with medium, the 3-way-connectors are switched in such a way that perfusion, which is defined as the flow from below the chip through the tissue, is achieved. In the perfusion configuration the flow is adjusted to the cell's needs which for hepatocytes typically ranges from 60-500 μl/min.
6. Sampling
During the experiment medium samples can be drawn. For this, syringes are connected to the sterile ports on top of the medium reservoir. After the sampling, the ports are sterilized with 70% isopropanol.
7. Isolation of intact cells from the chip for downstream applications
At the end of the experiment, the bioreactors are disconnected from the gas supply and the roller pump, transferred to the clean bench and disassembled as described earlier. With sterile forceps the chip is removed from the bioreactor housing, placed into a 3.5 cm petri dish and rinsed with PBS. Afterwards, the chip is incubated with trypsin/EDTA (0.25%/0.53mM) for 5-15 min in an incubator to detach the cells from the microstructured area. The collected cell suspension is centrifuged for 5 min at 600 g. The cells can then be used for conventional downstream applications, e.g., total RNA or protein isolation. Routinely, we isolate total RNA (PARIS kit, Ambion Inc., Austin, Texas, USA) for microarray analysis and real-time RT-PCR. Protein expression is analysed after immunohistochemical staining of the cells inside the chip with a laser scanning microscope, but can also be analysed otherwise, e.g., by flow cytometry.
Discussion
We have developed a chip-based platform for the three-dimensional cultivation of cells in actively perfused micro bioreactors. The chips can be manufactured from non-biodegradable as well as biodegradable polymers by micro injection molding, hot embossing as well micro thermoforming techniques 3. Depending on the experimental design, the surface of the polymer can be modified by UV-irradiation 4. Hepatocyte cell lines as well as primary rat hepatocytes can successfully be cultivated in these devices...
Disclosures
The authors have nothing to disclose.
Acknowledgements
We would like to thank Mechthild Herschbach and Anke Dech for excellent technical assistance.
Materials
| Material Name | Type | Company | Catalogue Number | Comment |
|---|---|---|---|---|
| Name | Company | Catalog Number | Comments | |
| Cells | Other | ATCC | HB-8065 | |
| Collagen I from rat tail | Reagent | Roche Diagnostics | 11 179 179 001 | |
| PARIS kit | Reagent | Ambion Inc. | AM1921 | |
| Syto16 | Reagent | Invitrogen | S7578 | |
| anti cytokeratin 18 | Antibody | Abcam plc | ab668 | Primary Ab, Mouse monoclonal, used 1/100 in PBS |
| Anti E-cadherin | Antibody | Abcam plc | ab1416 | Primary Ab, Mouse monoclonal, used 1/50 in PBS. |
| Goat anti-albumin | Reagent | Bethyl Laboratories | E80-129 | Primary Ab, goat anti-human Albumin, used 1/200 in PBS |
| Rabbit anti-mouse IgG1 | Antibody | Invitrogen | A11059 | Secondary Ab, Alexa Flour 488 conjugated, used 1/100 in PBS + 0.5 % BSA |
| Cy3 anti-goat IgG | Reagent | Jackson ImmunoResearch Lab | 705-165-003 | Cy3 AffiniPure donkey a-goat IgG Ab, used 1/700 in PBS + 0.5% BSA |
References
- Berry, M. N., Friend, D. S. High-yield preparation of isolated rat liver parenchymal cells: A biochemical and fine structural study. J. Cell Biol. 53, 506-520 (1969).
- Seglen, P. O. Preparation of rat liver cells. 3. Enzymatic requirements for tissue dispersion. Exp. Cell Res. 82, 391-398 (1973).
- Giselbrecht, S., Gietzelt, T., Gottwald, E., Trautmann, C., Truckenmueller, R., Weibezahn, K. F., Welle, A. 3D tissue culture substrates produced by microthermoforming of pre-processed polymer films. Biomed. Microdev. 8, 191-199 (2006).
- Welle, A., Gottwald, E. UV-based patterning of polymeric substrates for cell culture applications. Biomed. Microdev. 4, 33-41 (2002).
- Gottwald, E., Giselbrecht, S., Augspurger, C., Lahni, B., Dambrowsky, N., Truckenmueller, R., Piotter, V., Gietzelt, T., Wendt, O., Pfleging, W., Welle, A., Rolletschek, A., Wobus, A. M., Weibezahn, K. -. F. A chip-based platform for the in vitro generation of tissues in three-dimensional organization. Lab Chip. 7, 777-785 (2007).
- Eschbach, E., Chatterjee, S. S., Noldner, M., Gottwald, E., Dertinger, H., Weibezahn, K. -. F., Knedlitschek, G., G, Microstructured scaffolds for liver tissue with high density: Morphological and biochemical characterization of tissue aggregates. J. Cell. Biochem. 95, 243-255 (2005).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved