A subscription to JoVE is required to view this content. Sign in or start your free trial.
Detection of Lung Tumor Progression in Mice by Ultrasound Imaging
In This Article
Summary
This protocol describes the steps taken to induce KRAS lung tumors in mice as well as the quantification of formed tumors by ultrasound imaging. Small tumors are visualized in early timepoints as B-lines. At later timepoints, relative tumor volume measurements are achieved by the measurement tool in the ultrasound software.
Abstract
With ~1.6 million victims per year, lung cancer contributes tremendously to the worldwide burden of cancer. Lung cancer is partly driven by genetic alterations in oncogenes such as the KRAS oncogene, which constitutes ~25% of lung cancer cases. The difficulty in therapeutically targeting KRAS-driven lung cancer partly stems from having poor models that can mimic the progression of the disease in the lab. We describe a method that permits the relative quantification of primary KRAS lung tumors in a Cre-inducible LSL-KRAS G12D mouse model via ultrasound imaging. This method relies on brightness (B)-mode acquisition of the lung parenchyma. Tumors that are initially formed in this model are visualized as B-lines and can be quantified by counting the number of B-lines present in the acquired images. These would represent the relative tumor number formed on the surface of the mouse lung. As the formed tumors develop with time, they are perceived as deep clefts within the lung parenchyma. Since the circumference of the formed tumor is well-defined, calculating the relative tumor volume is achieved by measuring the length and width of the tumor and applying them in the formula used for tumor caliper measurements. Ultrasound imaging is a non-invasive, fast and user-friendly technique that is often used for tumor quantifications in mice. Although artifacts may appear when obtaining ultrasound images, it has been shown that this imaging technique is more advantageous for tumor quantifications in mice compared to other imaging techniques such as computed tomography (CT) imaging and bioluminescence imaging (BLI). Researchers can investigate novel therapeutic targets using this technique by comparing lung tumor initiation and progression between different groups of mice.
Introduction
As the leading cause of cancer-related deaths worldwide, lung cancer remains refractory to treatments, mainly due to lack of relevant pre-clinical models that can recapitulate the disease in the lab1. Around 25% of lung cancer cases are due to mutations in the KRAS oncogene2. KRAS-driven lung cancer is often associated with poor prognosis and low response to therapy, highlighting the importance of further studies in this disease2.
We optimized a method that allows the relative evaluation of lung tumor growth in real time in KRAS lung cancer-induced immune-competent mice. We use Lox-Stop-Lox KRAS G12D (LSL-KRAS G12D) mice in which the KRAS G12D oncogene can be expressed by Cre lentiviral vectors3,4. These vectors are driven by carbonic anhydrase 2, allowing the viral infection to take place specifically in alveolar epithelial cells5. In addition, to accelerate the initiation and progression of lung tumors, the lentiviral construct also expresses P53 shRNA from an U6/H1 promoter (the lentiviral construct herein will be referred to as Ca2Cre-shp53)6. The biological relevance of this method lies in the natural course of lung tumor development in mice as opposed to xenografts of non-orthotopic tumors in mice. An obstacle using the orthotopic method is monitoring lung tumor growth without sacrificing the mouse. To overcome this limitation, we optimized ultrasound imaging to permit the analysis of lung tumor progression in two-dimensional (2D) mode in this mouse model. Initiating tumors at 7 weeks post-infection are reflected as B-lines in ultrasound images, which can be counted, but will not reflect the exact number of tumors present on the lung. B-lines are characterized by laser-like vertical white lines arising from the pleural line in the lung parenchyma7,8. Large tumors can be visualized after 18 weeks of infection. The relative volume of these tumors is quantified by 2D measurements done on ultrasound.
This method is optimal for researchers investigating the effect of pharmacological drugs on lung tumor growth in the LSL-KRAS G12D mouse model. In addition, lung tumor progression can be compared between mice with different genetic lineages, to examine the importance of the presence or absence of certain genes/proteins on the development of lung tumor volume.
Protocol
Animal studies were performed in accordance with the Institutional Animal Care and Use Committee (IACUC) of McGill University and procedures were approved by the Animal Welfare Committee of McGill University (animal use protocol # 2009-5754).
1. Generation of CA2Cre-shp53 Lentiviral Titre
NOTE: The following protocol is the same as that described in Xia et al.6, with minor modifications.
- Preparation of lentivirus (for 15 cm x 10 cm dishes)
- On day 1, plate healthy HEK293T cells (7.5 x 106 cells per 10 cm dish) with 10 mL of Dulbecco's modified Eagle medium) (DMEM), 10% fetal bovine serum (FBS), and 1% pen/strep. Culture in a 37 °C, 5% CO2 incubator.
- Prepare the mix for calcium-phosphate transfection (mixture for 15 plates). Prepare tube A containing 225 µg (15 µg/plate) of the lentiviral vector (Ca2Cre-shp53), 75 µg (5 µg/plate) of PsPAX2 plasmid (containing HIV-1 gag and HIV-1 pol genes), 75 µg (5 µg/plate) of pMD2.G plasmid (containing VSV-G gene) for packaging, a final concentration of 0.15 M of CaCl2 and fill the tube with distilled H2O up to 3.75 mL. Prepare tube B containing 3.75 mL of 2x HEPES-buffered saline (HBS; 50 mM HEPES, pH 7.05, 280 mM NaCl, 10 mM KCl, 1.5 mM Na2HPO4·2H2O, and 12 mM D-dextrose).
- Vortex tube A and add it dropwise to tube B under continuous vortexing.
NOTE: The total volume will be 7.5 mL. - Incubate at room temperature in the dark for 20−30 min.
- Approximately 9 h after plating the cells, add 500 µL of the transfection mixture dropwise into the cell medium (7.5 mL/15 dishes: 0.5 mL per dish).
- Swirl gently each dish to mix, and incubate all dishes at 37 °C, 5% CO2.
- On day 2, 12−18 h after transfection, replace the media with 10 mL of antibiotic-free reduced serum media (Table of Materials) per 10 cm dish. Place dishes back in the 37 °C, 5% CO2 incubator.
- On day 3, collect the media containing lentiviruses expressing Ca2Cre-shp53 and filter through 0.45 μm filters. Replenish the media with fresh antibiotic-free reduced serum media.
NOTE: The collected media can be kept at 4 °C for no more than 3 days. - On day 4, collect, for a second time, the media containing the lentivirus and filter through a 0.45 µm filter. Keep at 4 °C (for no more than 3 days).
- Combine the virus supernatants from steps 1.1.8 and 1.1.9. Concentrate them through centrifugal filter units (Table of Materials) by centrifuging at 1,372 x g for 30 min at 4 °C. Repeat the process until all the collected media has passed through the columns.
NOTE: After each centrifugation, 100−200 μL of concentrated filtrate can be harvested. - Collect and mix the concentrated filtrates in a 15 mL ice-cold tube. Mix the concentrated lentiviruses well and then aliquot (e.g., 100 μL/ tube). Store at -80 °C.
- Lentiviral titration
NOTE: Immortalized mouse embryonic fibroblasts (MEFs) expressing a loxP-flanked allele of green fluorescent protein (GFP) are used in this protocol for the quantification of viral titer. However, any cell line with a loxP-GFP allele should be suitable for this step.- Culture cells expressing a loxP-flanked allele of GFP with DMEM, 10% FBS and 1% pen/strep at 37 °C, 5% CO2.
- Plate 2 x 105 cells in two 50 mm wells of a 6-well plate.
NOTE: The cells of one well will be used for lentiviral infection while that of the other will be used as a negative control. - The next day, replenish the cells with 2 mL of DMEM, 10% FBS, and 1% pen/strep 2 h before lentiviral infection.
- Add 20 µL of the CA2Cre-shp53 lentivirus (the volume may vary when needed) in the lentiviral infection well.
- After 3 days in culture, determine the frequency of the Cre-induced GFP-positive cells by flow cytometry, as shown in Figure 1. Wash cells with phosphate-buffered saline (PBS), detach by trypsinization and collect cells by centrifugation at 112 x g.
- Wash the cells twice with PBS. Finally, suspend the cells in 100 µL of PBS. Determine the percentage of GFP positive cells by a cell analyzer (Table of Materials).
- Calculate the lentivirus infectious/functional titer using the following formula:

where F is the frequency of GFP-positive cells and calculated by subtracting the frequency of GFP -positive cells after infection (Fi) from the frequency of GFP-positive cells (background) prior to infection (Fc) as shown in Figure 1 (F = Fi-Fc), Cn is the number of plated cells (2 x 105), V is the volume of the inoculum (mL), and DF is the virus dilution factor.
NOTE: The infectious/functional concentration = ~2 x 106 TU/mL.
2. Intratracheal Intubation of Lentiviruses in LSL-KRASG12D Mice
NOTE: The method of intratracheal intubation was used as described in the published protocol by Vandivort et al.9. In this protocol, mice LSL-KRASG12D mice in C57BL/6 background are used at age 6−8 weeks. A home-made working procedure board is used as described in Vandivort et al.9. The board is positioned in front of the experimenter in a convenient workspace (approximately 1 m2).
- Prepare a spirometer by removing the plunger of a 1 mL syringe and loading 60 µL of PBS into the syringe.
- Attach a 22 G catheter tip into the syringe and set aside.
- Anaesthetize the mice by intraperitoneal injection of a 1 µL/g of mouse body weight of ketamine (50 mg/mL)/xylazine (5 mg/mL)/acepromazine (1 mg/mL) cocktail. Ensure proper sedation of the mouse by a low respiratory rate (1 breath every 2 s).
- Aspirate 20 µL of CA2Cre-shp53 lentivirus into a pipettor and set aside.
- Position the sedated mouse on the working procedure board by hooking its upper incisors into the thread of the board.
NOTE: The mouse's dorsum should be flat against the platform. - Tape the caudal portion of the thoracic cavity to the platform to ensure alignment of the mouse during the procedure.
- Adjust a gooseneck illuminator between 80−100% intensity and place the light 1−2 cm from the skin surface.
- From behind the platform, draw the tongue out of the oral cavity of the mouse using sterile forceps.
- While securing the tongue, insert a depressor into the oral cavity of the mouse then release the tongue.
- Position the gooseneck on the main stem bronchi to illuminate trachea.
NOTE: The trachea may be visible through the action of respiration, causing fluctuation of the light. - When the trachea is clearly viewed, insert the spirometer prepared (syringe with PBS and catheter) into the tracheal path.
- Remove the depressor and observe the rise and fall of PBS in the syringe with each breath.
NOTE: This is an indicator that the catheter is properly positioned into the trachea. - Remove the syringe containing PBS while maintaining the catheter inside the trachea as the previous position.
- Deposit the 20 µL CA2Cre-shp53 lentivirus into the center of the catheter.
- While keeping the catheter in place, inject 300 µL of air into the catheter using an empty syringe to ensure proper distribution of lentivirus in the lungs.
- Keep the catheter in place and reinsert the spirometer into the catheter.
NOTE: The rise and fall of the PBS bubble will ensure that the procedure is successful. - Remove the catheter and tape. Place the animal in a warm and dry place until it is revived.
3. Ultrasound Imaging of Lung Tumors in Mice
NOTE: Ultrasound imaging was performed after 7 and 18 weeks of lentiviral intubation using the system listed in Table of Materials; however, any model can be used for the analysis.
- One day prior to imaging, remove fur from the chest area of the intubated mouse.
NOTE: Mouse should be sedated during this step by placing them in an induction chamber of 3% isoflurane and 2L/minute O2. - On the day of imaging, set up the workspace as shown in Figure 2. Turn on the heating pump for ultrasound gel and the temperature monitor.
- Set up a warm 33 °C incubator to place the mice in post-imaging.
- Place the three-dimensional (3D) motor (Figure 2F) on the integrated rail system.
- Make sure that the 3D motor and the transducer mounting system are securely in place.
- Connect a preferred transducer (frequency: 40 MHz; Figure 2E and Table of Materials) for tumor measurements perpendicular to the 3D motor.
- Start a new study on the ultrasound software.
- Select Study Browser, then select New at the bottom of the screen.
- Select New Study, a new window will appear that enables the addition of a Study Name in addition to further information about the study, i.e., date of study, name of researcher, etc.
- Fill in the information in Series Name, i.e., Animal ID, Strain, Weight, Date of Birth, etc.
- Select Done, the program will change for B-mode.
- Put a heating lamp in a convenient position above the animal platform.
- Place the mouse in the induction chamber (3.5% isoflurane).
NOTE: Proper anesthetization is confirmed by unconsciousness of the mouse, and slower respiratory rate of around 1 breath per 2 seconds. - When the mouse is sedated, change the connection of the anesthetic machine to be directed towards the animal platform, reduce isoflurane to 2.5%.
- Place the mouse on the animal platform in decubitus ventral, with its oral cavity directed towards the anesthetics tube.
- Apply lubricant on the eyes of the mouse.
- Place the mouse in decubitus dorsal and tape its hands and feet firmly onto the animal platform.
- Apply a small layer of ultrasound gel on the mouse chest.
- Lower the acquisition probe using the height-control knob to touch the surface of the mouse chest. Position the probe such that the heart of the mouse is approximately centered.
- Use the micro-knobs to acquire images of the whole chest, from both extremities, in the transverse orientation ideally gathering 500 frames per mouse (number of frames may vary depending on personal choice).
- Once imaging is done, remove the gel from the chest of the mouse and place the mouse in the warm incubator.
4. 2D Analysis of Ultrasound Images
- After opening acquired frames on the ultrasound software, scan the frames for tumors.
- For small initiating tumors, count the number of B-lines periodically every 10 frames for the full length of the 500 frames acquired.
Therefore, B-lines are counted in a total of 50 images, each image is separated by 10 frames. B-lines are characterized by longitudinal white straight lines fully traversing the screen. - For 2D measurements of large tumors, select the linear tool and measure the width and length of the tumor present.
- To calculate the volume of the tumors, use the following formula:
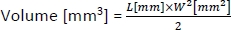
where L and W are the length and width of the tumor, respectively.
Results
After obtaining a lentiviral infectious titer of ~2 x 106 TU/mL (Figure 1), the Ca2Cre-shp53 lentivirus was intratracheally injected when LSL-KRAS G12D mice reached an appropriate age (6−8 weeks)9. Ultrasound imaging was performed after 7 weeks of infection upon initiation of tumors (Figure 3B). Imaging was done at 7 weeks in order to include the various types of precursor lesions that occur in the LSL-KR...
Discussion
We demonstrate a method that can assess lung tumor growth in the Cre-inducible LSL-KRAS G12D mouse model by ultrasound. This method can be used for evaluating the effect of pharmacological inhibitors on lung tumor growth. It can also be used to compare lung tumor growth between mice of different genetic backgrounds. Using this technique does not require specialized computational skills, however, it is important to be systematic in the number of frames used for analysis to allow for proper comparison if the method is used...
Disclosures
The authors have nothing to disclose.
Acknowledgements
We thank Dr. I. Verma for the lentiviral Ca2Cre-shp53 vector. The work was supported by funds from the Canadian Institutes of Health Research (CIHR MOP 137113) to AEK.
Materials
| Name | Company | Catalog Number | Comments |
| 0.45 μm Acrodisc Syringe Filters | Pall Corporation | PN 4614 | |
| 100-mm Cell Cultre Plate | CELLSTAR | 664 160 | |
| 6-well Cell Culture Plate | CELLSTAR | 657 160 | |
| Amicon Ultra - 15 Centrifugal Filter Units | Merck Millipore Ltd. | UFC910024 | |
| BD LSR-Fortessa | BD Biosciences | 649225B 3024 | |
| CA2Cre-shp53 lentiviral vector | From Dr. I Verma Laboratory | ||
| DMEM | Multicell | 319-005-CL | |
| FBS | Multicell | 80450 | |
| LSL-KRASG12D mouse | JAX Mice | 8179 | |
| MX550S; Centre Transmit: 40 MHz | FUJIFILM VisualSonics | 51070 | |
| OptiMEM | gibco | 11058-021 | |
| Pen/strep | Multicell | 450-201-EL | |
| pMD2.G | Addgene | 12259 | |
| PsPAX2 | Addgene | 12260 | |
| VEVO-3100 | FUJIFILM VisualSonics | 51072-50 |
References
- Eisenstein, M. Personalized medicine: Special treatment. Nature. 513, 8 (2014).
- Karachaliou, N., et al. KRAS mutations in lung cancer. Clinical Lung Cancer. 14 (3), 205-214 (2013).
- Jackson, E. L., et al. Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras. Genes & Development. 15 (24), 3243-3248 (2001).
- DuPage, M., Dooley, A. L., Jacks, T. Conditional mouse lung cancer models using adenoviral or lentiviral delivery of Cre recombinase. Nature Protocol. 4 (7), 1064-1072 (2009).
- Chen, J., Lecuona, E., Briva, A., Welch, L. C., Sznajder, J. I. Carbonic anhydrase II and alveolar fluid reabsorption during hypercapnia. American Journal of Respiratory Cell and Molecular Biology. 38 (1), 32-37 (2008).
- Xia, Y., et al. Reduced cell proliferation by IKK2 depletion in a mouse lung-cancer model. Nature Cell Biology. 17 (4), 532 (2015).
- Demi, L., et al. Determination of a potential quantitative measure of the state of the lung using lung ultrasound spectroscopy. Scientific Reports. 7, 12746 (2017).
- Mohanty, K., et al. Characterization of the Lung Parenchyma Using Ultrasound Multiple Scattering. Ultrasound in Medicine and Biology. 43, 993-1003 (2017).
- Vandivort, T. C., An, D., Parks, W. C. An Improved Method for Rapid Intubation of the Trachea in Mice. Journal of Visualized Experiments. (108), e53771 (2016).
- Saraogi, A. Lung ultrasound: Present and future. Lung India. 32 (3), 250-257 (2015).
- Gargani, L., Volpicelli, G. How I do it: lung ultrasound. Cardiovascular Ultrasound. 12, 25 (2014).
- Soldati, G., et al. On the Physical Basis of Pulmonary Sonographic Interstitial Syndrome. Journal of Ultrasound in Medicine. 35 (10), 2975 (2016).
- Raes, F., et al. High-Resolution Ultrasound and Photoacoustic Imaging of Orthotopic Lung Cancer in Mice: New Perspectives for Onco-Pharmacology. PLoS One. 11 (4), 15 (2016).
- Lakshman, M., Needles, A. Screening and quantification of the tumor microenvironment with micro-ultrasound and photoacoustic imaging. Nature Methods. 12 (4), 372 (2015).
- Chichra, A., Makaryus, M., Chaudhri, P., Narasimhan, M. Ultrasound for the Pulmonary Consultant. Clinical Medicine Insights: Circulatory Respiratory and Pulmonary Medicine. 10, 9 (2016).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved