Exon Skipping in Directly Reprogrammed Myotubes Obtained from Human Urine-Derived Cells
In This Article
Summary
In this article, we describe a detailed protocol for efficient modelling of Duchenne muscular dystrophy muscle using MYOD1-converted urine-derived cells to evaluate the restoration of dystrophin mRNA and protein levels after exon skipping.
Abstract
Duchenne muscular dystrophy (DMD), a progressive and fatal muscle disease, is caused by mutations in the DMD gene that result in the absence of dystrophin protein. To date, we have completed an investigator-initiated first-in-human study at the National Center of Neurology and Psychiatry based on the systemic injection of the morpholino oligonucleotides which are prone to exon-53 skipping. For the effective treatment of DMD, in vitro testing with myoblasts derived from DMD patients to screen drugs and assess patient eligibility before undertaking clinical trials is thought to be essential. Very recently, we reported a new MYOD1-converted urine-derived cell (UDC) treated with the histone methyltransferase inhibitor (3-deazaneplanocin A hydrochloride), as a cellular model of DMD. The new autologous UDC might show phenocopy of the disease-specific phenotypes of DMD, leading to the application of precision medicine in a variety of muscle-related diseases. In this article, we describe a detailed protocol for efficient modelling of DMD muscle cells using MYOD1-converted UDCs along with reverse transcriptase polymerase chain reaction (RT-PCR), Western blotting, and immunocytochemistry to evaluate the restoration of dystrophin mRNA and protein levels after exon skipping.
Introduction
Duchenne muscular dystrophy (DMD), a progressive, fatal muscle disease, is caused by frame-shift mutations in the DMD gene that result in the absence of dystrophin protein1. Antisense oligonucleotide-based exon skipping therapy is thought to be promising for DMD. This therapy is based on the conversion of the severer DMD phenotype to the milder Becker muscular dystrophy-like phenotype by altering pre-mRNA splicing to restore the DMD reading frame2. We have recently completed a first-in-human study based on repeated intravenous administration of the phosphorodiamidate morpholino oligomer (PMO) viltolarsen, which can induce exon 53 skipping in DMD, and demonstrated an excellent safety profile, promising efficacy, and acceptable pharmacokinetic parameters (registered as UMIN: 000010964 and ClinicalTrials.gov: NCT02081625)3.
However, to develop cost-effective and efficient treatments for the disease, in vitro tests using primary muscle cells obtained from DMD patients are essential for drug screening and patient eligibility verification before undertaking clinical trials, as well as biomarkers that reflect the efficacy of exon skipping therapies during human trials4. Very recently, we reported a novel technology to develop patient-specific MYOD1-converted urine-derived cells (UDCs)5,6 as a primary myoblast model of DMD7. Thus, to generate the myoblasts, only the collection of urine from patients is required and no invasive procedure is needed. In this article, we describe a detailed protocol for efficient modelling of DMD muscle using MYOD1-converted UDCs treated with 3-deazaneplanocin A hydrochloride to evaluate the restored dystrophin mRNA and protein after exon skipping.
Protocol
The Ethics Committee of the National Center of Neurology and Psychiatry approved this study (approval ID: A2017-018, A2018-029). All individuals gave informed consent before providing urine. All experiments were performed under the relevant guidelines and regulations.
1. Isolation and primary culture of UDCs
NOTE: UDCs were isolated according to a previously published protocol8,9,10 with some modifications.
- Collect urine samples during spontaneous micturition in sterilized plastic bottles.
NOTE: Sterilization of the external urethral orifice is not needed. Midstream urine is desirable to reduce the risk of viral contamination. If the stock time before the next procedure is >1 h, urine samples should be transferred to 4 °C to preserve the cell viability. However, a temperature of <4 °C should be avoided because insoluble precipitates may appear. - Centrifuge the entire urine sample at 400 x g for 10 min at room temperature.
- Aspirate the supernatant, leaving 1 mL in the tube.
- Resuspend the pellets individually in the remaining 1 mL of urine and then collect those in a single 50 mL tube.
- Add 10 mL of washing buffer consisting of 99 mL of PBS without calcium and magnesium, 1% penicillin/streptomycin (P/S), 0.5 µg/mL amphotericin B, and centrifuge the samples at 200 x g for 10 min at room temperature.
- Aspirate the supernatant, leaving 0.2 mL in the tube.
- Resuspend the cell pellets in 4.5 mL of primary medium composed of a 1:1 mixture of high glucose Dulbecco's modified Eagle medium (DMEM)without sodium pyruvate and Ham's F-12 nutrient mix supplemented with recombinant human epidermal growth factor (EGF), insulin, hydrocortisone, epinephrine, T3, transferrin, 10% tetracycline-free fetal bovine serum (FBS), 1% P/S, and 0.5 µg/mL amphotericin B.
- Seed the cells in three wells of gelatine-coated six well plates (total volume of each well, 1.5 mL). Culture humidified at 37 °C and 5% CO2 for 24 h.
- Add 1.5 mL of the primary medium daily for the next 3 days.
- On day 4, replace the medium with 1.5 mL of growth medium supplemented with recombinant human EGF, insulin, hydrocortisone, epinephrine, T3, transferrin, 15% tetracycline-free FBS, 0.5% L-alanine-L-glutamine, 0.5% nonessential amino acids, and 2.5 ng/mL fibroblast growth factor-basic (bFGF), recombinant human platelet-derived growth factor (PDGF), EGF, and 1% P/S.
- Change the growth medium every other day.
NOTE: UDC colonies appear within a week. - When the UDC culture becomes 80−90% confluent, remove the medium and wash cells with PBS, split all the cells using 0.25% trypsin-EDTA and seed at 3,000-5,000 cells/cm2 onto a new gelatine-coated 60 mm dish (passage 1).
NOTE: The UDCs can be stored in liquid nitrogen. UDCs are usually divided at 60−70% confluency in 60 mm culture dish into three stock tubes.
2. Retroviral construct
- Amplify the coding region of MYOD1 (NM_002478.4) plasmid by polymerase chain reaction (PCR).
NOTE: The mixture for MYOD1 amplification and conditions for the thermal cycler are shown in Table 1 and Table 2, respectively. - Detect a single band of about 1,000 bp size by 0.7% agarose gel electrophoresis using 1 µL of the amplified PCR product to confirm that the MYOD1 sequence is amplified successfully.
- Clean the PCR product using the clean-up kit and determine its concentration with a spectrophotometer.
- Incubate the mixture as shown in Table 3 at 37 °C overnight to digest retroviral vector with a Tet-on system and puromycin resistant gene at restriction enzyme-targeted regions in the multiple cloning site.
- Detect a single band by 0.7% agarose gel electrophoresis using 1 µL of the digested product to confirm that the retroviral vector was digested successfully.
- Clean the digested product using clean-up kit and determine its concentration by spectrophotometer.
- To clone the amplified MYOD1 fragment (produced by steps 2.1−2.3) into the digested retroviral vector (produced by steps 2.4−2.6), perform an in-fusion cloning reaction. Set up the reaction as shown in Table 4, incubate the reaction for 15 min at 50 °C, and then place on ice.
- Perform transformation using E. coli competent cells according to the manufacturer's instructions (Table of Materials).
- Select the transformed competent cells by culturing on an LB culture plate consisting of 10 g/L bacto tryptone, 5 g/L bacto yeast extract, 5 g/L NaCl, 15 g/L bacto agar, and 50 mg/L ampicillin.
- Pick up the selected colony and culture in LB culture medium without bacto agar at 200 rpm at 37 °C overnight.
- Purify the MYOD1-inserted retroviral vectors using a plasmid purification kit (Table of Materials) and quantify using a spectrophotometer.
- Confirm that MYOD1 is correctly inserted into the retroviral vector by direct sequencing of the PCR product amplified by the forward and reverse primers targeted respectively on both sides across the inserted MYOD1 sequence.
NOTE: The MYOD1 sequence can be detected sandwiched between retroviral vector sequences when in-fusion cloning is successful. - For retroviral production, seed the packaging cells at 50,000 cells/cm2 on 10 cm collagen-coated plates and culture in DMEM with 10% FBS humidified at 37 °C and 5% CO2 for 24 h.
- When the packaging cells proliferate to 80% confluency, mix 30 µg of MYOD1-inserted retroviral vectors, 30 µg of packaging vectors, and transfection reagent containing cell-penetrating peptide (Table of Materials) by vortexing, and incubate them for 10 min.
- Add the incubated mixture to the medium of packaging cells and culture humidified at 37 °C and 5% CO2.
NOTE: Collagen coating is necessary. Transfection may be better at 24 h or more after seeding because packaging cells easily detach from the culture plate. - After 4 h or overnight, change the medium to fresh growth medium.
- Collect the viral supernatant and replace with fresh medium at 24 and 48 h after cotransfection and combine the supernatant.
- To concentrate the viral supernatant, mix it with the concentrator reagent and incubate at 37 °C overnight, then centrifuge at 1,500 x g for 45 min at 4 °C.
- Filter the retrovirus supernatant through a PVDF filter with 0.45 µm pores.
- Check the titer of the retroviral vector using a quantitative PCR kit and thermal cycler system according to the manufacturer's instructions.
- Divide the viral supernatant into small aliquots and stock them at -80 °C.
3. Infection with MYOD1-retroviral vector in UDCs
- Seed the UDCs at 3,000-5,000 cells/cm2 on a gelatine-coated 60 mm dish.
- After 24 h of seeding, infect the thawed retrovirus (step 2.21) at a multiplicity of infection of 200 by adding hexadimethrine bromide at a concentration of 8 µg/mL.
- After a 24 h incubation humidified at 37 °C and 5% CO2, replace the culture medium with fresh growth medium containing 1 µg/mL puromycin to select the MYOD1-transduced cells. Change the medium every other day.
NOTE: When selecting for MYOD1-positive cells, 1 µg/mL puromycin is usually used for selection. The appropriate dose should be determined. Use a plate containing untransfected cells and choose the dose that kills all the cells in 3−5 days. MYOD1-positive cells should be selected within 7−10 days after adding puromycin. The MYOD1-transduced UDCs can be stored in liquid nitrogen.
4. Myogenic differentiation of MYOD1-transduced UDCs treated with 3-deazaneplanocin A hydrochloride (DZNep)
NOTE: Recently, it has been reported that DZNep, a histone methyltransferase inhibitor, could significantly promote the expression of MYOGENIN, one of the late muscle regulatory factors, and also lead to myotube differentiation7.
- Plate MYOD1-transduced UDCs in the collagen-coated wells at a density of 3.5 x 104 cells/cm2. Culture humidified at 37 °C and 5% CO2.
- After 24 h, change the growth medium to differentiation medium composed of high glucose DMEM with L-alanine-L-glutamine, 5% horse serum, ITS supplement, 1 µg/mL doxycycline, and 5 µM DZNep.
NOTE: The 10 mM DZNep solution can be stored at -80 °C for 3 months. Use a defrost freezer and avoid repeated freeze-thaw cycles. Both MYOD1 activated by doxycycline and DZNep suppress proliferation and promote the myogenic differentiation of UDCs. Therefore, it is recommended that doxycycline and DZNep are added after the induction of myogenic differentiation. DZNep promotes myogenic differentiation of the MYOD1-UDCs in a dose-dependent manner. On the other hand, it shows cytotoxicity at a high concentration. Therefore, determine the appropriate concentration of DZNep, ranging from 1−10 µM depending on its effects on myogenic differentiation and cellular bioavailability. - After 3 days, change the differentiation medium to fresh differentiation medium without DZNep. Then, change the medium every 3 days.
NOTE: UDCs fuse to each other and form myotubes within 1−2 weeks after differentiation.
5. Exon skipping in MYOD1-converted UDCs
NOTE: Here, three protocols are described to evaluate exon skipping in patient-derived cells: 1) reverse transcriptase polymerase chain reaction (RT-PCR) of dystrophin mRNA; 2) semiquantification of restored dystrophin protein signal by Western blot; and 3) semiquantification of restored dystrophin fluorescence signal by immunocytochemistry. All the methods can detect exon skipping in a dose-dependent manner.
- Evaluation of exon skipping efficiency by RT-PCR
- To transfect antisense oligonucleotide (ASO) into MYOD1-converted UDCs obtained from DMD patients on the day 7 after differentiation, mix ASO, transfection reagent (Table of Materials), and differentiation medium to a final concentration of 1−10 µM. Culture humidified at 37 °C and 5% CO2.
- After 72 h of incubation with ASO, change the medium to fresh differentiation medium without ASO.
- From 3−7 days after ASO transfection, remove differentiation medium and wash 1x with PBS. Add cell lysis buffer, lyse the UDCs and harvest the total RNA using an RNA extraction kit.
- Measure the RNA concentration with a spectrophotometer.
- Combine the required reagents for one-step RT-PCR reaction in PCR tubes according to Table 5.
- Place the PCR tubes with the mixture in a thermocycler. Run the thermocycler, according to Table 6.
- Perform microchip electrophoresis and calculate the exon skipping efficiency using the molar concentration as below.
Exon skipping efficiency (%) = skipped band / (skipped band + non-skipped band) x 100
NOTE: Store the PCR product in a refrigerator at 4 °C for short-term storage or -20 °C for long-term storage.
- Detection of dystrophin after exon skipping by Western blotting
- Transfect ASO and culture MYOD1-UDCs according to steps 5.1.1 and 5.1.2.
- Change the medium every 3 days.
- After 2 weeks of differentiation, extract the total protein from the cultured cells using radioimmunoprecipitation assay (RIPA) buffer containing protease inhibitors.
- Sonicate the lysates on ice and centrifuge at 14,000 x g for 15 min at 4 °C.
- Collect the supernatant and determine protein concentrations using a BCA protein assay kit.
- Add 15 µg of total protein in a 0.5 mL tube and dilute by adding the RIPA buffer containing protease inhibitors to a total volume of 10 µL. Run 15 µg of total protein per lane.
- Add sample buffer, reducing agent, and deionized water as shown in Table 7. Denature the cell lysates at 70 °C for 10 min.
- Prepare the tris-acetate running buffer containing 8.95 g/L tricine, 6.06 g/L tris base, 1.0 g/L sodium dodecyl sulfate (SDS).
- Load the sample (20 µL) onto tris-acetate 3−8% gel and perform electrophoresis at 150 V for 75 min.
- Prepare the blotting buffer without methanol.
- Soak the PVDF membrane for 20 s in methanol and then in the blotting buffer until use (at least 10 min). Cut the PVDF membrane to a size of 6 x 8 cm using mini gels and 8 x 12 cm using midi gels.
- Cut the blotting papers to the same size as that of the PVDF membrane and soak those in the blotting buffer until use.
- After electrophoresis, cut the gel to the same size as that of the PVDF membrane and soak the gel in distilled water.
- Place the blotting papers, PVDF membrane, and gel on the semidry transfer apparatus (Figure 1). Transfer at 4 mA/cm2 for 30 min.
- Rinse the membrane 2x with distilled water.
- Prepare anti-dystrophin (1:500) and anti-α-tubulin (1:1,200) antibody as the primary antibodies.
- Prepare HRP-conjugated anti-mouse antibody (1:100) as a secondary antibody.
- Incubate the membranes with the primary antibodies, wash with wash buffer, then incubate with the secondary antibody using an automated Western-processing device (Table of Materials) at room temperature.
NOTE: Anti-α-tubulin antibody is usually used as a loading control. The mixed primary antibody solutions, including 1:500 anti-dystrophin and 1:1,200 anti-α-tubulin antibodies work well when the antibody reaction is performed simultaneously. - Rinse the membrane in distilled water.
- Detect the proteins using chemiluminescent detection reagent and a charge-coupled device (CCD) camera-based imager.
- Analyse the data using appropriate software.
- Detection of dystrophin after exon skipping by immunocytochemistry
- Directly reprogram the UDCs into the myotubes in collagen-coated 96 well plate according to steps 4.1−4.3.
- Transfect the ASO and culture MYOD1-UDCs according to steps 5.1.2 and 5.1.3.
- After 2 weeks of differentiation, wash the cells with PBS and fix them in 4% paraformaldehyde for 10 min at 4 °C.
- Permeabilize MYOD1-UDCs in 0.1% nonionic detergent for 10 min at room temperature and block those with 10% goat serum for 15 min at 37 °C.
- Incubate the cells with a primary antibody overnight at 4 °C.
- Wash the cells with PBS and incubate those with the secondary antibody for 30 min at room temperature.
NOTE: Here, mouse anti-dystrophin (1:30) is used as the primary antibody, anti-mouse IgG is used as the secondary antibody, and Hoechst (1:10,000) is used for nuclei staining. - Image the plates using a fluorescent microscope and use an analyzer to semiquantify the fluorescence signal automatically in every well under the same condition.
Representative Results
We could collect the UDCs easily and non-invasively. UDCs formed colonies within a week after starting primary cell culture we observed a marked proliferative ability. The culture of UDCs was straightforward, and bacterial or fungal contamination was rare when the procedure was performed correctly.
Figure 2 shows representative phase-contrast images of the UDC colony a week after primary culture (Figure 2A) and MYOD1-UDCs a week after differentiation (Figure 2B). Figure 3 shows the successful detection of exon skipping in UDCs obtained from DMD patients by RT-PCR. Figure 3A shows RT-PCR analysis of dystrophin after antisense oligonucleotide treatment in DZNep-treated MYOD1-UDCs derived from a 6-year-old male with an exon 45-54 deletion in the DMD gene. The open reading frame was restored by exon 44 skipping. On day 14 following differentiation, we confirmed the induction of exon skipping in a dose-dependent manner (Figure 3B). The upper bands denote native products, and the lower bands denote exon 44-skipped products that restored the open reading frame.
Figure 4 shows the successful detection of dystrophin after exon skipping in the UDCs obtained from DMD patients by Western blotting in a dose-dependent manner. We also detected the restored dystrophin expression using immunocytochemistry (Figure 5). We measured the intensities of dystrophin with a fluorescent microscope 1 week after the antisense oligonucleotide (ASO) transfection on a 96 well plate (Figure 5A). Markedly higher fluorescent signals were observed in MYOD1-UDCs treated with ASO than in MYOD1-UDCs treated with control ASO (Figure 5B).
These results suggest that our new assay can evaluate exon skipping efficiently in MYOD1-UDCs obtained from DMD patients at the mRNA and protein level.
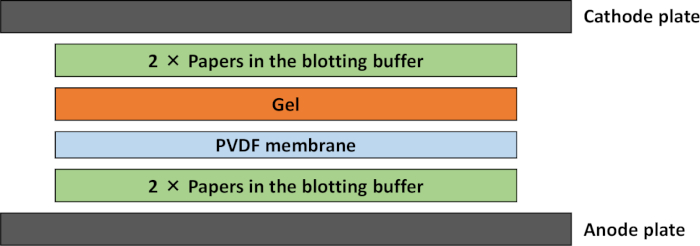
Figure 1: Schematic representation of the transfer stack for semidry Western blot. Two papers soaked in the blotting buffer were laid down at the negative terminal, and two papers soaked in the buffer were stacked on top of this. The gel, which has been soaked in the buffer, was laid gently over the PVDF membrane. Please click here to view a larger version of this figure.
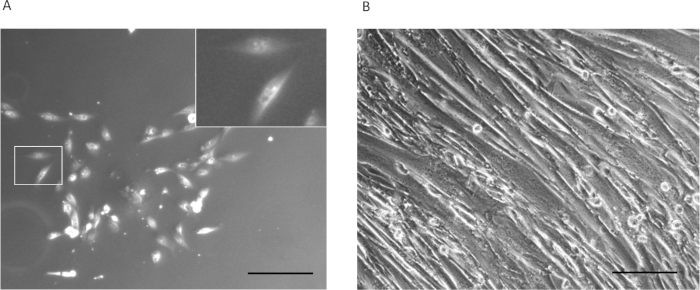
Figure 2: Representative images of the UDCs. (A) Phase-contrast image of UDCs a week after primary culture. Scale bar = 200 µm. Inset: A magnified image of the area in the white rectangle. (B) Phase-contrast image of MYOD1-UDCs a week after differentiation. Scale bar = 50 µm. This figure has been modified from Takizawa et al.7. Please click here to view a larger version of this figure.
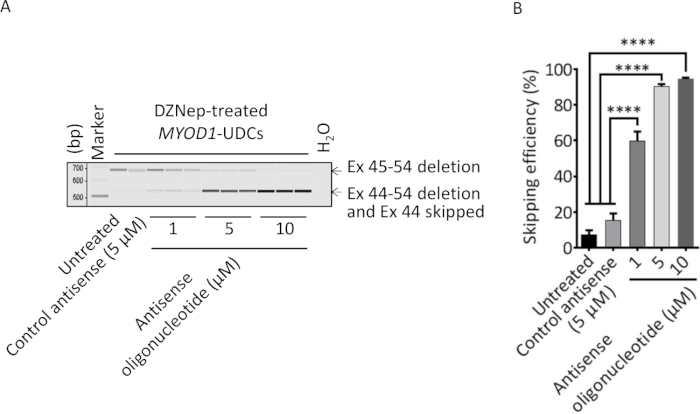
Figure 3: Successful evaluation of exon skipping in urine-derived cells (UDCs) obtained from DMD patients by RT-PCR. (A) RT-PCR analysis of dystrophin after antisense oligonucleotide treatment in 3-deazaneplanocin A hydrochloride (DZNep)-treated MYOD1-UDCs derived from Duchenne muscular dystrophy (DMD) patient with an exon 45-54 deletion. DZNep-treated MYOD1-UDCs were also treated with the control antisense at 1-10 µM concentration as controls. The upper bands were unskipped products (Ex 45-54 deletion) that remained out of the reading frame. The lower bands were the exon 44-skipped products (Ex 44-54 deletion and Ex 44 skipped) that restored the open reading frame. (B) Skipping efficiency was calculated as (exon 44-skipped transcript molarity)/(native + exon 44-skipped transcript molarity [marked with arrows]) x 100% using a microchip electrophoresis system. One-way ANOVA followed by Bonferroni's post hoc test was used to compare the skipping efficiencies (n = 3 for each group, ****P < 0.0001). The data are expressed as the mean ± SEM. This figure has been modified from Takizawa et al.7. Please click here to view a larger version of this figure.
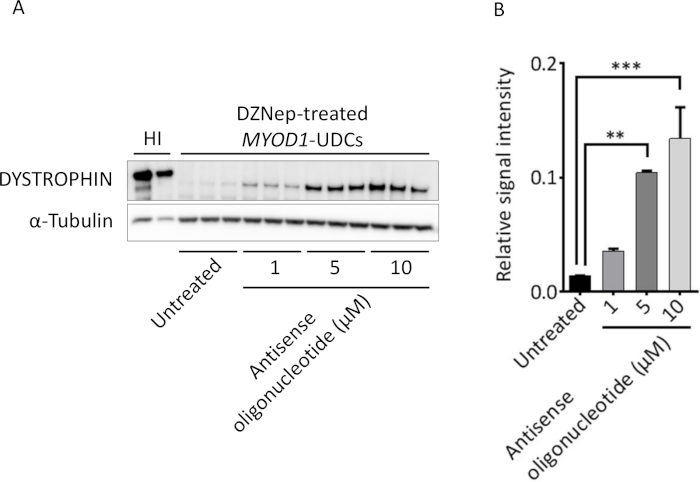
Figure 4: Successful evaluation of exon skipping in urine-derived cells (UDCs) from DMD patients by Western blot. (A) Representative Western blot for dystrophin in DZNep-treated MYOD1-UDCs from DMD patient with an exon 45-54 deletion after exon 44 skipping. For dystrophin detection, anti-dystrophin (against C-terminal) was used. (B) The relative intensities of the bands normalized to α-tubulin expression were compared in patient-derived cells with and without antisense oligonucleotide treatment by performing one-way ANOVA followed by Bonferroni's post hoc test (n = 3 for each group, **P < 0.01, ***P < 0.001, HI = healthy individual). This figure has been modified from Takizawa et al.7. Please click here to view a larger version of this figure.
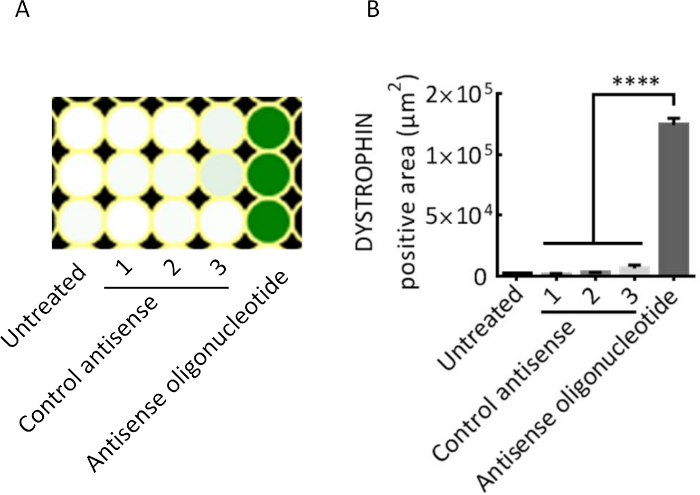
Figure 5: Heatmaps of immunocytochemistry for dystrophin after antisense oligonucleotide treatment in DZNep-treated MYOD1-UDCs obtained from DMD patient with exon 45-54 deletion. (A) Deletion of exon 45-54 restored the open reading frame based on the exon skipping of exon 44. (B) The signal intensity was quantified using a fluorescent microscope after 1 week of antisense oligonucleotide transfection on a 96 well plate. One-way ANOVA followed by Bonferroni's post hoc test was used for the comparison (n = 3-4 for each group, ****P < 0.0001). This figure has been modified from Takizawa et al.7. Please click here to view a larger version of this figure.
| Reagent | Volume | Final concentration |
| 2x PCR premix | 12.5 μL | 1x |
| Forward primer | 5 pmol | 0.2 μM |
| Reverse primer | 5 pmol | 0.2 μM |
| Template | 80 ng | |
| Sterilized distilled water | up to 25 μL | |
| Total volume per reaction | 25 μL |
Table 1: Mixture for MYOD1 amplification by RT-PCR.
| 98 °C | 10 s | |
| 55 °C | 10 s | } 35 cycles |
| 72 °C | 10 s |
Table 2: Conditions for the thermal cycler for MYOD1 amplification.
| Reagent | Volume |
| 10x K buffer | 2 μL |
| Retroviral vector (500 ng/μL) | 2 μL |
| Restriction enzyme 1 (2−15 U) | 1 μL |
| Restriction enzyme 2 (2−15 U) | 1 μL |
| Sterilized distilled water | 14 μL |
| Total volume | 20 μL |
Table 3: Mixture for a tube to digest retroviral vector.
| Reagent | Volume |
| Purified MYOD1 fragment | 100 ng |
| Digested retroviral vector | 100 ng |
| 5x Enzyme premix | 4 μL |
| Sterilized distilled water | up to 20 μL |
| Total volume | 20 μL |
Table 4: Mixture for the in-fusion cloning reaction.
| Solution | Volume/Reaction (μL) | Final concentration |
| RNase-free water | Variable | - |
| One-step RT-PCR buffer | 4 | 1x |
| dNTP mix (containing 10 mM of each dNTP) | 0.8 | 400 mM of each dNTP |
| Forward primer (10 mM) | 1.2 | 0.6 mM |
| Reverse primer (10 mM) | 1.2 | 0.6 mM |
| One-step RT-PCR enzyme mix | 0.8 | - |
| RNase inhibitor (optional) | Variable | 5−10 units/reaction |
| Template RNA | 50−400 ng | |
| Total volume | 20 |
Table 5: Necessary compounds for one reaction of the one-step RT-PCR.
| 1 cycle | Reverse transcription | 30 min | 50 °C |
| 1 cycle | Initial PCR activation step | 15 min | 95 °C |
| 1 cycle | Denaturation | 1 min | 94 °C |
| Annealing | 1 min | 60 °C | |
| Extension | 1 min | 72 °C | |
| 1 cycle | Final extension | 7 min | 72 °C |
| Hold | ∞ | 4 °C |
Table 6: Thermal cycler condition for one-step RT-PCR.
| Reagent | Volume |
| Protein (15 μg) | 10 μL |
| Sample buffer (4x) | 5 μL |
| Reducing agent (10x) | 2 μL |
| Deionized water | 3 μL |
| Total volume | 20 μL |
Table 7: Preparation of samples for sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE).
Discussion
Here, we describe a detailed protocol of exon skipping in MYOD1-converted UDCs obtained from DMD patients. Using the assay system, we screened optimal antisense sequences efficiently. We assume that MYOD1-converted UDCs can be useful for the investigation of the pathophysiology of the disease.
Evaluation of exon skipping using patient-derived cells at the mRNA level is indispensable for screening new drugs and assessing patient eligibility before undertaking clinical trials. Calculation of exon skipping efficiency can be evaluated only at an mRNA level.
Evaluation of exon skipping at the protein level is also important because dystrophin restoration is important as a surrogate biomarker to predict the benefits of exon skipping. To date, screening of antisense oligonucleotide sequences is often performed using primary muscle cell lines or immortalized myoblast cell lines including human rhabdomyosarcoma (RD) cells, but we cannot measure the recovery of dystrophin levels using muscle cell lines or RD cell lines because they express this protein endogenously. We can clearly detect the restoration of dystrophin in DMD patient-derived MYOD1-UDCs in a dose-dependent manner. In our new assay, we consider that the evaluation of restored protein by Western blotting is superior in quantifiability. On the other hand, evaluation by immunocytochemistry using 96 well plates is ideal for screening many candidate compounds simultaneously.
In this article, we describe a detailed protocol for an efficient modelling DMD muscle using MYOD1-converted UDCs along with RT-PCR, Western blotting, and immunocytochemistry to evaluate the restored dystrophin at the mRNA and protein levels after exon skipping. UDCs can be collected noninvasively and easily. Therefore, we assume that the brand-new in vitro assay can be applied to a wide range of basic and translational studies regardless of the type of muscular disorders.
Acknowledgements
This work was supported by the Japan Society for the Promotion of Science Grant-in-Aid for Scientific Research (C) [grant no. 18K07544 to Y.A.], Grants-in-Aid for Research on Nervous and Mental Disorders [grant no. 28-6 to Y.A.], and the Japan Agency for Medical Research and Development [grant nos. 18ek0109239h0002, 18lm0203066h0001, and 18lm0203069h0001 to Y.A.].
Materials
| Name | Company | Catalog Number | Comments |
| 1% P/S Solution Stabilized | Thermo Fisher | 15070-063 | Cell culture |
| Amphotericin B | Sigma Aldrich | A2942 | |
| Anti-dystrophin | Abcam | ab15277 | Western blot (WB) |
| Anti-dystrophin | Leica | NCL-DYS1 | Immunocytochemistry(ICC) |
| Anti-mouse IgG, Dylight 488 | Vector Laboratories | DK-2488 | ICC |
| Anti-α-tubulin | Sigma | T6199 | Western bot and ICC |
| BZ-X800 | KEYENCE | BZ-800 | Fluorescent microscope |
| CELLBANKER | ZENOAQ | CB011 | Cell stock in liquid nitrogen |
| ChemiDoc MP Imaging System | Bio-Rad | 170-8280J1 | WB |
| CloneAmp HiFi PCR premix | Clontech | 639298 | Retroviral production |
| cOmplete Protease Inhibitor Cocktail | Roche | 4693116001 | Protein extraction for WB |
| E.coli DH5 α Competent Cells | TAKARA | 9057 | |
| ECL Prime Western Blotting Detection Reagent | GE healthcare | RPN2232 | WB |
| EGF | Peprotech | AF-100-15 | Cell culture |
| Endo-Porter | GeneTools | 2922498000 | ASO transfection |
| Extra Thick Blot Filter Paper | Bio-Rad | 1703965 | WB |
| EzFastBlot HMW | Atto | AE-1460 | WB |
| fibroblast growth factor-basic | Sigma-Aldrich | F0291 | Cell culture |
| Glutamax | Thermo Fisher Scientific | 35050-061 | Cell culture |
| GP2-293 packaging cells | Clontech | 631458 | Retroviral production |
| Ham's F-12 Nutrient Mix | Thermo Fisher Scientific | 11765-054 | Cell culture |
| High glucose DMEM with GlutaMAX-I | Thermo Fisher Scientific | 10569-010 | Cell culture |
| High glucose DMEM without sodium pyruvate | GE Healthcare | SH30022.01 | Cell culture |
| HiSpeed Plasmid Purification Kit | QIAGEN | 12643 | Retroviral production |
| Histofine Simple Stain MAX PO | NICHIREI BIOSCIENCE INC. | 424151 | WB |
| Hoechst 33342 | Thermo Fisher Scientific | H3570 | ICC |
| Human PDGF-AB | Peprotech | 100-00AB-10UG | Cell culture |
| iBind Flex Solution | Thermo Fisher Scientific | SLF2020 | WB |
| iBind Flex Western Device | Thermo Fisher Scientific | SLF2000 | WB (Automated mestern-processing device) |
| Immobilon-P Transfer Membrane (PVDF) | MERCK | IPVH304F0 | WB |
| In-Fusion HD cloning Kit | Clontech | 639648 | Retroviral production |
| ITS Liquid Media Supplement | Sigma-Aldrich | I3146 | Cell culture |
| MILTEX HV 0.45 μm filter | MERCK | SLHV033RS | Retroviral production |
| MultiNA | SHIMADZU | MCE-202 | Microchip electrophoresis |
| MYOD1 (GFP-tagged) | ORIGENE | RG209108 | Retroviral production |
| NanoDrop | Thermo Fisher | ND-ONE-W | Spectrophotometer |
| Nonessential amino acids | Thermo Fisher | 11140-050 | Cell culture |
| NucleoSpin Gel and PCR Clean-Up Kit | Clontech | 740986.20 | PCR clean up |
| NuPAGE 3-8% Tris-Acetate Protein Gels | Invitrogen | EA03785BOX | WB |
| NuPAGE Antioxidant | Invitrogen | NP0005 | WB |
| NuPAGE LDS Sample Buffer | Invitrogen | NP0007 | WB |
| NuPAGE Sample Reducing Agent | Invitrogen | NP0009 | WB |
| NuPAGE Tris-Acetate SDS Running Buffer | Invitrogen | LA0041 | WB |
| One Step TB Green PrimeScript RT-PCR Kit | TAKARA | RR066A | Titer check of retroviral vector |
| PBS | Thermo Fisher Scientific | 14190-250 | Cell culture |
| Pierce BCA Protein Assay Kit | Thermo Scientific | 23227 | WB |
| Polybrene Infection / Transfection Reagent | Sigma-Aldrich | TR-1003 | Retroviral infection |
| pRetroX-TetOne-Puro Vector | Clontech | 634307 | Retroviral vecor |
| Puromycin | Clontech | 631305 | Cell culture |
| QIAGEN OneStep RT-PCR Kit | Qiagen | 210212 | PCR |
| REGM Bullet Kit | Lonza | CC-3190 | Material for growth medium of UDCs |
| REGM SingleQuots | Lonza | CC-4127 | Material for primary medium of UDCs |
| Retrovirus Titer Set | TAKARA | 6166 | Titer check of retroviral vector |
| Retro-X Concentrator | Clontech | 631455 | Retroviral production |
| RIPA buffer | Thermo Fisher Scientific | 89901 | WB |
| RNeasy kit | Qiagen | 74104 | RNA extraction for PCR |
| Tetracycline-free foetal bovine serum | Clontech | 631106 | Cell culture |
| Triton-X | MP Biomedicals | 9002-93-1 | ICC |
| Trypsin-EDTA (0.05%) | Gibco | 25300054 | Cell culture |
| XCell SureLock Mini-Cell | Invitrogen | EI0001 | WB |
| Xfect transfection reagent | Clontech | 631317 | Transfection of plasmids into packaging cells |
References
- Hoffman, E. P., Brown, R. H., Kunkel, L. M. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 51, 919-928 (1987).
- Cirak, S., et al. Exon skipping and dystrophin restoration in patients with Duchenne muscular dystrophy after systemic phosphorodiamidate morpholino oligomer treatment: an open-label, phase 2, dose-escalation study. Lancet. 378, 595-605 (2011).
- Komaki, H., et al. Systemic administration of the antisense oligonucleotide NS-065/NCNP-01 for skipping of exon 53 in patients with Duchenne muscular dystrophy. Science Translational Medicine. 10 (437), (2018).
- Antoury, L., et al. Analysis of extracellular mRNA in human urine reveals splice variant biomarkers of muscular dystrophies. Nature Communications. 9, 3906 (2018).
- Rahmoune, H., et al. Glucose transporters in human renal proximal tubular cells isolated from the urine of patients with non-insulin-dependent diabetes. Diabetes. 54, 3427-3434 (2005).
- Zhang, Y., et al. Urine derived cells are a potential source for urological tissue reconstruction. The Journal of Urology. 180, 2226-2233 (2008).
- Takizawa, H., et al. Modelling Duchenne muscular dystrophy in MYOD1-converted urine-derived cells treated with 3-deazaneplanocin A hydrochloride. Scientific Reports. 9, 3807 (2019).
- Zhou, T., et al. Generation of human induced pluripotent stem cells from urine samples. Nature Protocols. 7, 2080-2089 (2012).
- Chen, W., et al. Skeletal myogenic differentiation of human urine-derived cells as a potential source for skeletal muscle regeneration. Journal of Tissue Engineering and Regenerative Medicine. 11, 334-341 (2017).
- Kim, E. Y., Page, P., Dellefave-Castillo, L. M., McNally, E. M., Wyatt, E. J. Direct reprogramming of urine-derived cells with inducible MyoD for modeling human muscle disease. Skeletal Muscle. 6, 32 (2016).
This article has been published
Video Coming Soon
ABOUT JoVE
Copyright © 2024 MyJoVE Corporation. All rights reserved