A subscription to JoVE is required to view this content. Sign in or start your free trial.
Tuning Oxide Properties by Oxygen Vacancy Control During Growth and Annealing
* These authors contributed equally
In This Article
Summary
Oxide materials show many exotic properties that can be controlled by tuning the oxygen content. Here, we demonstrate the tuning of oxygen content in oxides by varying the pulsed laser deposition parameters and by performing postannealing. As an example, electronic properties of SrTiO3-based heterostructures are tuned by growth modifications and annealing.
Abstract
Electrical, optical, and magnetic properties of oxide materials can often be controlled by varying the oxygen content. Here we outline two approaches for varying the oxygen content and provide concrete examples for tuning the electrical properties of SrTiO3-based heterostructures. In the first approach, the oxygen content is controlled by varying the deposition parameters during a pulsed laser deposition. In the second approach, the oxygen content is tuned by subjecting the samples to annealing in oxygen at elevated temperatures after the film growth. The approaches can be used for a wide range of oxides and nonoxide materials where the properties are sensitive to a change in the oxidation state.
The approaches differ significantly from electrostatic gating, which is often used to change the electronic properties of confined electronic systems such as those observed in SrTiO3-based heterostructures. By controlling the oxygen vacancy concentration, we are able to control the carrier density over many orders of magnitude, even in nonconfined electronic systems. Moreover, properties can be controlled, which are not sensitive to the density of itinerant electrons.
Introduction
The oxygen content plays a vital role in the properties of oxide materials. Oxygen has a high electronegativity and, in the fully ionic limit, attracts two electrons from neighboring cations. These electrons are donated to the lattice when an oxygen vacancy is formed. The electrons can be trapped and form a localized state, or they can become delocalized and capable of conducting a charge current. The localized states are typically located in the band gap between the valence and conduction band with a total angular momentum that can be nonzero1,2,3. The localized states can, thus, form localized magnetic moments and have a large impact on, for instance, the optical and magnetic properties1,2,3. If the electrons become delocalized, they contribute to the density of itinerant charge carriers. In addition, if an oxygen vacancy or other defects are formed, the lattice adapts to the defect. The presence of defects can, thus, naturally lead to local strain fields, symmetry breaking, and a modified electronic and ionic transport in oxides.
Controlling the oxygen stoichiometry is, therefore, often key to tune, for instance, the optical, magnetic, and transport properties of oxide materials. A prominent example is that of SrTiO3 and SrTiO3-based heterostructures, where the ground state of the material systems is very sensitive to the oxygen content. Undoped SrTiO3 is a nonmagnetic insulator with a band gap of 3.2 eV; however, by introducing oxygen vacancies, SrTiO3 changes the state from insulating to metallic conducting with an electron mobility exceeding 10,000 cm2/Vs at 2 K4. At low temperatures (T < 450 mK), superconductivity may even be the favored ground state5,6. Oxygen vacancies in SrTiO3 have also been found to render it ferromagnetic7 and result in an optical transition in the visible spectrum from transparent to opaque2. For more than a decade, there has been a large interest in depositing various oxides, such as LaAlO3, CaZrO3, and γ-Al2O3, on SrTiO3 and examining the properties arising at the interface8,9,10,11,12,13. In some cases, it turns out that the properties of the interface differ markedly from those observed in the parent materials. An important result of the SrTiO3-based heterostructures is that the electrons can be confined to the interface, which makes it possible to control the properties related to the density of itinerant electrons using electrostatic gating. In this way, it becomes possible to tune, for instance, the electron mobility14,15, superconductivity11, electron pairing16, and magnetic state17 of the interface, using electric fields.
The formation of the interface also enables a control of the SrTiO3 chemistry, where the deposition of the top film on SrTiO3 can be used to induce a redox reaction across the interface18,19. If an oxide film with a high oxygen affinity is deposited on SrTiO3, oxygen can transfer from the near-surface parts of SrTiO3 to the top film, thereby reducing SrTiO3 and oxidizing the top film (see Figure 1).
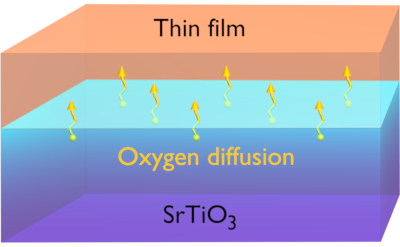
Figure 1: Oxygen vacancy formation in SrTiO3. Schematic illustration of how oxygen vacancies and electrons are formed in the interface-near region of SrTiO3 during the deposition of a thin film with a high oxygen affinity. Reprinted figure with permission from a study by Chen et al.18. Copyright 2011 by the American Chemical Society. Please click here to view a larger version of this figure.
In this case, oxygen vacancies and electrons are formed near the interface. This process is expected to be the origin of the conductivity formed during the deposition at the interface between SrTiO3 and room-temperature-grown metal films or oxides such as amorphous LaAlO318,20 or γ-Al2O310,21,22,23. Thus, the properties of these SrTiO3-based interfaces are highly sensitive to the oxygen content at the interface.
Here, we report the use of postdeposition annealing and variations in the pulsed laser deposition parameters to control the properties in oxide materials by tuning the oxygen content. We use γ-Al2O3 or amorphous LaAlO3 deposited on SrTiO3 at room temperature as examples on how the carrier density, electron mobility, and sheet resistance can be changed by orders of magnitude by controlling the number of oxygen vacancies. The methods offer some benefits beyond those obtained with electrostatic gating, which is typically used to tune the electrical9,11,14 and in some cases the magnetic15,17 properties. These benefits include forming a (quasi-)stable final state and avoiding the use of electric fields, which requires electrical contact to the sample and may cause side effects.
In the following, we review general approaches for tuning the properties of oxides by controlling the oxygen content. This is done in two ways, namely, 1) by varying the growth conditions when synthesizing the oxide materials, and 2) by annealing the oxide materials in oxygen. The approaches can be applied to tune a range of properties in many oxide and some monoxide materials. We provide a concrete example on how to tune the carrier density at the interface of SrTiO3-based heterostructures. Ensure that a high level of cleanliness is exercised to avoid contamination of the samples (e.g., by using gloves, tube furnaces dedicated to SrTiO3, and nonmagnetic/acid resistant tweezers).
Protocol
1. Controlling properties by varying growth conditions
- Preparation of high-quality surfaces of SrTiO3
- Purchase mixed terminated SrTiO3 substrates (e.g., of 5 mm x 5 mm x 0.5 mm in size) with a typical surface angle of 0.05°–0.2° with respect to the (001) crystal planes.
NOTE: The miscut angle determines the flatness of the surface, which is important for epitaxial growth on the substrate, as well as for the resulting properties at the interface. - Clean the desired number of substrates by ultrasonication in acetone for 5 min and ethanol for 5 min at room temperature in a standard ultrasonicator.
- Ultrasonicate the substrates for 20 min at 70 °C in clean water, which dissolves SrO24 or form Sr-hydroxide complexes at surface domains terminated with SrO25, while leaving the chemically stable TiO2-terminated domains unchanged26.
- Ultrasonicate the substrates in a 3:1:16 HCl:HNO3:H2O acidic solution (e.g., 9:3:48 mL) at 70 °C for 20 min in a fume hood to selectively etch SrO due to the basic nature of SrO surface domains, the acidity of TiO2, and the presence of the Sr-hydroxide complexes.
- Remove the residual acid from the substrates by ultrasonication in 100 mL of clean water for 5 min at room temperature in a fume hood.
NOTE: TiO2-terminated SrTiO3 can be purchased commercially or prepared in various ways based on the selective etching of SrO on the surface24,27. The conventional etching in HF also leads to TiO2-terminated SrTiO3, but this is avoided here due to safety concerns and a risk of unintentional F-doping of SrTiO328. - Thermally treat the substrates in an atmosphere of 1 bar of oxygen for 1 h at 1,000 °C with a heating and cooling rate of 100 °C/h in a ceramic tube furnace, to relax the substrate surface into a state with low energy.
- Purchase mixed terminated SrTiO3 substrates (e.g., of 5 mm x 5 mm x 0.5 mm in size) with a typical surface angle of 0.05°–0.2° with respect to the (001) crystal planes.
- Deposition of the thin film(s) on the substrate
- Mount the substrates on the heater or a chip carrier, depending on whether in situ transport measurements during the deposition are to be performed.
NOTE: A silver paste that cures at room temperature can be conveniently used for substrate mounting. - Connect the four corners of the SrTiO3 surface to a chip carrier electrically using, for instance, standard wedge wire bonding with 20 µm-thick Al wires, if in situ transport measurements are desired. Mount the chip carrier onto a chip carrier holder where wires connect the sample to an electrical measurement setup through a vacuum-compatible connector.
- Place the TiO2-terminated substrate 4.7 cm from the single-crystalline Al2O3 target for a typical deposition of Al2O3 on SrTiO3.
- Start sheet resistance measurements using the Van der Pauw geometry29, if in situ transport measurements are to be performed.
- Heat the substrate to 650 °C at a rate of 15 °C/min or keep the substrate at room temperature.
- Prepare for ablating from a single-crystalline Al2O3 target in an oxygen pressure of 1 x 10-5 mbar using, for instance, a nanosecond-pulsed KrF laser with a wavelength of 248 nm, a laser fluence of 3.5 J/cm2, and a frequency of 1 Hz. Tune the properties using the oxygen content by using an oxygen deposition pressure in the range of 10-6 to 10-1 mbar or by varying other deposition parameters.
- Deposit the desired thickness of γ-Al2O3 (typically 0–5 unit cells).
NOTE: This can be determined using, for instance, reflective high-energy electron diffraction (RHEED) oscillations or atomic force microscopy measurements, where the latter is measured as the height difference produced by preventing the deposition of γ-Al2O3 on the part of the substrate using a physical mask. - Cool down the γ-Al2O3/SrTiO3 heterostructure at a rate of 15 °C/min at the deposition pressure without performing an additional annealing step if a high-temperature deposition is done.
- Remove the sample from the deposition chamber and stop the electrical measurements.
- Store the sample in vacuum, nitrogen or, alternatively, at ambient conditions. The sample degradation is slowest when stored in vacuum or nitrogen20.
- Mount the substrates on the heater or a chip carrier, depending on whether in situ transport measurements during the deposition are to be performed.
2. Controlling properties by thermal annealing
- Mount the sample with silver paste on a chip carrier.
- Connect the sample electrically to the chip carrier using, for instance, wedge wire bonding of Al wires in the Van der Pauw geometry29.
- Connect the chip carrier electrically to the measurement equipment, using a connector and wires with thermally resistant insulation.
- Start the sheet resistance measurements.
- Place the chip carrier equipped with the sample in a closed furnace.
- Flush thoroughly with the gas used for the annealing while checking whether the sample resistance is sensitive to a change in the atmosphere.
- Anneal the sample using the desired annealing profile. Typical annealing temperatures are 50–250 °C and 100–350 °C for a-LaAlO3/SrTiO3 and γ-Al2O3/SrTiO3 heterostructures, respectively, depending on the thickness of the top film and the desired rate of oxygen incorporation.
NOTE: Use more heat-compatible options than Al wires and standard ceramic chip carriers if temperatures above 350–400 °C are needed. - Abort the annealing when a desired change in the sheet resistance has occurred.
- Cool down the sample by ramping down the temperature, or take out the sample.
- Stop the electrical measurements.
NOTE: The resistance is generally temperature dependent, which must be taken into account if specific transport properties at a certain temperature are the goal.
Results
Controlling properties by varying growth conditions
Varying the deposition parameters during the deposition of oxides can lead to a large change in the properties, in particular for SrTiO3-based heterostructures, as shown in Figure 2.
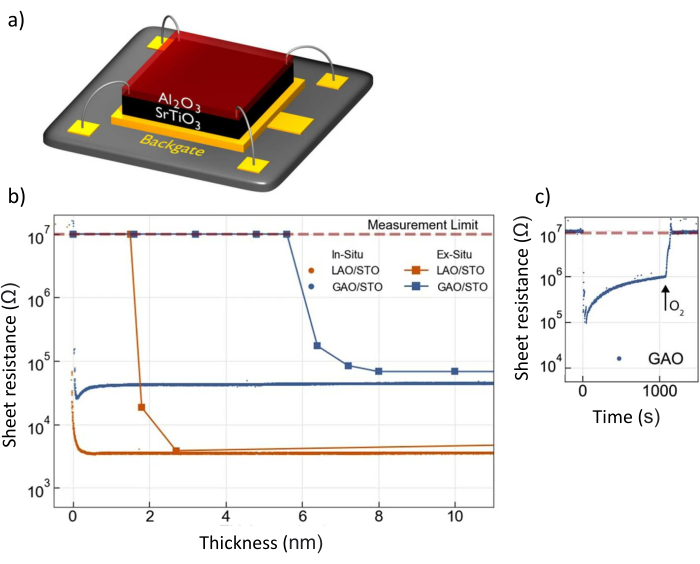
Figure 2: Controlling the transpor...
Discussion
The methods described here rely on using the oxygen content to control oxide properties, and the oxygen partial pressure and operating temperature are, thus, critical parameters. If the total oxidation state of the system is tuned in a way where the system remains in a thermodynamic equilibrium with the surrounding atmosphere (i.e., changed pO2 at high temperature), the changes can be reversible. However, in the case of SrTiO3-based heterostructures, interfacial oxygen vacancies are typically formed...
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors thank J. Geyti from the Technical University of Denmark for his technical assistance. F. Trier acknowledges support by research grant VKR023371 (SPINOX) from VILLUM FONDEN. D. V. Christensen acknowledges the support of Novo Nordisk Foundation NERD Programme: New Exploratory Research and Discovery, Superior Grant NNF21OC0068015.
Materials
| Name | Company | Catalog Number | Comments |
| SrTiO3 | Crystec | Single crystalline (001) oriented, 0.05-0.2 degree miscut angle | |
| LaAlO3 | Shanghai Daheng Optics and Fine Mechanics Co.Ltd. | Single crystalline | |
| Al2O3 | Shanghai Daheng Optics and Fine Mechanics Co.Ltd. | Single crystalline | |
| Chemicals and gases | Standard suppliers | ||
| Silver paste | SPI Supplies, Structure Probe Inc | 05001-AB, High purity silver paint | |
| Ultrasonicator | VWR | USC500D HF45kHz/100W | |
| Wedge wire bonder | Shenzhen Baixiangyuan Science & Technology Co.,Ltd. | HS-853A Aluminum wire bonder | |
| Pulsed laser deposition | Twente Solid State Technologies (TSST) | PLD from TSST with software version V3.0.29, equipped with a 248 nm KrF nanosecond laser (Compex Pro 205 F) from Coherent | |
| Resistance measurement setup | Custom made | Based on the following electrical instruments and custom written software: Keithley 6221 DC and AC current source Keithley 2182A nanovoltmeter Keithley 7001 switch system with a matrix card Keithley 6487 picoammeter | |
| Hall measurements | Cryogenics | Based on the following electrical instruments and custom written software: Keithley 2400 DC current source Keithley 2182A nanovoltmeter Keithley 7001 switch system with a matrix card | |
| Furnace | Custom made | Custom written software control of a FTTF 500/70 tube furnace from Scandia Ovnen AS and a eurotherm 2216e temperature controller |
References
- Pavlenko, N., Kopp, T., Tsymbal, E. Y., Sawatzky, G. A., Mannhart, J. Magnetic and superconducting phases at the LaAlO3/SrTiO3 interface: The role of interfacial Ti 3d electrons. Physical Review B. 85 (2), 020407 (2012).
- Schütz, P., et al. Microscopic origin of the mobility enhancement at a spinel/perovskite oxide heterointerface revealed by photoemission spectroscopy. Physical Review B. 96, 161409 (2017).
- Choi, H., Song, J. D., Lee, K. -. R., Kim, S. Correlated Visible-Light Absorption and Intrinsic Magnetism of SrTiO3 Due to Oxygen Deficiency: Bulk or Surface Effect. Inorganic Chemistry. 54 (8), 3759-3765 (2015).
- Frederikse, H. P. R., Hall Hosler, W. R. Mobility in SrTiO3. Physical Review. 161 (3), (1967).
- Schooley, J. F., Hosler, W. R., Cohen, M. L. Superconductivity in Semiconducting SrTiO3. Physical Review Letters. 12 (17), 474-475 (1964).
- Schooley, J. F., et al. Dependence of the Superconducting Transition Temperature on Carrier Concentration in Semiconducting SrTiO3. Physical Review Letters. 14 (9), 305-307 (1965).
- Coey, J. M. D., Venkatesan, M., Stamenov, P. Surface magnetism of strontium titanate. Journal of Physics: Condensed Matter. 28 (48), 485001 (2016).
- Ohtomo, A., Hwang, H. Y. A high-mobility electron gas at the LaAlO3/SrTiO3 heterointerface. Nature. 427 (6973), 423-426 (2004).
- Thiel, S., Hammerl, G., Schmehl, A., Schneider, C. W., Mannhart, J. Tunable quasi-two-dimensional electron gases in oxide heterostructures. Science. 313 (5795), 1942-1945 (2006).
- Chen, Y. Z., et al. A high-mobility two-dimensional electron gas at the spinel/perovskite interface of γ-Al2O3/SrTiO3. Nature Communications. 4, 1371 (2013).
- Caviglia, A. D., et al. Electric field control of the LaAlO3/SrTiO3 interface ground state. Nature. 456 (7222), 624-627 (2008).
- Christensen, D. V., et al. Electric field control of the γ-Al2O3/SrTiO3 interface conductivity at room temperature. Applied Physics Letters. 109 (2), 021602 (2016).
- Chen, Y., et al. Creation of High Mobility Two-Dimensional Electron Gases via Strain Induced Polarization at an Otherwise Nonpolar Complex Oxide Interface. Nano Letters. 15 (3), 1849-1854 (2015).
- Bell, C., et al. Dominant Mobility Modulation by the Electric Field Effect at the LaAlO3/SrTiO3 Interface. Physical Review Letters. 103 (22), 226802 (2009).
- Niu, W., et al. Giant Tunability of the Two-Dimensional Electron Gas at the Interface of γ-Al2O3/SrTiO3. Nano Letters. 17, 6878 (2017).
- Cheng, G., et al. Electron pairing without superconductivity. Nature. 521 (7551), 196-199 (2015).
- Bi, F., et al. Room-temperature electronically-controlled ferromagnetism at the LaAlO3/SrTiO3 interface. Nature Communications. 5, (2014).
- Chen, Y., et al. Metallic and Insulating Interfaces of Amorphous SrTiO3-Based Oxide Heterostructures. Nano Letters. 11 (9), 3774-3778 (2011).
- Chen, Y. Z., et al. On the origin of metallic conductivity at the interface of LaAlO3/SrTiO3. Applied Surface Science. 258 (23), 9242-9245 (2012).
- Trier, F., et al. Degradation of the interfacial conductivity in LaAlO3/SrTiO3 heterostructures during storage at controlled environments. Solid State Ionics. 230, 12-15 (2013).
- Christensen, D. V., Smith, A. Is γ-Al2O3 polar. Applied Surface Science. , 887-890 (2017).
- Gunkel, F., et al. Thermodynamic Ground States of Complex Oxide Heterointerfaces. ACS Applied Materials & Interfaces. 9 (1), 1086-1092 (2017).
- Christensen, D. V., et al. Controlling the carrier density of SrTiO3-based heterostructures with annealing. Advanced Electronic Materials. 1700026. , (2017).
- Connell, J. G., Isaac, B. J., Ekanayake, G. B., Strachan, D. R., Seo, S. S. A. Preparation of atomically flat SrTiO3 surfaces using a deionized-water leaching and thermal annealing procedure. Applied Physics Letters. 101 (25), 251607-251607 (2012).
- Koster, G., Kropman, B. L., Rijnders, G. J., Blank, D. H., Rogalla, H. Quasi-ideal strontium titanate crystal surfaces through formation of strontium hydroxide. Applied Physics Letters. 73 (20), 2920-2922 (1998).
- Komiyama, M., Gu, M. Atomic force microscopy images of MgO (100) and TiO2 (110) under water and aqueous aromatic molecule solutions. Applied Surface Science. 120 (100), 125-128 (1997).
- Kawasaki, M., et al. Atomic control of the SrTiO3 crystal surface. Science. 266 (5190), 1540-1542 (1994).
- Chambers, S. A., Droubay, T. C., Capan, C., Sun, G. Y. Unintentional F doping of SrTiO3(001) etched in HF acid-structure and electronic properties. Surface Science. 606 (001), 554-558 (2012).
- vander Pauw, L. J. A method of measuring specific resistivity and Hall effect of discs of arbitrary shape. Philips Research Reports. 13, 1-9 (1958).
- Chen, Y. Z., et al. Room Temperature Formation of High-Mobility Two-Dimensional Electron Gases at Crystalline Complex Oxide Interfaces. Advanced Materials. 26, (2013).
- Christensen, D. V., et al. Electron Mobility in γ-Al2O3/SrTiO3. Physical Review Applied. 9 (5), 054004 (2018).
- Cen, C., et al. Nanoscale control of an interfacial metal-insulator transition at room temperature. Nature Materials. 7 (4), 298-302 (2008).
- Cen, C., Thiel, S., Mannhart, J., Levy, J. . Oxide Nanoelectronics on Demand. Science. 323 (5917), 1026-1030 (2009).
- Xu, C., et al. Disentanglement of growth dynamic and thermodynamic effects in LaAlO3/SrTiO3 heterostructures. Scientific Reports. 6, 22410 (2016).
- Chambers, S. A. Understanding the mechanism of conductivity at the LaAlO3/SrTiO3(001) interface. Surface Science. 605 (001), 1133-1140 (2011).
- Nakagawa, N., Hwang, H. Y., Muller, D. A. Why some interfaces cannot be sharp. Nature Materials. 5 (3), 204-209 (2006).
- Sambri, A., et al. Plasma plume effects on the conductivity of amorphous-LaAlO3/SrTiO3 interfaces grown by pulsed laser deposition in O2. and Ar. Applied Physics Letters. 100 (23), 231605 (2012).
- Biscaras, J., et al. Limit of the electrostatic doping in two-dimensional electron gases. of LaXO3(X = Al, Ti)/SrTiO3. Scientific Reports. 4, 6788 (2014).
- Christensen, D. V., et al. Controlling interfacial states in amorphous/crystalline LaAlO3/SrTiO3 heterostructures by electric fields. Applied Physics Letters. 102 (2), 021602 (2013).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved