Light-driven Molecular Motors on Surfaces for Single Molecular Imaging
In This Article
Summary
The manuscript describes how to synthesize and graft a molecular motor on surfaces for single molecular imaging.
Abstract
The design and synthesis of a synthetic system that aims for the direct visualization of a synthetic rotary motor at the single molecule level on surfaces are demonstrated. This work requires careful design, considerable synthetic effort, and proper analysis. The rotary motion of the molecular motor in solution is shown by 1H NMR and UV-vis absorption spectroscopy techniques. In addition, the method to graft the motor onto an amine-coated quartz is described. This method helps to gain more insight into molecular machines.
Introduction
In living organisms, there are abundant molecular motors functioning to sustain daily life. They are able to perform various tasks such as fuel production, transport, mobility, etc.1. Drawing from the inspiration of these fascinating examples in Nature, scientists have developed a series of artificial molecular motors over the last several decades to convert different types of energy into controlled motion at the molecular level2,3,4,5,6,7,8,9,10. The Nobel Prize in Chemistry in 2016 was awarded to three pioneers in this field. Ben Feringa, one of the laureates, has developed the light-driven molecular motor that is able to undergo continuous unidirectional rotary motion.
However, at a molecular level, Brownian motion, also known as the random motion due to molecular collisions and vibrations, is usually the major obstacle for further application of these molecular motors. Brownian motion can disrupt any directed motion. Confining the molecular motors on surfaces may be one of the options to overcome this problem. By doing so, the relative rotation of one part of the molecule with respect to the other is converted to the absolute rotation of the rotor relative to the surface11. In addition, use of the single molecular imaging technique may help visualize the motion. Therefore, the results obtained by this work will help to gain more insight into the synthetic molecular motor.
The pioneering work of Yoshida and Kinosita (Figure 2a)12,13 has served as the inspiration for the design in the current work, shown in Figure 2b. The lower half of a light-driven molecular motor is attached to a surface to serve as the stator. The rotor part is functionalized with a rigid arm and fluorescent label. When applying two different irradiation wavelengths to the system, one will trigger the rotation of the motor, while the other will excite the fluorescent tag. In principle, the rotary motion of the rotor part triggers the rotation of the fluorescent group. Therefore, the rotation of the fluorescent tag can be followed by defocused wide-field fluorescence microscopy. This method provides, for the first time, a method to convert the relative rotation of a molecular motor into absolute rotation, and therefore a way to visualize the rotation of a synthetic motor.
This article provides details on the design, total synthesis, and solution isomerization studies of a molecular motor that is used for single molecular imaging. The molecular structure is shown in Figure 3. In addition, the method to attach molecular motors on quartz surface is described.
Protocol
NOTE: Organic synthesis is the major core of this project. Figure 1 shows the key steps and how to obtain the target molecule.
1. Preparation of 1b
NOTE: Solvents were purchased in analytical grade.
- Synthesis of ketone (3)
NOTE: At first, a five-member ring ketone 2 with an iodo group is functionalized because it is more reactive. This reaction is carried out under argon atmosphere.- In a sealed tube (100 mL) containing 2 (640 mg, 2.3 mmol), CuI (219 mg, 1.1 mmol), and NaI (3.44 g, 23 mmol), add 1,4-dioxane (50 mL) and N,N'-dimethyl ethylenediamine (202 mg, 2.3 mmol).
- Set the reaction temperature to 140 °C and stir for 24 h.
- Cool the mixture to room temperature (RT), remove the solvent in vacuo, and purify the remaining material by flash chromatography: SiO2 (40 g); eluent: pentane:EtOAc = 10:1 (total = 550 mL). The product should be obtained as a yellow, sticky oil (642 mg, 91%).
- Synthesis of motor 5
NOTE: Use the Barton-Kellogg reaction to form the central double bond. This reaction is carried out under argon.- In a 50 mL round-bottom flask, add Lawesson's reagent (415 mg, 1.1 mmol), ketone 3 (219 mg, 0.68 mmol), and toluene (10 mL).
- Heat the mixture at reflux for 2 h and subsequently evaporate the solvent.
- Purify the residue by flash column: SiO2 (24 g), eluent: pentane:ethyl acetate = 30:1 (total = 155 mL) to obtain the corresponding thioketone, which is subsequently redissolved in 20 mL of THF to yield a blue solution.
- Add a THF solution (20 mL) of diazo compound 4 (476 mg, 1.37 mmol) to the blue solution, and stir the newly mixed solution at 50 °C overnight.
NOTE: This is the most crucial step of the entire synthesis. The diazo compound and thioketone solution must be fresh and should be made prior to the reaction. - Evaporate the solvent and purify the residue by chromatography: SiO2 (24 g), eluent: pentane:CH2Cl2 = 10:1 (total = 220 mL) to yield motor 5 (250 mg, 50%) as a red solid.
- Synthesis of motor 6
NOTE: When the central double bond is formed, replace the iodo group by an acetylene moiety.- To a 20 mL Schlenk tube, add 3 (165 mg, 0.26 mmol), Pd(PPh3)2Cl2 (2.5 mole %), CuI (5 mole %). Then, add THF (10 mL) and (i-Pr)2NH (2 mL) that should be bubbled with argon for 10 min before.
- Stir the mixture at RT for 10 min. Then, add triisopropylsilyl acetylene (42 mg, 0.27 mmol).
- Stir the mixture for 15 h, then pour it into an aqueous saturated NH4Cl solution (25 mL).
- Extract the mixture with CH2Cl2 (3 x 20 mL). Further wash the combined organic layers with saturated brine and dry (Na2SO4).
- Remove the solvent and purify the residue by flash chromatography: SiO2 (12 g), eluent: pentane:CH2Cl2 = 10:1 (220 mL) to yield 8 as a brown oil (171 mg, 99%).
- Synthesis of motor 8
NOTE: This reaction is carried out under argon.- Stir a mixture of 6 (161 mg, 0.24 mmol), pinacol ester 7 (240 mg, 0.71 mmol), K3PO4 (300 mg, 1.44 mmol), and Pd(PPh3)4 (98 mg, 0.096 mmol) in 1,4dioxane (20 mL) at 90 °C in a 50 mL Schlenk tube for 16 h.
- Cool the mixture to RT, dilute it with ethyl acetate (30 mL), and perform the filtration with a glass filter.
- Remove the solvent. Purify the residue by flash column chromatography: SiO2 (12 g), eluent: pentane: CH2Cl2= 1:6 (total = 122 mL) to yield ester 8 as a brown oil (156 mg, 56%).
- Synthesis of motor 9
- Add TBAF (0.1 mL) to a solution of 8 (120 mg, 0.13 mmol) in THF (10 mL) in a 20 mL Schlenk tube at 0 °C.
- Stir the mixture at 0 °C for 1 h, then pour it into an aqueous saturated NH4Cl solution (20 mL).
- After extraction with CHCl3 (3 x 10 mL), wash the combined organic layers with saturated brine and dry (Na2SO4).
- Remove the solvent and purify the residue by flash chromatography: SiO2 (12 g), eluent: pentane:ethyl acetate = 1:3 (124 mL) to yield 9 as a dark red oil (116 mg, 95%).
- Synthesis of motor 12
NOTE: This reaction is carried out under argon.- To a 20 mL Schlenk tube, add motor 9 (75 mg, 0.10 mmol), PBI 11 (68 mg, 0.10 mmol) Pd(PPh3)2Cl2 (2.5 mol%), CuI (5 mol%). Then, add THF (10 mL) and (i-Pr)2NH (2 mL) that should be bubbled with argon for 10 min before.
- Stir the mixture overnight and pour it into an aqueous saturated NH4Cl solution.
- After extraction with CHCl3 (3 x 20 mL), wash the combined organic layers with saturated brine and dry (Na2SO4).
- Remove the solvent and purify the residue by flash chromatography: SiO2 (12 g), eluent: CHCl3 (100 mL) to yield motor 12 as a dark red solid (66 mg, 57%).
- Synthesis of motor 1b
NOTE: When the ester compound is obtained, hydrolyze it to make the final target molecule.- Dissolve the ester 12 (90 mg, 0.038 mmol) in THF (5 mL), MeOH (5 mL), and NaOH(aq.) (1 M, 5 mL) in a 50 mL flask and heat the mixture to 75 °C for 16 h.
- Cool the mixture to RT and add 5 mL of water. Remove the THF and MeOH by rotary evaporation.
- Titrate the mixture with HCl(aq.) (1 M) until reaching a pH of 1 to form a brown precipitate. Filtrate the mixture and wash the brown solid with cold water (10 mL) and dry in vacuo. This brown solid is the target molecule 1b (55 mg, 85%).
2. Preparation of motor functionalized monolayer MS-1b
- Clean quartz slides by immersing them in a piranha solution (3:7 ratio of 30% H2O2 in H2SO4) at 90 °C for 1 h. Rinse copiously with doubly distilled water 3x, then rinse with MeOH. Dry the slides under a stream of N2 before surface modification.
- Silanize the piranha-cleaned quartz slides by immersing in a 1 mM solution of 3-aminopropyl(diethoxy)methylsilane in freshly distilled toluene at RT for 12 h. Rinse copiously with toluene and MeOH.
- Sonicate the quartz first in toluene, then in MeOH, and dry them under a stream of argon.
- Immerse the amine-coated slides in DMF solution of 1b (10-4 M) at RT for 12 h.
- Wash the slides with DMF, water, and MeOH, then dry them under a stream of argon. After drying, the slides are ready for use.
Results
Irradiation of the molecular motor is performed with UV light (λmax = 365 nm). Upon irradiation, a photo-induced E-Z isomerization around the central double bond occurs. During this process, the molecule is transformed from a stable to an unstable isomer. A thermally activated helix inversion step then follows to release the strain of the whole molecule. This results in the original stable state. 1H NMR spectroscopy is then employed to evaluate the rotary process (Figure 4a). A solution of the sample is prepared in an NMR tube, then a lamp of UV-light (λmax = 365 nm) is placed next to the tube. After 2 h of irradiation, distinct changes can be found in the 1H NMR spectrum. These changes indicate the generation of a new isomer that is considered to be unstable-1b (Figure 4b). It is seen in the 1H NMR spectroscopy that Ha shifts from 2.9 ppm (doublet) to 3.3 ppm (double doublet). The signal at 1.4 ppm can be assigned as the absorption of the methyl group, and it downfield shifts from 1.4 ppm to 1.6 ppm. When the sample is kept overnight at room temperature in the dark, the original spectrum can be recovered (Figure 4a). It indicates the process of the thermal helix inversion that converts unstable-1b to stable-1b.
In order to study the rotary motion of motor 1b on surfaces, the surface-attached motor assemblies MS-1b (MS = motor on surfaces) are prepared. The quartz slides are first functionalized with amine. After this step, the quartz is immersed in a DMF solution (10-4 M) of 1b at RT overnight. The resultant quartz is rinsed with DMF, water, and MeOH. The prepared quartz slides are then submitted for UV/vis studies. A UV/vis absorption spectrum of MS-1b (solid line) is shown in Figure 5b. As seen in the spectrum, the major absorption band and absorption profile are similar to that observed in solution (Figure 5a). It also shows the characteristic absorptions for motor (420 nm) and PBI (456 nm, 490 nm, 524 nm). These peaks suggest the successful attachment of motor 1b to the amine-coated surfaces. In addition, the quartz slide is irradiated for 15 min, and spectral changes are observed similar to that of the solution, indicating generation of the unstable MS-1b.
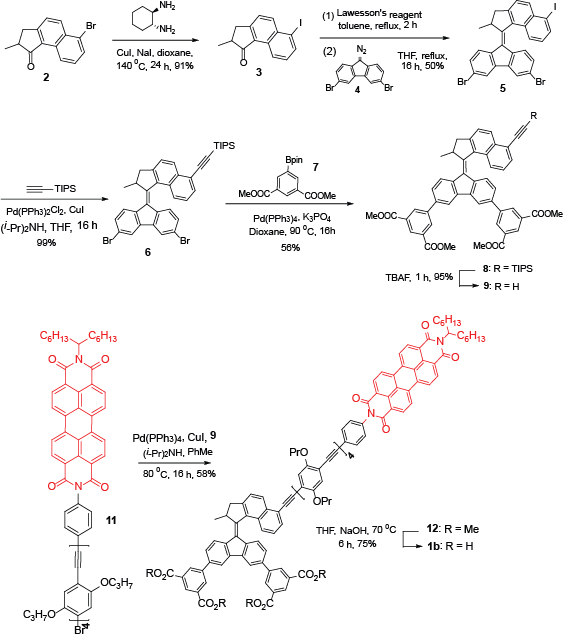
Figure 1: Synthetic scheme towards the preparation of target molecule 1b. The scheme shows the reagents, solvents, and reaction conditions that are used in each step.
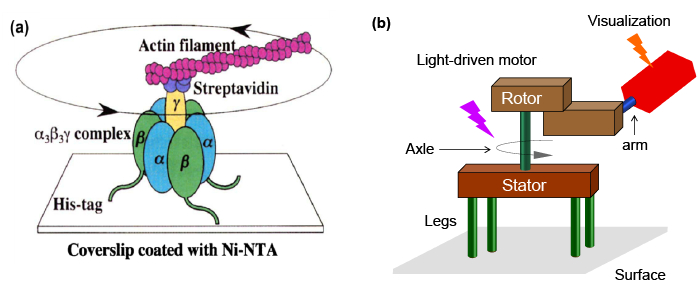
Figure 2: (a) Schematic illustration of the structure of F0F1-ATPase grafted on a surface for visualization of unidirectional rotation (reproduced with permission12). (b) Conceptual design of a synthetic surface-bound light-driven molecular motor for single molecule imaging.
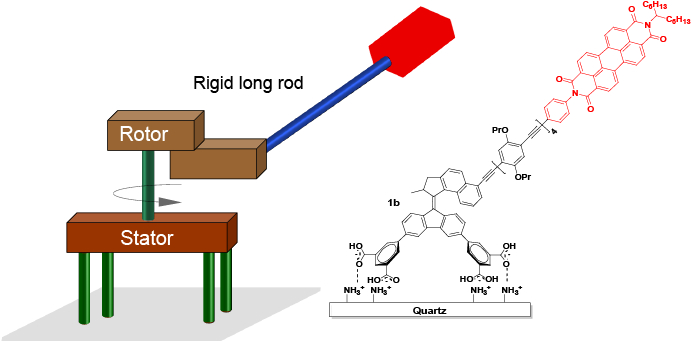
Figure 3: Structure of a surface-bound molecular motor 1b, bearing a rigid long arm between the motor core and PBI label.
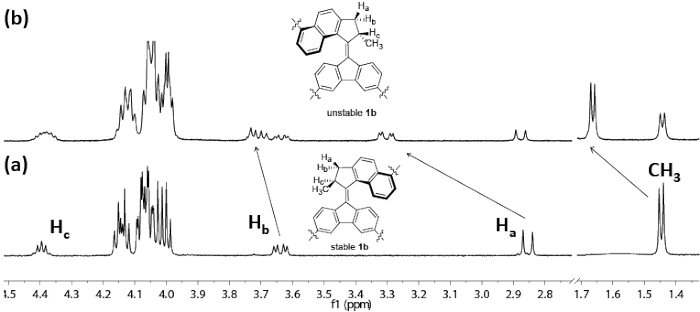
Figure 4: Aliphatic region of the 1H-NMR spectra of motor 1b (CD2Cl2, -20 °C, c = 10-3 M) (a) stable-1b, before irradiation (365 nm). (b) Photo stationary state mixture after irradiation. Please click here to view a larger version of this figure.
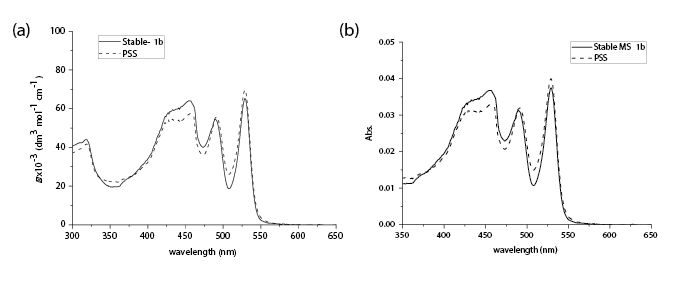
Figure 5: UV/vis absorption spectra. UV/vis absorption spectra of (a) motor 1b (CH2Cl2, 0 °C), stable isomer (solid line) and unstable isomer (dashed line) at PSS. (b) MS-1b (quartz, 4 °C) before (solid line) and after (dashed line) irradiation.
Discussion
This project involves a significant amount of synthetic work; therefore, the most critical step is organic synthesis towards the final molecule. Among the total synthesis, the Barton-Kellogg reaction is the key step, since it is the reaction in which the central double bond of the molecular motor is formed. Currently, several methods have been used to form these types of structures. Here, diazo-thioketone coupling is used, and the upper and lower halves have been prepared as the diazo and thioketone compounds, respectively. Thioketone and diazo compounds are usually not stable under air; therefore, the reaction requires fast operating under a strictly inert atmosphere.
Existing methods to confine molecular motors on the surfaces are mostly based on bipodal systems. However, the isomerization processes of previously designed bipodal motors were obstructed due to intermolecular interactions. In addition, some of the bipodal examples requires further activation prior to attachment. The current method accomplishes this in a tetrapodal manner, which provides robust attachment of the motor on surfaces with sufficient isolated space.
A limitation of this method is the choice of fluorescent tag. Only dyes with specific wavelengths are allowed, as the rotation of motor is triggered by the 365 nm wavelength and thus should not be overlapped. In addition, the synthetic route employed in the described protocol towards the target molecule requires several steps in which harsh conditions are needed for completion of the reaction. In the future, a more facile synthetic design is probably needed if a more advanced molecule for single molecular imaging is required.
In conclusion, the design and synthesis of a highly functionalized light-driven molecular motor is described for the first time. Some details of the synthetic effort are discussed, as well. Furthermore, methods to graft the motor onto a quartz slide surface are demonstrated, and the sample can be further tested for visualization of single molecular motion14.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported financially by the Netherlands Organization for Scientific Research (NWO-CW), the European Research Council (ERC; advanced grant no. 694345 to B.L.F.), and the Ministry of Education, Culture and Science (Gravitation program no. 024.001.035).
Materials
| Name | Company | Catalog Number | Comments |
| NMR spectrometer | Varian | AMX400 | for proton nmr study |
| Reagent for organic reactions | Sigma | analytical grade | reagent for organic reactions |
| Silica gel | Merck | 230-400 mesh ASTM | Flash chromatography |
| Solvent | Acros | spectrophotometric grade | Flash chromatography |
| UV lamp | ENB | 280C | for UV-vis irradation |
| UV-vis absorption spectrophotometer | JASCO | V-630 | UV-vis measurment |
References
- Berg, J. M., Tynoczko, J. L., Styer, L. . Biochemistry 5th ed. , (2006).
- Erbas-Cakmak, S., Leigh, D. A., McTernan, C. T., Nussbaumer, A. L. Artificial Molecular Machines. Chemical Reviews. 115, 10081-10206 (2015).
- Feringa, B. L. The Art of Building Small: From Molecular Switches to Motors. Angewandte Chemie: International Edition. 56, 11059-11078 (2017).
- Stoddart, J. F. Molecular Machines. Accounts of Chemical Research. 34, 410-411 (2001).
- Kinbara, K., Aida, T. Toward Intelligent Molecular Machines: Directed Motions of Biological and Artificial Molecules and Assemblies. Chemical Reviews. 105, 1377-1400 (2005).
- Kay, E. R., Leigh, D. A., Zerbetto, F. Synthetic Molecular Motors and Mechanical Machines. Angewandte Chemie: International Edition. 46, 72-191 (2007).
- Kottas, G. S., Clarke, I. L., Horinek, D., Michl, J. Artificial Molecular Rotors. Chemical Reviews. 105, 1281-1376 (2005).
- Watson, M. A., Cockroft, S. L. Man-made Molecular Machines: Membrane Bound. Chemical Society Reviews. 45, 6118-6129 (2016).
- Kassem, S., et al. Artificial Molecular Motors. Chemical Society Reviews. 46, 2592-2621 (2017).
- Sauvage, J. P. . Molecular Machines and Motors. , (2001).
- van Delden, R. A., et al. Unidirectional molecular motor on a gold surface. Nature. 437, 1337-1340 (2005).
- Noji, H., Yasuda, R., Yoshida, M., Kinosita, K. Direct observation of the rotation of F1-ATPase. Nature. 386, 299-302 (1997).
- Hutchison, J. A., et al. A surface-bound molecule that undergoes optically biased Brownian rotation. Nature Nanotechnology. 9, 131-136 (2014).
- Krajnik, B., et al. Defocused Imaging of UV-Driven Surface-Bound Molecular Motors. Journal of the American Chemical Society. 139, 7156-7159 (2017).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved