Microinjectrode System for Combined Drug Infusion and Electrophysiology
In This Article
Summary
We present a microinjectrode system designed for electrophysiology and assisted delivery of experimental probes (i.e., nanosensors, microelectrodes), with optional drug infusion. Widely available microfluidic components are coupled to a cannula containing the probe. A step-by-step protocol for microinjectrode construction is included, with results during muscimol infusion in macaque cortex.
Abstract
This microinjectrode system is designed for drug infusion, electrophysiology, and delivery and retrieval of experimental probes, such as microelectrodes and nanosensors, optimized for repeated use in awake, behaving animals. The microinjectrode system can be configured for multiple purposes: (1) simple arrangement of the cannula for placement of an experimental probe that would otherwise be too fragile to penetrate the dura mater, (2) microfluidic infusion of a drug, either independently or coupled to a cannula containing an experimental probe (i.e., microelectrode, nanosensor). In this protocol we explain the step by step construction of the microinjectrode, its coupling to microfluidic components, and the protocol for use of the system in vivo. The microfluidic components of this system allow for delivery of volumes on the nanoliter scale, with minimal penetration damage. Drug infusion can be performed independently or simultaneously with experimental probes such as microelectrodes or nanosensors in an awake, behaving animal. Applications of this system range from measuring the effects of a drug on cortical electrical activity and behavior, to understanding the function of a specific region of cortex in the context of behavioral performance based on probe or nanosensor measurements. To demonstrate some of the capabilities of this system, we present an example of muscimol infusion for reversible inactivation of the frontal eye field (FEF) in rhesus macaque during a working memory task.
Introduction
Electrophysiology and drug injection methods are widely used in neuroscience to study neuronal activity and behavior, in vivo, in rodents and primates. Over the last three decades, improvements of the early injectrode models allowed a more precise and less invasive technique, and simultaneous recording and drug injection at specific brain sites1,2,3. For primates in particular, the ability to precisely deliver small volumes with minimal tissue damage is critical if the technique is to be used for the study of advanced cognitive functions that require highly trained animals. Recent advances include chronic electrophysiological and chemical measurements in combination with stimulation using implanted probes4, and combined recording and microfluidic drug delivery has recently been piloted in rodents5. The injectrode system described here allows electrophysiological recording, stimulation, and precise drug delivery, and it has already been successfully implemented in multiple primate labs6,7,8.
The increasing availability of delicate, specialized sensors, such as nanosensors9,10 with neuroscience applications, demands a reliable method for getting the probe through the dura mater without damaging the fragile nanoscale devices or microelectrode tips.
We designed a microinjectrode system that overcomes the technical challenges of combining these methods using readily available, low-cost components, and facilitates two main functions: (i) The ability to place a fragile experimental probe, such as a microelectrode or nanosensor, through the dura mater and neural tissue, protected from any damage. This functionality allows placement of the experimental probe at targeted locations, delivered by using the cannula as a guide through the neural tissue. (ii) The ability to use a microelectrode to perform experiments combining electrophysiology recordings and electrical stimulation with drug injection.
Our system uses a guide tube to penetrate the dura, along with a cannula which functions both for drug delivery (when using the system for microinfusion) and provides additional protection for the microelectrode or nanosensor (both when passing through the dura and neural tissue). This system can be easily constructed with widely commercially available components, which are inexpensive and easy to find. We minimize penetration damage by using a small diameter cannula (outer diameter OD = 235 µm, inner diameter ID = 108 µm).
Here we present step-by-step instructions for the microinjectrode construction and configuration of the microfluidic system. We explain the steps needed for use of the microinjectrode, either independently or coupled to the microfluidic system for drug injection. A similar approach can be applied with any fragile experimental probe, such as a nanosensor9,10. The probe can be front- or back-loaded into the cannula (depending on design), and will be protected from damage when penetrating the dura and neural tissue. We provide example data from an in vivo experiment with non-human primates, in which we used a tungsten microelectrode to perform electrical stimulation, and subsequently injected muscimol in the frontal eye field (FEF) while the animal performed a memory guided saccade (MGS) task.
Protocol
Experimental procedures followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals and the Society for Neuroscience Guidelines and Policies. Protocols for experimental and behavioral procedures were approved by the University of Utah Institutional Animal Care and Use Committee.
1. Construction of the Microinjectrode for Stimulation and Recording (Figure 1a)
- Measure the length of the cannula and the probe (in this example a nanosensor). Probe must be longer than the cannula by the length it is to protrude from the cannula tip (depending on probe design) plus approximately 2 cm.
- Under a magnifier or a microscope (~10x magnification), load the probe into the cannula; if possible a back-loading is preferable to protect the tip of the probe.
NOTE: This step, performed manually, is challenging. It is recommended to practice with a microelectrode under a magnifying glass before attempting with an actual experimental probe. - Pass the cannula (containing the probe) through the top ferrule, T-junction, and bottom ferrule.
- If the probe is just a single wire without any attachments, back-load it into the canula and insert the assembly into the T-junction from the bottom ferrule. Top of cannula (flat-end side) should be positioned in the middle of the T-junction, within the bottom but not the top ferrule. The experimental probe or biosensor should protrude above the top of the top ferrule.
NOTE: Custom-made ferrules can also be made by drilling a hole in the ferrule plugs using micro drill bits, the size of the hole being based upon the diameter needed for tightening the cannula to the T-junction.
- If the probe is just a single wire without any attachments, back-load it into the canula and insert the assembly into the T-junction from the bottom ferrule. Top of cannula (flat-end side) should be positioned in the middle of the T-junction, within the bottom but not the top ferrule. The experimental probe or biosensor should protrude above the top of the top ferrule.
- Use the ferrule wrench to tighten the ferrules on the top and bottom of the T-junction. Do not over-tighten. A small piece of tubing can be added to strengthen the electrode support within the top ferrule.
- Solder gold pins to each of the probe terminals (signal, ground, etc.), according to the specifications of the probe.
- Adjust relative position of probe and cannula. Measure the distance that the probe is protruding from cannula under magnification, and adjust manually from the top end (probe can slide freely within ferrules).
- Add epoxy glue between the gold pins and the top ferrule to attach the probe to the ferrule.
- Unscrew the top ferrule to retract probe inside cannula. Visually confirm that the probe is fully within the cannula under magnification.
- Attach the injectrode to the microdrive.
2. Construction of the Microinjectrode for Drug Infusion (Figure 1b)
- Attach the "non-beveled" or flat-end of the cannula to the bottom of the T-junction using a ferrule. Use the ferrule wrench to tighten the ferrule.
- Attach a small piece of capillary tubing (~1.5 cm) to the top of the T-junction by passing it through the standard ferrule. Tighten with a ferrule wrench.
- Back-load the microelectrode through the capillary tubing, T-junction, cannula and corresponding ferrules.
- Make sure that the back-end of the electrode protrudes less than 1 cm from the back of the capillary tubing, and the tip of the electrode protrudes from the cannula at the desired distance on the bottom side. Electrode position can be manually adjusted from the top-end.
- Solder a gold pin to the microelectrode terminal.
- Add epoxy glue between the gold pin and the top ferrule to attach the microelectrode to the ferrule.
- Unscrew the top ferrule to retract the probe inside cannula. Visually confirm that the microelectrode is fully retracted into the cannula.
3. Construction of the Microfluidic Circuit (Figure 2)
- Place a breadboard on a stable surface. Place the two three-way valves parallel to the longest sides of the breadboard, about 6 in. apart with one port (the one that is always open) facing each other. Use screws to fix the valves to the breadboard.
- Place a ruler next to the valves (to measure and track movement of fluids inside the capillary tubing).
- Load a mixture of 1:1 low viscosity oil and food coloring (marker) into the gastight syringe and place in the Marker pump. Cut one piece of capillary tubing, and use standard ferrules and Luer-lock connectors to connect the syringe to one of the ports on the Input valve. This is the "marker line".
- Cut a short piece of capillary tubing for the "ruler line". Use standard ferrules to tighten to the facing ports of the valves.
- Cut two longer pieces of capillary tubing to connect the Output valve to the microinjectrode, and to connect the Drug pump to the Input valve (use standard ferrules).
NOTE: The length of these two lines depends on the experimental setup, one must be long enough to reach from the infusion apparatus to the animal, and the other one from the Drug pump to the Input valve. Use a cleaving stone to cut the capillary tubing.
4. Mounting the Microinjectrode to the Microdrive (Figure 3)
- Make sure the microelectrode/experimental probe is retracted in the cannula prior to mounting.
NOTE: The guide tube should be in position in microdrive. - Attach a custom-made adapter to the microinjectrode.
- Top-load the microinjectrode through the guide tube and secure it to the adapter using screws.
- Measure the microdrive position (depth) at which the microinjectrode protrudes from the guide tube, then retract it ~1 cm to prepare for insertion.
- For microinfusion experiments, connect the "brain line" to the unused T-junction opening of the microinjectrode. Use a standard ferrule and tighten with the ferrule wrench.
5. Flushing and Preparation of the Microfluidic System
- Position the microdrive with the microinjectrode over a waste beaker.
- Load chlorhexidine (e.g., nolvasan; dissolved at 20 g/L) into the 1 mL gastight syringe and place it in the Drug pump. Turn the flow direction of the valves such that fluid goes from the Drug pump through the valve to the valve line and out the "brain line".
- Flush the circuit with chlorhexidine using a low flow rate (50-200 µL/min) for a minimum of 10 min. Repeat steps 5.2 through 5.3 with sterile saline and then air.
NOTE: It is important to check for leaks at this stage. Gently apply lint-free wipes at the junctions to help reveal any liquid leaks through the ferrules. - Load the drug in the 500 µL gastight syringe, compress the air and then place in the Drug pump. Flow at 50 µL/min until a few drops flow from the microinjectrode.
- Soak the guide tube in chlorhexidine (dissolved at 20 g/L) for 15 min.
- Turn the direction of the Output valve towards the "flushing line". Advance the Marker pump until a clear edge of color and oil is observed on the ruler line. Make sure there is always oil between the drug and the color in order to not mix the two water-soluble materials and lose the sharp edge between them. Mark the starting position of this oil/dye line (with a piece of tape or marker).
- Turn the direction of the Output valve towards the brain line.
6. Performing Recording or an Infusion Experiment
NOTE: Animal handling steps will vary depending on the lab and experiment. The following steps are to be performed after the necessary surgical set up and preparation has been performed to expose the dura. Following the experiment, all necessary post-procedure steps must be performed in accordance with institutionally approved protocols.
- Attach the microdrive to the recording chamber. Lower the guide tube to penetrate the dura.
NOTE: The guide tube should not penetrate any further than the dura in order to avoid damaging the cortex. - Lower the microinjectrode to about 2 mm above the site for recording/injection in the brain.
- Tighten the top ferrule (protruding microelectrode/biosensor) and connect the gold pins to the recording system. Keep advancing the microinjectrode to the target site.
NOTE: Remember to include the distance that the microelectrode extends beyond the cannula in the calculations. - For infusion experiments, use the manual microsyringe pump to move the column of oil by 1 cm every 3 min (~60 nL/min). Once the desired volume has been infused, switch the Output valve towards the flushing line.
NOTE: The volume infused will vary based on model species and brain area targeted. Faster flow rates may damage neural tissue. - When the experiments are complete, retract the microinjectrode within the guide tube (leave the probe protruded). Then remove the microdrive for flushing. Flush the microfluidic system as described in steps 5.1-5.5. to prepare for reuse.
NOTE: In our experience, the microinjectrode will last for several uses if proper care is taken. Electrophysiological recording quality drops faster than the capability of injection.
Representative Results
We performed injection of a GABAa agonist (muscimol) for reversible inactivation of the frontal eye field (FEF), while the animal performed a memory guided saccade task11. In this task, the animal fixates and a peripheral visual target is presented. The animal maintains fixation while remembering the target location, and once the fixation point disappears, executes a saccadic eye movement to the remembered location to receive a reward. The microinjectrode was built according to instructions in Figure 1b. The infusion volume for the example experiment was 850 nL. Behavioral performance on the memory guided saccade (MGS) task at various locations and times relative to the muscimol infusion is shown in Figure 4. The largest performance deficits were observed at 2 to 3 h post-infusion.
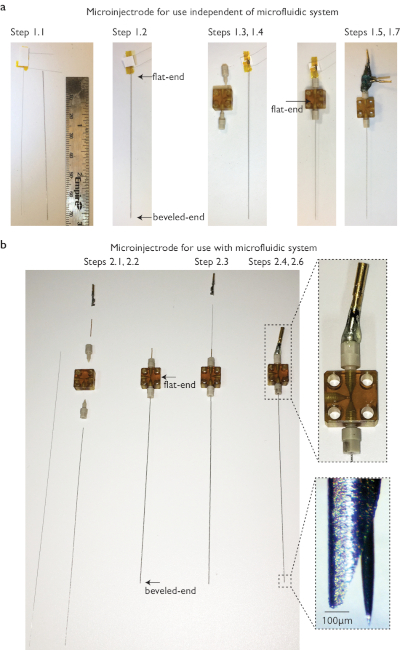
Figure 1: Step by step fabrication of microinjectrode. (a) Configuration for use independent of microfluidic system. Cannula and probe are measured in order to confirm that the tip of the probe can be protruded at the desired length (e.g., 150 µm). The probe is front loaded into the cannula. The cannula is passed through the T-junction and attached on the bottom side, with the flat end in the middle of the T-junction; the back end of the probe continues through the top ferrule. The microinjectrode is finalized by soldering gold pins on each of the probe terminals and adding glue between them and the top ferrule for stability. Connection to the acquisition system depends upon the design of the probe. In this example, our probe is a nanosensor with three leads. (b) Configuration for use with microfluidic system. To couple the microinjectrode to the microfluidic system, a piece of capillary tubing is used for the top side of the T-junction. The probe can be front or back loaded. The microfluidic line is then plugged to the third T-junction opening. In this example we used a microelectrode. See the zoomed picture of the tip of a cannula in which the microelectrode was protruded by tightening the top ferrule. See the Table of Materials for a list of items used in construction. Please click here to view a larger version of this figure.
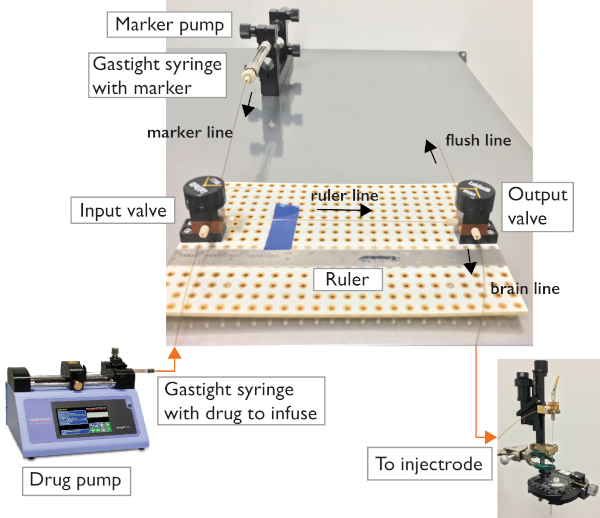
Figure 2: Microfluidic system. The two-valve configuration allows control of flow direction towards the microinjectrode or towards the flushing line for troubleshooting. The circuit relies on two 3-port valves connected using capillary tubing and standard ferrules. Gastight syringes are used to carry and inject the infusion drug and the marker. A programmable syringe pump allows for automatic flushing of the system and loading of the drug. A manual microsyringe pump allows for controlled injection and visualization. Please click here to view a larger version of this figure.
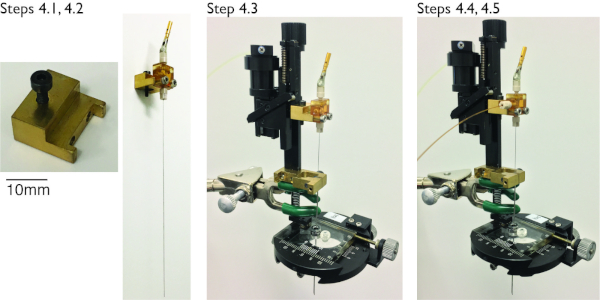
Figure 3: Mounting of microinjectrode to a hydraulic microdrive with and without injection capacity. Step 4.1: A custom-made adapter allows for attachment of the microinjectrode to the microdrive. A single screw attaches the adapter to the microdrive; two screws secure microinjectrode to the adapter. The top ferrule should be unscrewed at least 2 turns in order to protect the tip of the microelectrode/experimental probe when loading the microinjectrode in the guide tube of the microdrive. Step 4.3: Insert microinjectrode into guide tube from the top. Step 4.4: If performing microinfusion, plug the drug line to the third T-junction opening using a plastic ferrule. Please click here to view a larger version of this figure.
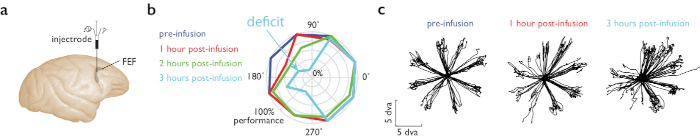
Figure 4: Memory guided saccade task during muscimol infusion in FEF. (a) The microinjectrode was placed in the right hemisphere, FEF area. (b) Behavioral performance during an MGS task in which eight targets are placed peripherally. We ran 4 blocks of the MGS task, before and at three times after injection. Polar plot shows performance (eccentricity) at each of these times (color), for different locations relative to the fixation point (angle on polar plot). Performance clearly decreased in the left visual hemifield 2 h after injection (blue trace, left half of polar plot). (c) Saccade traces for 8 peripheral memory locations before (left) and after muscimol injection in the FEF (right, 1 and 3 h post-infusion). Saccade accuracy in the left visual hemifield (left half of polar plots) decreased after muscimol injection. Scale in degrees of visual angle (dva). Please click here to view a larger version of this figure.
Discussion
Several methods are currently available to perform simultaneous drug delivery and electrophysiology. Our system is intended to have the flexibility to be used for recordings either independently or in combination with drug injection, and to have the ability to precisely place any fragile experimental probe, such as a nanosensor or a microelectrode, protected from any damage, through the dura mater and neural tissue. The system allows precise control of drug infusion volumes with the naked eye (17 nL precision shown in previous studies in our lab3).
There are more specialized systems for pressure injection with smaller diameters12. Those systems allow for multiple recording sites, but the complex setup of software and hardware required for control of the system carries higher costs for each of the components, and has less flexibility to interface with experimental probes that are not yet commercialized on a large scale. Moreover, our injectrode does not require a chronic implant and provides a great degree of flexibility: compatible with biosensors to measure chemical and electrophysiological signals, and capable of infusing drugs as well, with the potential to measure the effect of localized drug infusions on these responses.
The design allows the experimental probe to be protruded after dura penetration in order to avoid damage to the structure of the probe. This feature allows for the multifunctionality of the device, to penetrate the dura without risking damage of any experimental probe such as nanometer-scale nanosensors10. However, there is a limitation of the length that can be protruded, restricted by the number of turns of the ferrule, limited to ~1 mm for the standard ferrules. There is minimal tissue damage due to the small cannula diameter (228 µm).
In the experiment we showed, the system was used to perform controlled delivery of muscimol for reversible inactivation of FEF, simultaneously with either electrical stimulation or extracellular recording (single neuron, local field potential) using a microelectrode. This experiment in FEF requires microstimulation of the FEF to confirm saccade vectors prior to inactivation, and the drug was infused to study working memory during reversible FEF inactivation. It is unlikely that a recording from the same isolated single neuron can be maintained before and after the drug injection; however, we were able to record local field potentials before and after infusion. Here, we show an experiment combining injection, recording, and electrical stimulation.
Once it is set up, the method is very reliable and robust. However, due to precipitation of small molecules (e.g., salt) within the small tube and ports, a thorough flushing is required after each experiment in order to keep the microfluidics free of obstructions and leaks. Due to the simplicity of the entire circuit, each component can be replaced independently for easy troubleshooting.
Although the method was demonstrated in the FEF area in a non-human primate, the principle can be applied to any other brain area where some combination of electrical stimulation, recording, and drug injection are desired, in species of rodent size or larger.
Acknowledgements
This work was supported by funding from the National Institutes of Health (NIH), grants EY026924 and EY014800 (to B.N.), an Unrestricted Grant from Research to Prevent Blindness, Inc., New York, NY to the Department of Ophthalmology and Visual Sciences, University of Utah, and the start-up funds provided to R.E. by the Henry Samueli School of Engineering and the Department of Electrical Engineering at the University of California, Irvine. This method is based on a previous report of a similar method developed in Dr. Tirin Moore’s lab, published in Noudoost & Moore 2011, Journal of Neuroscience Methods. The authors thank Dr. Kelsey Clark for her comments on the manuscript.
Materials
| Name | Company | Catalog Number | Comments |
| 3-port manual valves | LabSmith | Manual 3-Port Selector Valve (MV201-C360) | https://products.labsmith.com/mv201-manual-3-port-selector-valve/#.XNYEC9NKh26 |
| Cannulae | Vita Needle Company | 304 Stainless steel tubing, Outer Diameter 228μm, Inner Diameter 165μm | https://www.vitaneedle.com/assets/files/Vita_Needle_Master_Tubing_Gauge_Chart.pdf |
| Cleaving stone | Molex | Cleaving stone 1" x 1" (part No. 1068680064) | Highly recommended to follow method for cleaving capillary tubing: https://www.cmscientific.com/info_sheets/cleaving_procedure.pdf |
| Clorhexidine diacetate | Walmart | Nolvasan solution disinfectant (AAP311) | Used for microfluidic circuit flushing, dissolved at 20 g/L |
| Custom adapter | Custom provider | - | Custom machined adapter to connect microinjectrode to hydraulic microdrive |
| Driver | LabSmith | T7 TORX driver for installing breadboard screws (LS-TORX Driver) | https://products.labsmith.com/ls-torx-driver/#.XO8sndNKh25 |
| Epoxy glue | LabSmith | Two-part high-strength epoxy adhesive (LS-EPOXY) for metal and plastic bonding | https://products.labsmith.com/ls-epoxy-12ml-epoxy-adhesive/#.XO8t89NKh24 |
| Ferrule | LabSmith | One-Piece Fitting (C360-100) for connecting capillary, thru hole sized for 360μm OD capillary | https://products.labsmith.com/one-piece-fitting#.XNYEaNNKh24 |
| Ferrule plug | LabSmith | One-Piece Plug (C360-101) for use in any -C360 port | https://products.labsmith.com/one-piece-fitting-plug/#.XNYFl9NKh24 |
| Ferrule wrench | LabSmith | 1/8" hex wrench for installing one-piece fittings and plugs (LS-HEX 1/8" Hex Wrench) | https://products.labsmith.com/ls-hex-1-8-hex-wrench/#.XO8sqtNKh24 |
| Gastight syringe | Hamilton Company | 500μL gastight syringe model 1750 (81220) and 1mL gastight syringe model 1001 (81320) | https://www.hamiltoncompany.com/laboratory-products/syringes/81220#top |
| Gold pins | Aim-Cambridge | Male gold plated crimp-on connector pin (40-9856M) | https://www.masterelectronics.com/aim-cambridge-cinch-connectivity-solutions/409856m-10109145.html |
| Lint-free wipes | Kimberly Clark | Kimtech Science Kimwipes Delicate Task | Lint-free wipes, used to identify leaks in the system |
| Liquid food color | McCormick & Co. | Water based, black liquid food color (52100581873) | https://www.mccormick.com/spices-and-flavors/extracts-and-food-colors/food-colors/black-food-color |
| Low viscosity oil | Clearco Products Co. | Pure Silicone Fluid Octamethyltrisiloxane with a viscosity of 1cSt at 25°C (PSF-1cSt) | http://www.clearcoproducts.com/pure-silicone-super-low-viscosity.html |
| Luer-Lock connector | LabSmith | Luer-Lock Adapter (C360-300), female fitting for connecting Luer Lock syringe to 360μm capillary tubing | https://products.labsmith.com/luer-lock-adapter-assembly#.XO81MtNKh24 |
| Micro drill bits | Grainger | Micro drill bit, 0.23mm (414H85) | https://www.grainger.com/category/machining/drilling-and-holemaking/drill-bits/machining-drill-bits/micro-drill-bits |
| Microelectrode | FHC | Metal microelectrode, tungsten with epoxy insulation | https://www.fh-co.com/category/metal-microelectrodes |
| Oil hydraulic micromanipulator | Narishige Group | Oil Hydraulic Micromanipulator with guide tube attached (MO-96) | http://products.narishige-group.com/group1/MO-96/chronic/english.html |
| Polymicro Capillary Tubing | Molex | Polymicro Flexible Fused Silica Capillary Tubing (TSP150375), Outer Diameter 375µm, Inner Diameter 150µm | https://www.molex.com/webdocs/datasheets/pdf/en-us/1068150024_CAPILLARY_TUBING.pdf |
| Programmable syringe pump | Harvard Apparatus | Standard Infuse/Withdraw Pump, programmable (70-2213) | https://www.harvardapparatus.com/standard-infuse-withdraw-pump-11-pico-plus-elite-programmable-syringe-pump.html |
| Ruler | Empire | Stainless steel 6" Stiff ruler (27303) | http://www.empirelevel.com/rulers.php |
| Screw set | LabSmith | Valve mounting screw set (LS-SCREWS .25), thread-forming screws (2-28 x 1/4”) to mount valves to breadboard | https://products.labsmith.com/ls-screws-25#.XO8widNKh24 |
| Standard Breadboard | LabSmith | 4" x 6" platform (LS600), with 0.25" hole spacing for mounting fluid circuit | https://products.labsmith.com/standard-breadboard/#.XO8xDdNKh24 |
| Sterile saline (sodium chloride) 0.9%. | Baxter | 0.9% Sodium Chloride sterile | Sterile Intravenous Infusion |
| Sterile syringe filters | Millipore Sigma | MilliporeSigma™ Millex™-GP Sterile Syringe Filters with PES Membrane (SLGPM33RS) | https://www.fishersci.com/shop/products/emd-millipore-millex-sterile-syringe-filters-pes-membrane-green-4/slgpm33rs |
| Stoelting manual microsyringe pump | Stoelting Company | Manual infusion/withdrawal pump (51222) | https://www.stoeltingco.com/manual-infusion-withdrawal-pump-2649.html |
| T-junction | LabSmith | Interconnect tee (C360-203) for combining flow streams, for use with 360μm OD capillary tubing | https://products.labsmith.com/interconnect-tee#.XO8z8dNKh24 |
References
- Chen, L. T. L., Goffart, L., Sparks, D. L. A simple method for constructing microinjectrodes for reversible inactivation in behaving monkeys. Journal of Neuroscience Methods. 107 (1-2), 81-85 (2001).
- Crist, C. F., Yamasaki, D. S. G., Komatsu, H., Wurtz, R. H. A grid system and a microsyringe for single cell recording. Journal of Neuroscience Methods. 26 (2), 117-122 (1988).
- Noudoost, B., Moore, T. A reliable microinjectrode system for use in behaving monkeys. Journal of Neuroscience Methods. 194 (2), 218-223 (2011).
- Zhang, S., et al. Real-time simultaneous recording of electrophysiological activities and dopamine overflow in the deep brain nuclei of a non-human primate with Parkinson's disease using nano-based microelectrode arrays. Microsystems & Nanoengineering. 4, (2018).
- Altuna, A., et al. SU-8 based microprobes for simultaneous neural depth recording and drug delivery in the brain. Lab on a Chip. 13 (7), 1422-1430 (2013).
- Noudoost, B., Clark, K. L., Moore, T. A Distinct Contribution of the Frontal Eye Field to the Visual Representation of Saccadic Targets. Journal of Neuroscience. 34 (10), 3687-3698 (2014).
- Rajalingham, R., DiCarlo, J. J. Reversible Inactivation of Different Millimeter-Scale Regions of Primate IT Results in Different Patterns of Core Object Recognition Deficits. Neuron. 102 (2), 493 (2019).
- Katz, L. N., Ates, J. L. Y., Pillow, J. W., Huk, A. C. Dissociated functional significance of decision-related activity in the primate dorsal stream. Nature. 535 (7611), 285 (2016).
- Esfandyarpour, R., Esfandyarpour, H., Javanmard, M., Harris, J. S., Davis, R. W. Microneedle biosensor: A method for direct label-free real time protein detection. Sensors and Actuators B-Chemical. 177, 848-855 (2013).
- Esfandyarpour, R., Yang, L., Koochak, Z., Harris, J. S., Davis, R. W. Nanoelectronic three-dimensional (3D) nanotip sensing array for real-time, sensitive, label-free sequence specific detection of nucleic acids. Biomedical Microdevices. 18 (1), (2016).
- Bahmani, Z., Daliri, M. R., Merrikhi, Y., Clark, K., Noudoost, B. Working Memory Enhances Cortical Representations via Spatially Specific Coordination of Spike Times. Neuron. 97 (4), 967-979 (2018).
- Veith, V. K., Quigley, C., Treue, S. A Pressure Injection System for Investigating the Neuropharmacology of Information Processing in Awake Behaving Macaque Monkey Cortex. JoVE: Journal of Visualized Experiments. (109), (2016).
This article has been published
Video Coming Soon
ABOUT JoVE
Copyright © 2024 MyJoVE Corporation. All rights reserved