A subscription to JoVE is required to view this content. Sign in or start your free trial.
Generation, Amplification, and Titration of Recombinant Respiratory Syncytial Viruses
In This Article
Summary
We describe a method for generating and amplifying genetically modified respiratory syncytial viruses (RSVs) and an optimized plaque assay for RSVs. We illustrate this protocol by creating two recombinant viruses that respectively allow quantification of RSV replication and live analysis of RSV inclusion bodies and inclusion bodies-associated granules dynamics.
Abstract
The use of recombinant viruses has become crucial in basic or applied virology. Reverse genetics has been proven to be an extremely powerful technology, both to decipher viral replication mechanisms and to study antivirals or provide development platform for vaccines. The construction and manipulation of a reverse genetic system for a negative-strand RNA virus such as a respiratory syncytial virus (RSV), however, remains delicate and requires special know-how. The RSV genome is a single-strand, negative-sense RNA of about 15 kb that serves as a template for both viral RNA replication and transcription. Our reverse genetics system uses a cDNA copy of the human RSV long strain genome (HRSV). This cDNA, as well as cDNAs encoding viral proteins of the polymerase complex (L, P, N, and M2-1), are placed in individual expression vectors under T7 polymerase control sequences. The transfection of these elements in BSR-T7/5 cells, which stably express T7 polymerase, allows the cytoplasmic replication and transcription of the recombinant RSV, giving rise to genetically modified virions. A new RSV, which is present at the cell surface and in the culture supernatant of BSRT7/5, is gathered to infect human HEp-2 cells for viral amplification. Two or three rounds of amplification are needed to obtain viral stocks containing 1 x 106 to 1 x 107 plaque-forming units (PFU)/mL. Methods for the optimal harvesting, freezing, and titration of viral stocks are described here in detail. We illustrate the protocol presented here by creating two recombinant viruses respectively expressing free green fluorescent protein (GFP) (RSV-GFP) or viral M2-1 fused to GFP (RSV-M2-1-GFP). We show how to use RSV-GFP to quantify RSV replication and the RSV-M2-1-GFP to visualize viral structures, as well as viral protein dynamics in live cells, by using video microscopy techniques.
Introduction
Human RSV is the leading cause of hospitalization for acute respiratory tract infection in infants worldwide1. In addition, RSV is associated with a substantial disease burden in adults comparable to influenza, with most of the hospitalization and mortality burden in the elderly2. There are no vaccines or specific antivirals available yet against RSV, but promising new drugs are in development3,4. The complexity and the heaviness of the techniques of quantification of RSV multiplication impede the search for antivirals or vaccines despite current considerable efforts. The quantification of RSV multiplication in vitro is generally based on laborious, time-consuming, and expensive methods, which consist mostly in the analysis of the cytopathic effect by microscopy, immunostaining, plaque reduction assays, quantitative reverse transcriptase (qRT)-polymerase chain reaction (PCR), and enzyme-linked immunosorbent assay tests. Viruses with modified genomes and expressing reporter genes, such as those coding for the GFP, are more suitable for such screenings. Coupled to the use of automated plate readers, reporter gene-carrying recombinant viruses can make these assays more suitable for standardization and high-throughput purposes.
RSV is an enveloped, nonsegmented negative-sense RNA virus that belongs to the Orthopneumovirus genus of the Pneumoviridae family, order Mononegavirales5. The RSV genome is a single-strand, negative-sense RNA of about 15 kb, which contains a noncoding region at the 3' and 5' extremities called Leader and Trailer and 10 transcriptional units encoding 11 proteins. The genes are ordered as follows: 3'-NS1, NS2, N, P, M, SH, G, F, M2 (encoding for M2-1 and M2-2 proteins) and L-5'. The genomic RNA is tightly packaged by the nucleoprotein N. Using the encapsidated genomic RNA as a template, viral RNA-dependent RNA polymerase (RdRp) will ensure transcription and replication of the viral RNA. Viral RdRp is composed of the large protein L which carries the nucleotide polymerase activity per se, its mandatory cofactor the phosphoprotein P and the M2-1 protein which functions as a viral transcription factor6. In infected cells, RSV induces the formation of cytoplasmic inclusions called inclusion bodies (IBs). Morphologically similar cytoplasmic inclusions have been observed for several Mononegavirales7,8,9,10. Recent studies on rabies virus, vesicular stomatitis virus (VSV), Ebola virus, and RSV showed that viral RNA synthesis occurs in IBs, which can thus be regarded as viral factories8,9,11,12. The virus factories concentrate the RNA and viral proteins required for viral RNA synthesis and also contain cellular proteins13,14,15,16,17. IBs exhibit a functional subcompartment called IB-associated granules (IBAGs), which concentrate the newly synthetized nascent viral mRNA together with the M2-1 protein. The genomic RNA and the L, P, and N are not detected in IBAGs. IBAGs are small dynamic spherical structures inside IBs that exhibit the properties of liquid organelles12. Despite the central role of IBs in viral multiplication, very little is known about the nature, internal structure, formation, and operation of these viral factories.
The expression of the genome of a poliovirus from a cDNA enabled the production of the first infectious viral clone in 198118. For single-stranded negative RNA viruses, it was not until 1994 that the production of a first rabies virus following transfection of plasmids into cells19 took place. The first plasmid-based reverse genetic system for RSV was published in 199520. Reverse genetics have led to major advances in the field of virology. The possibility of introducing specific modifications into the viral genome has provided critical insights into the replication and pathogenesis of RNA viruses. This technology has also greatly facilitated the development of vaccines by allowing specific attenuation through targeted series of modifications. Genome modifications allowing a rapid quantification of viral multiplication greatly improved the antiviral screening and study of their mode of action.
Although previously described, obtaining genetically modified RSVs remains delicate. Here, we detail a protocol to create two types of recombinant HRSV, respectively expressing RSV-GFP or RSV-M2-1-GFP. In this protocol, we describe the transfection conditions needed to rescue the new recombinant viruses, as well as their amplification to obtain viral stocks with high titer, suitable for reproducible experimentations. The construction of the reverse genetics' vectors per se is not described here. We do describe methods for the optimal harvesting and freezing of viral stocks. The most accurate method to quantify viral infectious particles remains plaque assay. Cells are infected with serial dilutions of the analyzed suspension and incubated with an overlay that prohibits the diffusion of free viral particles in the supernatant. In such conditions, the virus will only infect contiguous cells forming a "plaque" for each initial infectious particle. In the conventional RSV titration assay, plaques are revealed by immunostaining and counted under microscopic observation. This method is expensive and time-consuming. Here we described a very simple protocol for an RSV plaque assay using microcrystalline cellulose overlay that enables the formation of plaques visible to the naked eye. We show how RSV-GFP can be used to measure RSV replication and, thus, to quantify the impact of antivirals. Combining reverse genetics and live imaging technology, we demonstrate how RSV-M2-1-GFP allows scientists to visualize M2-1 in live cells and to follow the dynamics of intracellular viral structures, such as IBs.
Protocol
1. Material Preparation
- Purchase cell media (reduced serum media, minimum essential media [MEM], 10x MEM, and Dulbecco’s modified Eagle’s medium [DMEM]), transfection reagent, and microcrystalline cellulose (see Table of Materials).
- Obtain the following vectors for reverse genetics: the genomic vector(s) and the expression vectors encoding the N protein and the polymerase complex proteins. The genomic vectors contain the full cDNA genome of RSV-GFP (p-RSV-GFP) and of RSV-M2-1GFP (p-RSV-M2-1GFP) downstream from the bacteriophage T7 RNA polymerase (T7 pol) promoter. The expression vectors (designated as p-N, p-P, p-L, and p-M2-1) contain the coding sequence of N, P, L, or M2-1 downstream the T7 pol (see Rincheval et al.12 and Rameix-Welti et al.21 for details regarding the plasmid constructs).
- Prepare media for a cell culture in a sterile environment and for the transfection and infection. Use DMEM with 2 mM L-glutamine supplemented with 10% fetal calf serum (FCS), 1,000 units/mL penicillin, and 1 mg/mL streptomycin (or without antibiotics) and MEM with 2 mM L-glutamine supplemented with 0%, 2%, or 10% FCS, 1,000 units/mL penicillin, and 1 mg/mL streptomycin, designated as “complete medium” in the following protocol.
- Obtain BSRT7/522 cells and make stocks in complete medium supplemented with 10% dimethyl sulfoxide (DMSO) at 1 to 2 x 106 cells/mL. Conserve the cell stocks in liquid nitrogen. Obtain HEp-2 cells. Culture BSRT7/5 cells in complete DMEM and HEp-2 cells in complete MEM at 37 °C and 5% CO2 in a sterile environment.
- Prepare a 10x RSV conservation solution (0.5 M HEPES and 1 M MgSO4 [pH 7.5] in water) in a sterile environment.
- Obtain an inverted fluorescence microscope compatible with GFP fluorescence measurements and compatible with live imaging if it is necessary to monitor an infection. Obtain a microplate reader compatible with GFP fluorescence measurements for the quantitation of RSV-GFP replication.
2. Rescue and First Passage of Recombinant Virus
NOTE: Perform all the following steps in a sterile environment, using a class II safety cabinet.
- The day before transfection, make a suspension of the BSRT7/5 cell line at 5 x 105 cells/mL in complete medium. Distribute 2 mL of cell suspension per well in a 6-well plate. Prepare one well per virus that is going to be rescued and one additional well for negative control. Incubate the plate at 37 °C and 5% CO2. Check that the cells are at a 80%–90% confluence the next day.
- Unfreeze the reverse genetics vectors (from step 1.2) p-RSV-GFP and p-RSV-M2-1-GFP, as well as p-N, p-P, p-L, and p-M2-1. Mix, for each virus to rescue, 1 µg of p-N and p-P, 0.5 µg of p-L, 0.25 µg of p-M2-1, and 1.25 µg of p-RSV (GFP or M2-1 GFP) in a tube.
NOTE: Different expression vectors for N, P, L, and M2-1 may be used; however, the ratio between the proteins has to be maintained. Perform the negative control by replacing the p-RSV vector with an empty vector. - Proceed to transfection, following the transfection reagent manufacturer’s protocol (see Table of Materials).
- Add 250 µL of reduced serum medium to the mixed vectors. In another tube, dilute 10 µL of the transfection reagent in 250 µL of reduced serum medium. Gently vortex both tubes and wait for 5 min. Mix the contents of both tubes and wait for 20 min at room temperature.
- Rinse the BSRT7/5 cells with 1 mL of reduced serum medium and distribute 1.5 mL of MEM with 10% FCS without antibiotics per well. If necessary, incubate at 37 °C and 5% CO2 until the incubation described in step 2.3.1 is completed.
- Add 500 µL of the transfection mix prepared in step 2.3.1 to a well when the 20 min incubation time is over. Place the cells in the incubator at 37 °C and 5% CO2 for 3 days. Do not change the culture medium of the cells during the transfection.
- Observe GFP fluorescence (excitation at 488 nm and emission at 515–535 nm) under an inverted fluorescence microscope at 20x magnification 1x per day to monitor the rescue efficiency, using the GFP filter.
- On the second day after the transfection, seed the cells for the first passage of the rescued viruses. Prepare a suspension of HEp-2 cells at 5 x 105 cells/mL in complete medium. Distribute 2 mL of cell suspension per well in a 6-well plate (one well per virus to rescue and one negative control).
- On the third day of transfection, scratch cells in each well of the transfected BSRT7/5 6-well plate, using a different scraper for each well. Transfer each well content (cells and supernatant) into a sterile 2 mL microcentrifuge tube. Vortex each tube vigorously for at least 30 s to release the rescued virus from the cell membranes.
NOTE: This corresponds to passage 0 (P0) of the rescued virus (Figure 1). - Use the fresh viral P0 suspension to perform the first amplification of the rescued viruses.
- Remove the culture medium from the HEp-2 6-well plate seeded the day before (see step 2.5) and quickly add 500 µL of the P0 suspension (from step 2.6) per well. Place the HEp-2 plate at 37 °C on a see-saw rocker for soft agitation for 2 h.
- Remove and discard the 500 µL of inoculum and add 2 mL of MEM with 2% FCS. Incubate the plate at 37 °C and 5% CO2 for 3 days. This will produce the first passage (P1) of the rescued viruses (Figure 1).
- Add 1/10 of the volume of 10x RSV conservation solution (0.5 M HEPES and 1 M MgSO4 [pH 7.5]) into the remaining P0 suspension (from step 2.6). Vortex the microtubes vigorously for 5 s and aliquot the contents in cryogenic tubes labeled with alcohol-resistant tags. Immerse the tubes for at least 1 h in alcohol precooled at -80 °C, and store them at -80 °C.
- Titrate the P0 stock (see step 2.6) of each rescued virus (see section 4 for the microcrystalline cellulose titration).
- Observe GFP fluorescence (excitation at 488 nm and emission at 515–535 nm) of the HEp-2 cells infected with the P0 suspension under an inverted fluorescence microscope at 20x magnification 1x per day to monitor the infection. Observe under a brightfield microscope the appearance of small syncytia and cell detachment which reflects the RSV cytopathogenic effect (CPE) (see Figure 2).
- Note that the rescue has failed if neither fluorescence nor CPE is visible after 2–3 days.
- Collect the first passage (P1) at day 3 or 4 as described in step 2.6. In brief, scrape the cells, collect the cells and supernatant together, vortex them, add the conservation solution as described in step 2.8, aliquot the sample, and freeze it.
- Titrate (see section 4 for the titration assay) and amplify the first passage (see section 3 for the amplification).
3. Amplification of the Rescued Viruses
NOTE: The following protocol describes the amplification of the rescued viruses in a 75 cm2 flask. Adapt the flask size to the volume needed and the required multiplicity of infection (MOI). Table 1 indicates volumes for different flasks. Perform all the following steps in a sterile environment in a class II safety cabinet.
- Prepare a suspension of HEp-2 cells at 5 x 105 cells/mL in complete medium, the day prior to the amplification. Distribute 15 mL of the cell suspension per 75 cm2 flask and incubate the flasks at 37 °C and 5% CO2. Prepare one flask per virus to amplify.
- The day after the start of the incubation, check that the cells are 80%–100% confluent.
- Dilute the viral suspension from step 2.12 in MEM without FCS to obtain a 3 mL suspension at 50,000 PFU/mL (corresponding to an MOI of 0.01 PFU/cell).
- Remove the medium and quickly add the 3 mL viral suspension. Place the flask at 37 °C on a see-saw rocker for soft agitation for 2 h.
- Remove and discard the inoculum and add 15 mL of MEM with 2% FCS. Incubate at 37 °C and 5% CO2 for 2–4 days.
- Check the cell morphology and GFP fluorescence (excitation at 488 nm and emission at 515–535 nm) under an inverted fluorescence microscope at 20x magnification in order to estimate the right time to harvest the viruses. Note that this is usually when 50%–80% of the HEp-2 cell layer is detached due to the RSV CPE that occurs between 48 and 72 h postinfection (p.i.) (see Figure 3).
- Scrape all the cells using a cell scraper. Collect both the cells and the supernatant together and transfer them to a 50 mL centrifuge tube.
- Add 1/10 of the volume of the 10x RSV conservation solution (0.5 M HEPES and 1 M MgSO4 [pH 7.5]). Vortex the tubes vigorously for 5 s and clarify the suspension by a 5 min centrifugation at 200 x g.
- Transfer the supernatant to a 50 mL tube. Vortex briefly and aliquot the suspension in cryogenic tubes labeled with alcohol-resistant tags. Immerse the tubes in precooled -80 °C alcohol for at least 1 h and store them at -80 °C.
- Unfreeze one of the aliquots to titrate the viral suspension (see section 4).
4. Plaque Titration Assay
- Prepare 12-well plates for titration the day before the titration assay is performed (six wells will be required to titrate one tube of virus). Seed the wells with 1 mL of HEp-2 cells at 5 x 105 cells/mL in complete medium.
- The next day, prepare a sterile microcrystalline cellulose suspension (2.4% [w/v] in water) (see Table of Materials).
- Disperse 2.4 g of microcrystalline cellulose powder in 100 mL of distilled water, using a standard magnetic stirrer, until complete dissolution of the powder (usually 4–12 h). Autoclave the suspension at 121 °C for 20 min and store it at room temperature before use.
NOTE: Under such conditions, the suspension is stable for 1 year. - After opening the solution in a sterile environment, store it at 4 °C for 6 months. Always mix the suspension before use (by hand shaking or vortexing) to make sure it is homogeneous.
- Disperse 2.4 g of microcrystalline cellulose powder in 100 mL of distilled water, using a standard magnetic stirrer, until complete dissolution of the powder (usually 4–12 h). Autoclave the suspension at 121 °C for 20 min and store it at room temperature before use.
- Prepare the 2x MEM in a sterile environment. Dilute commercial MEM 10x with sterile water and add L-glutamine, 1,000 units/mL penicillin, and 1 mg/mL streptomycin. Shake the dilution vigorously and store it at 4 °C.
NOTE: Perform steps 4.4–4.10 in a sterile environment using a class II safety cabinet. - Prepare six tubes containing 900 µL of MEM without FCS per virus to be titrated (the titration tubes). Thaw the virus aliquots, vortex them vigorously for 5 s, and transfer 100 µL to the first titration tube.
- Perform a tenfold dilution 6x, as follows. Add 100 μL of virus to 900 μL of medium in the first tube, put the cap on the tube, and mix its contents by vortexing for a few seconds. Change the tip on the pipette, add 100 µL of the first dilution to 900 µL of medium in the second tube, put the cap on the tube, and vortex. Repeat the procedure until the sixth tube.
NOTE: It is very important to change the tip for each dilution. - Write the virus name and the fold dilutions on the HEp-2 12-well plates. Add a mark to match the plate and its cover because they may be separated during staining (step 4.9). Remove the medium from the plates and distribute 400 µL of one dilution per well. Incubate the plates at 37 °C for 2 h, for virus adsorption.
NOTE: Change the pipette tip between each inoculum or proceed from lowest to highest concentration with the same tip. Inoculate a limited series of plates (1 to 2) simultaneously to avoid the cells drying. - Prepare the microcrystalline cellulose overlay during the virus adsorption (extemporaneous preparation). To obtain 100 mL of overlay, mix 10 mL of 2.4% microcrystalline cellulose suspension, 10 mL of 2x MEM, and 80 mL of MEM with 2% FCS.
- Adjust the pH of the 2x MEM to around 7.2 with a sterile sodium bicarbonate solution at 7.5%, following the color indicator. Add the microcrystalline cellulose suspension and the MEM and mix vigorously.
- At the end of the 2 h incubation, add 2 to 3 mL of overlay to each well of the 12-well plates without removing the inoculum. Be careful to avoid the contamination of the adjacent wells with high viral titer inoculums. Incubate the plate at 37 °C and 5% CO2 for 6 days. Do not move the plate and do not move the incubator during incubation.
- Proceed to stain the cells, using crystal violet solution (8% crystal violet [v/v], 2% formaldehyde [v/v], and 20% ethanol [v/v] in water).
- Protect the work surface of the biosafety cabinet with a sheet (the crystal violet strongly colors surfaces).
- Gently shake the plates to take off the microcrystalline cellulose overlay. Remove the supernatants and wash the cells 2x with 1x phosphate-buffered saline (PBS). Handle the plates one by one to avoid the cells drying. Add 1–2 mL of the crystal violet solution and wait 10–15 min. Remove the solution, which can be reused for subsequent plate staining.
- Immerse the plates and lids in fresh bleach for a few seconds and, then, wash them thoroughly with tap water. Note that the plates and covers are decontaminated by the bleach.
- Put the plates and lids on paper towels and let them dry. Dry the plates at an ambient temperature after the water rinsing and store them at room temperature. For long storage periods (months), keep the plates protected from light to protect the color. Note that if the cells lose their coloration, they can be stained again with crystal violet.
- Calculate virus titers. Count the plaques in the wells of the dry plates, which are visible to the naked eye. Check that the number of plaques of the different dilutions is coherent (factor 10 between each dilution). Choose the well on which the plaques are the easiest to count. Assess the number of plaques versus the inoculum volume and the dilution.
NOTE: On the example provided in Figure 4, 21 plaques are counted at the 10-5 dilution. These correspond to a titer of
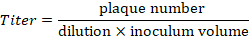
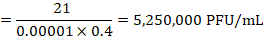
5. The Use of HRSV-GFP Recombinant Virus to Monitor Viral Replication in Cells Treated with Small Interfering RNA or Antivirals
NOTE: Perform all steps except 5.1 and 5.2.5 in a sterile environment using a class II safety cabinet.
- Monitoring the effect of cellular gene silencing on RSV multiplication
NOTE: The transfection protocol depends on the reagent (see Table of Materials).- Prepare 96-well plates for the GFP measurement. Two days before the assay, for given small interfering RNA (siRNA), prepare a solution of reduced serum media containing siRNA at a concentration of 100 nM and an siRNA transfection reagent diluted at 1/500. Incubate the solution for 30 min at room temperature.
- Add 25 µL of the solution to the wells of the plate prepared in 5.1.1 (in triplicate). Seed the wells with 75 µL of a suspension of A549 cells at 4 x 105 cells/mL in complete medium without antibiotics to obtain a final cell concentration of 3 x 105 cells/mL. Incubate the plate for 48 h at 37 °C and 5% CO2.
NOTE: The final siRNA concentration is 25 nM and the final transfection reagent volume is 0.5 µL/well). - Infect the cells as follows. Remove the medium from the wells. Add 100 µL of RSV-GFP suspension at 50,000 PFU/mL and incubate for 2 h at 37 °C and 5% CO2. Remove the viral suspension and add 100 µL of DMEM with 2% FCS and without phenol red. Incubate the plate at 37 °C and 5% CO2.
- At 24 h and 48 h p.i., measure the fluorescence, using a spectrofluorometer set to excitation and emission wavelengths of 488 and 520 nm, respectively (fluorescence is expressed in relative fluorescence units). Use noninfected A549 cells as standards for fluorescence and background levels.
NOTE: The cells need to be fixed with 4% paraformaldehyde (PFA) before measuring them without the plate cover.
- Assessment of the drug inhibition using RSV-GFP
- Prepare 96-well plates for the GFP measurement. The day before the assay, seed the wells with 100 µL of a suspension of HEp-2 cells at 5 x 105 cells/mL in complete medium without phenol red.
- Prepare a serial dilution of the tested drugs (AZ4316 in this example) in MEM complemented with 2% FCS and antibiotics (50 µL per well). Prepare a viral suspension at 10,000 PFU/mL in MEM without stromal vascular factor (SVF) and without phenol red (50 µL per well).
- Remove the medium from the 96-well HEp-2 plate and add 50 µL of the drug suspension and 50 µL of the viral suspension (in triplicate). Perform a mock infection in parallel as a control.
NOTE: The drug dilution and viral suspension may be mixed before adding them on the cells, or they can be added sequentially. - Incubate the plate for 48 h at 37 °C and 5% CO2.
- Measure the fluorescence, using a spectrofluorometer as described in step 5.1.4. Use mock-infected HEp-2 cells as standards for fluorescence background levels.
6. Characterization of M2-1 Localization In Vivo with the RSV-M2-1-GFP Recombinant Virus
NOTE: Perform steps 6.1 and 6.2 in a sterile environment, using a class II safety cabinet.
- Prepare a suspension of HEp-2 cells at 5 x 105 cells/mL in complete medium. Seed 1.5 mL of the cell suspension in a 35 mm Petri dish permeant to CO2 and adapted for live imaging.
- Perform the infection the day after seeding with the RSV-M2-1-GFP virus at MOI 1, as described in steps 3.3–3.5 (remove the medium, add 500 µL to 1 mL of inoculum, and incubate the sample at 37 °C while gently shaking for 2 h; remove the inoculum and add 1.5 mL of MEM with 2% FCS). Incubate the cells at 37 °C and 5% CO2 for the desired time (IBs will start to appear from 10 h p.i.).
- Preheat the incubation chamber of an inverted microscope equipped with 40x to 100x objectives at 37 °C, prior to placing the Petri dish containing the infected cells on the stage. Open the CO2 supply and wait for focus stabilization.
- Perform imaging with GFP-compatible filters, under a low excitation intensity and image frequency (from 1 to 0.1 image per min) to minimize phototoxicity.
Results
In this work, we described a detailed protocol to produce recombinant RSV viruses expressing a fluorescent protein (Figure 2). In pRSV-GFP, the GFP gene was introduced between the P and M genes, as described for the Cherry gene in previously published work21. In the pRSV-M2-1-GFP, the M2 gene was left untouched and an additional gene coding for M2-1-GFP was inserted between SH and G genes12. The first step, corr...
Discussion
Here we present a method of rescue of recombinant RSVs from five plasmids, and their amplification. The ability to manipulate the genome of viruses has revolutionized virology research to test mutations and express an additional gene or a tagged viral protein. The RSV we have described and used as an example in this article is a virus expressing a reporter gene, the RSV-GFP (unpublished), and expresses an M2-1 protein fused to a GFP tag12. RSV rescue is challenging and requires practice. The trans...
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors thank Dr. Qin Yu from AstraZeneca R&D Boston, MA, USA, for providing the AZD4316 drug. The authors are grateful to the Cymages platform for access to the ScanR Olympus microscope, which was supported by grants from the region Ile-de-France (DIM ONE HEALTH). The authors acknowledge support from the INSERM and the Versailles Saint-Quentin University.
Materials
| Name | Company | Catalog Number | Comments |
| 35mm µ dish for live cell imaging | Ibidi | 81156 | |
| A549 | ATCC | ATCC CCL-185 | |
| Avicel RC-591 | FMC BioPolymer | Avicel RC-591 | Technical and other information on Avicels is available at http://www.fmcbiopolymer.com. Store at room temperature. Protocol in step 4 is optimized for this reagent. |
| BSRT7/5 | not commercially available | See ref 22. Buchholz et al, 1999 | |
| Crystal violet solution | Sigma | HT90132 | |
| Fluorescence microscope for observations | Olympus | IX73 Olympus microscope | |
| Fluorescence microscope for videomicroscopy | Olympus | ScanR Olympus microscope | |
| HEp-2 | ATCC | ATCC CCL-23 | |
| HEPES ≥99.5% | Sigma | H3375 | |
| L-Glutamine (200 mM) | ThermoFisher Scientific | 25030024 | |
| LIPOFECTAMINE 2000 REAGENT | ThermoFisher Scientific | 11668019 | Protocol in step 2.3. is optimized for this reagent. |
| MEM (10X), no glutamine | ThermoFisher Scientific | 11430030 | |
| MEM, GlutaMAX Supplement | ThermoFisher Scientific | 41090-028 | |
| MgSO4 ReagentPlus, ≥99.5% | Sigma | M7506 | |
| Opti-MEM I Reduced Serum Medium | ThermoFisher Scientific | 51985-026 | |
| Paraformaldehyde Aqueous Solution, 32%, EM Grade | Electron Microscopy Sciences | 15714 | |
| Penicillin-Streptomycin (10,000 U/mL) | ThermoFisher Scientific | 15140122 | |
| Plasmids | not commercially available | see ref 21. Rameix-Welti et al, 2014 | |
| See Saw Rocker | VWR | 444-0341 | |
| Si RNA GAPDH | Dharmacon | ON-TARGETplus siRNA D-001810-10-05 | SMARTpool and 3 of 4 individual siRNAs designed by Dharmacon. |
| Si RNA IMPDH2 | Dharmacon | ON-TARGETplus siRNA IMPDH2 Pool- Human L-004330-00-0005 | SMARTpool of 4 individual siRNAs designed by Dharmacon. Individual references and sequences J-004330-06: GGAAAGUUGCCCAUUGUAA; J-004330-07: GCACGGCGCUUUGGUGUUC; J-004330-08: AAGGGUCAAUCCACAAAUU; J-004330-09: GGUAUGGGUUCUCUCGAUG; |
| Si RNA RSV N | Dharmacon | ON-TARGETplus custom siRNA | UUCAGAAGAACUAGAGGCUAU and UUUCAUAAAUUCACUGGGUUA |
| SiRNA NT | Dharmacon | ON-TARGETplus Non-targeting Pool | |
| SiRNA transfection reagent | Dharmacon | DharmaFECT 1 Ref: T-2001-03 | Protocol in steps 5.1.and 5.1.2 are optimized for this reagent. |
| Sodium Bicarbonate 7.5% solution | ThermoFisher Scientific | 25080094 | |
| Spectrofluorometer | Tecan | Tecan infinite M200PRO |
References
- Shi, T., et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. The Lancet. 390 (10098), 946-958 (2017).
- Falsey, A. R., Hennessey, P. A., Formica, M. A., Cox, C., Walsh, E. E. Respiratory Syncytial Virus Infection in Elderly and High-Risk Adults. The New England Journal of Medicine. 352 (17), 1749-1759 (2005).
- DeVincenzo, J. P., et al. Activity of Oral ALS-008176 in a Respiratory Syncytial Virus Challenge Study. The New England Journal of Medicine. 373 (21), 2048-2058 (2015).
- DeVincenzo, J. P., et al. Oral GS-5806 Activity in a Respiratory Syncytial Virus Challenge Study. The New England Journal of Medicine. 371 (8), 711-722 (2014).
- Afonso, C. L., et al. Taxonomy of the order Mononegavirales: update 2016. Archives of Virology. 161 (8), 2351-2360 (2016).
- Collins, P. L., Hill, M. G., Cristina, J., Grosfeld, H. Transcription elongation factor of respiratory syncytial virus, a nonsegmented negative-strand RNA virus. Proceedings of the National Academy of Sciences of the United States of America. 93 (1), 81-85 (1996).
- Hoenen, T., et al. Inclusion bodies are a site of ebolavirus replication. Journal of Virology. 86 (21), 11779-11788 (2012).
- Heinrich, B. S., Cureton, D. K., Rahmeh, A. A., Whelan, S. P. Protein expression redirects vesicular stomatitis virus RNA synthesis to cytoplasmic inclusions. PLoS Pathogens. 6 (6), e1000958 (2010).
- Lahaye, X., et al. Functional Characterization of Negri Bodies (NBs) in Rabies Virus-Infected Cells: Evidence that NBs Are Sites of Viral Transcription and Replication. Journal of Virology. 83 (16), 7948-7958 (2009).
- Kolesnikova, L., Mühlberger, E., Ryabchikova, E., Becker, S. Ultrastructural organization of recombinant Marburg virus nucleoprotein: comparison with Marburg virus inclusions. Journal of Virology. 74 (8), 3899-3904 (2000).
- Dolnik, O., Stevermann, L., Kolesnikova, L., Becker, S. Marburg virus inclusions: A virus-induced microcompartment and interface to multivesicular bodies and the late endosomal compartment. European Journal of Cell Biology. 94 (7-9), 323-331 (2015).
- Rincheval, V., et al. Functional organization of cytoplasmic inclusion bodies in cells infected by respiratory syncytial virus. Nature Communications. 8 (1), 563 (2017).
- Santangelo, P. J., Bao, G. Dynamics of filamentous viral RNPs prior to egress. Nucleic Acids Research. 35 (11), 3602-3611 (2007).
- Lifland, A. W., et al. Human Respiratory Syncytial Virus Nucleoprotein and Inclusion Bodies Antagonize the Innate Immune Response Mediated by MDA5 and MAVS. Journal of Virology. 86 (15), 8245-8258 (2012).
- Garcia, J., Garcia-Barreno, B., Vivo, A., Melero, J. A. Cytoplasmic inclusions of respiratory syncytial virus-infected cells: formation of inclusion bodies in transfected cells that coexpress the nucleoprotein, the phosphoprotein, and the 22K protein. Virology. 195 (1), 243-247 (1993).
- Brown, G., et al. Evidence for an association between heat shock protein 70 and the respiratory syncytial virus polymerase complex within lipid-raft membranes during virus infection. Virology. 338 (1), 69-80 (2005).
- Radhakrishnan, A., et al. Protein analysis of purified respiratory syncytial virus particles reveals an important role for heat shock protein 90 in virus particle assembly. Molecular & Cellular Proteomics. 9 (9), 1829-1848 (2010).
- Racaniello, V. R., Baltimore, D. Cloned poliovirus complementary DNA is infectious in mammalian cells. Science. 214 (4523), 916-919 (1981).
- Schnell, M. J., Mebatsion, T., Conzelmann, K. K. Infectious rabies viruses from cloned cDNA. The EMBO Journal. 13 (18), 4195-4203 (1994).
- Collins, P. L., et al. Production of infectious human respiratory syncytial virus from cloned cDNA confirms an essential role for the transcription elongation factor from the 5' proximal open reading frame of the M2 mRNA in gene expression and provides a capability for vaccine. Proceedings of the National Academy of Sciences of the United States of America. 92 (25), 11563-11567 (1995).
- Rameix-Welti, M. -. A., et al. Visualizing the replication of respiratory syncytial virus in cells and in living mice. Nature Communications. 5, 5104 (2014).
- Buchholz, U. J., Finke, S., Conzelmann, K. K. Generation of bovine respiratory syncytial virus (BRSV) from cDNA: BRSV NS2 is not essential for virus replication in tissue culture, and the human RSV leader region acts as a functional BRSV genome promoter. Journal of Virology. 73 (1), 251-259 (1999).
- Cianci, C., Meanwell, N., Krystal, M. Antiviral activity and molecular mechanism of an orally active respiratory syncytial virus fusion inhibitor. Journal of Antimicrobial Chemotherapy. 55 (3), 289-292 (2005).
- Derscheid, R. J., et al. Human respiratory syncytial virus memphis 37 grown in HEp-2 cells causes more severe disease in lambs than virus grown in vero cells. Viruses. 5 (11), 2881-2897 (2013).
- McKimm-Breschkin, J. L. A simplified plaque assay for respiratory syncytial virus - direct visualization of plaques without immunostaining. Journal of Virological Methods. 120, 113-117 (2004).
- Matrosovich, M., Matrosovich, T., Garten, W., Klenk, D. New low-viscosity overlay medium for viral plaque assays. Virology Journal. 7, 1-7 (2006).
- Novina, C. D., Sharp, P. A. The RNAi revolution. Nature. 430 (6996), 161-164 (2004).
- Sintchak, M. D., Nimmesgern, E. The structure of inosine 5'-monophosphate dehydrogenase and the design of novel inhibitors. Immunopharmacology. 47 (2-3), 163-184 (2000).
- Beaucourt, S., Vignuzzi, M. Ribavirin: A drug active against many viruses with multiple effects on virus replication and propagation. Molecular basis of ribavirin resistance. Current Opinion in Virology. 8, 10-15 (2014).
- Hruska, J. F., Bernstein, J. M., Douglas, R. G., Hall, C. B. Effects of Ribavirin on Respiratory Syncytial Virus in vitro. Antimicrobial Agents and Chemotherapy. 17 (5), 770-775 (1980).
- Simões, E. A. F., et al. Past, Present and Future Approaches to the Prevention and Treatment of Respiratory Syncytial Virus Infection in Children. Infectious Diseases and Therapy. 7 (1), 87-120 (2018).
- Alvarez, R., et al. RNA interference-mediated silencing of the respiratory syncytial virus nucleocapsid defines a potent antiviral strategy. Antimicrobial Agents and Chemotherapy. 53 (9), 3952-3962 (2009).
- DeVincenzo, J., et al. A randomized, double-blind, placebo-comtrolled study of an RNAi-based therapy directed against respiratory syncytial virus. Proceedings of the National Academy of Sciences of the United States of America. 107 (19), 8800-8805 (2010).
- Zhou, Y., et al. High-throughput screening of a CRISPR/Cas9 library for functional genomics in human cells. Nature. 509 (7501), 487-491 (2014).
- Nikolic, J., et al. Negri bodies are viral factories with properties of liquid organelles. Nature Communications. 8 (1), 58 (2017).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved