Loop-Mediated Isothermal Amplification for Screening Salmonella in Animal Food and Confirming Salmonella from Culture Isolation
In This Article
Summary
Loop-mediated isothermal amplification (LAMP) is an isothermal nucleic acid amplification test (iNAAT) that has attracted broad interest in the pathogen detection field. Here, we present a multi-laboratory-validated Salmonella LAMP protocol as a rapid, reliable, and robust method for screening Salmonella in animal food and confirming presumptive Salmonella from culture isolation.
Abstract
Loop-mediated isothermal amplification (LAMP) has emerged as a powerful nucleic acid amplification test for the rapid detection of numerous bacterial, fungal, parasitic, and viral agents. Salmonella is a bacterial pathogen of worldwide food safety concern, including food for animals. Presented here is a multi-laboratory-validated Salmonella LAMP protocol that can be used to rapidly screen animal food for the presence of Salmonella contamination and can also be used to confirm presumptive Salmonella isolates recovered from all food categories. The LAMP assay specifically targets the Salmonella invasion gene (invA) and is rapid, sensitive, and highly specific. Template DNAs are prepared from enrichment broths of animal food or pure cultures of presumptive Salmonella isolates. The LAMP reagent mixture is prepared by combining an isothermal master mix, primers, DNA template, and water. The LAMP assay runs at a constant temperature of 65 °C for 30 min. Positive results are monitored via real-time fluorescence and can be detected as early as 5 min. The LAMP assay exhibits high tolerance to inhibitors in animal food or culture medium, serving as a rapid, reliable, robust, cost-effective, and user-friendly method for screening and confirming Salmonella. The LAMP method has recently been incorporated into the U.S. Food and Drug Administration’s Bacteriological Analytical Manual (BAM) Chapter 5.
Introduction
Loop-mediated isothermal amplification (LAMP) is a novel isothermal nucleic acid amplification test (iNAAT) invented in 2000 by a group of Japanese scientists1. Through the formation of a target-specific stem-loop DNA structure during initial steps, LAMP uses a strand-displacing DNA polymerase to efficiently amplify this starting material quasi-exponentially, resulting in 109 copies of target in less than 1 h1. Compared to polymerase chain reaction (PCR), a widely used NAAT, LAMP possesses several advantages. First, LAMP reactions are carried out under isothermal conditions. This obviates the need for a sophisticated thermal cycling instrument. Second, LAMP is highly tolerant to culture media and biological substances2 with robustness demonstrated for both clinical and food applications3,4. This simplifies sample preparation and minimizes false negative results5. Third, LAMP is amenable to multiple detection platforms, such as turbidity, colorimetry, bioluminescence, fluorescence, and microfluidics6. Fourth, LAMP is highly specific as it uses four to six specially designed primers to target six to eight specific regions1,7. Fifth, LAMP is ultrasensitive and numerous studies have reported its superior sensitivity to PCR or real-time PCR8. Finally, LAMP is faster with many assays now adopting a 30 min standard run time while PCR-type assays usually take 1−2 h8.
These attractive features fueled the application of LAMP in broad pathogen detection areas, including in vitro diagnostics9, animal disease diagnostics10, and food and environmental testing11. Notably, a TB-LAMP (LAMP for Mycobacterium tuberculosis) has been recommended by WHO as a valid replacement test for sputum-smear microscopy for pulmonary tuberculosis diagnoses in peripheral settings12. LAMP application also expands beyond microbial identification to include the detection of allergens, animal species, drug resistance, genetically modified organisms, and pesticides13.
Nontyphoidal Salmonella is a zoonotic pathogen of substantial food safety and public health concern worldwide14. It has also been identified as an important microbial hazard in food for animals (i.e., animal food)15,16. To prevent Salmonella illnesses/outbreaks from contaminated human food and animal food, it is imperative to have rapid, reliable, and robust methods for testing Salmonella in a variety of matrices. In the past decade, considerable efforts have been made internationally on the development and application of Salmonella LAMP assays in a wide array of food matrices, as recently summarized in an extensive review8. Several Salmonella LAMP assays, including the one presented here, have successfully completed multi-laboratory validation following well-established international guidelines17,18,19,20.
Our Salmonella LAMP assay specifically targets the Salmonella invasion gene invA (GenBank accession number M90846)21 and is rapid, reliable, and robust in multiple food matrices4,22,23,24,25,26. The method has been validated in six animal food matrices in a precollaborative study26 and in dry dog food in a multi-laboratory collaborative study19. As a result, the Salmonella LAMP method presented here has recently been incorporated into the U.S. Food and Drug Administration (FDA)’s Bacteriological Analytical Manual (BAM) Chapter 5 Salmonella27 to serve two purposes, one as a rapid screening method for the presence of Salmonella in animal food and two as a reliable confirmation method for presumptive Salmonella isolated from all foods.
Protocol
NOTE: A LAMP reaction mix contains DNA polymerase, buffer, MgSO4, dNTPs, primers, DNA template, and water. The first four reagents are contained in an isothermal master mix (Table of Materials). Primers are premixed in-house to become a primer mix (10x). DNA templates can be prepared from enrichment broths of animal food samples for screening purpose or from cultures of presumptive Salmonella isolates for confirmation purpose. In addition, a positive control (DNA extracted from any Salmonella reference strains, e.g., Salmonella enterica serovar Typhimurium ATCC 19585 [LT2]) and a no template control (NTC; sterile molecular grade water) are included in every LAMP run.
1. Preparation of DNA templates
- To prepare DNA templates from animal food enrichments, follow these steps.
- Aseptically weigh 25 g of animal food sample (e.g., dry cat food, dry dog food, cattle feed, horse feed, poultry feed, and swine feed) into a sterile filter bag (Table of Materials), or equivalent. Place the bag into a large container or rack for support during incubation.
- Add 225 mL of sterile buffered peptone water (BPW). Mix well by swirling and brief hand-massage. Let stand at room temperature for 60 ± 5 min.
- Mix well by swirling and determine pH with a test paper. Adjust pH, if necessary, to 6.8 ± 0.2 with sterile 1 N NaOH or 1 N HCl. Incubate at 35 ± 2 °C for 24 ± 2 h.
- Mix well by swirling the bag containing animal food enrichment broths. Transfer 1 mL from the filtered side of the bag to a microcentrifuge tube. Vortex briefly.
- Extract DNA using a sample preparation reagent (Table of Materials) as follows.
- Centrifuge at 900 x g for 1 min to remove large particles and transfer supernatant to a new microcentrifuge tube.
- Centrifuge at 16,000 x g for 2 min and discard supernatant.
- Suspend the pellet in 100 µL of the sample preparation reagent and heat at 100 ± 1 °C for 10 min in a dry heat block.
- Cool to room temperature and store sample DNA extracts at -20 °C.
- To prepare DNA templates from presumptive Salmonella cultures, follow these steps.
- Obtain presumptive Salmonella isolates from culture isolation in all foods following FDA’s BAM Chapter 5 Salmonella section D: Isolation of Salmonella27.
- Inoculate presumptive Salmonella isolates on a nonselective agar plate (e.g., blood agar, nutrient agar, and trypticase soy agar) and incubate at 35 ± 2 °C for 24 ± 2 h.
- Transfer several single colonies to 5 mL of trypticase soy broth (TSB) or brain heart infusion (BHI) broth and incubate at 35 ± 2 °C for 16 ± 2 h.
NOTE: This step can be optional if the presumptive Salmonella culture is pure. In that case, DNA templates can be prepared by suspending several single colonies in 5 mL of TSB and heat 500 μL of the suspension at 100 ± 1 °C for 10 min in a dry heat block. Continue with step 5 below. - Transfer 500 µL of the overnight culture to a microcentrifuge tube and heat at 100 ± 1 °C for 10 min in a dry heat block.
- Cool to room temperature and store isolate DNA extracts at -20 °C.
- To prepare positive control DNA, follow similar steps as above for preparing DNA templates from presumptive Salmonella cultures with one extra dilution step.
- Inoculate S. Typhimurium ATCC 19585 (LT2) or any Salmonella reference strains on a nonselective agar plate (e.g., blood agar, nutrient agar, and trypticase soy agar) and incubate at 35 ± 2 °C for 24 ± 2 h.
- Transfer several single colonies to 5 mL of TSB or BHI broth and incubate at 35 ± 2 °C for 16 ± 2 h to reach ~109 CFU/mL.
- Serially dilute the overnight culture in 0.1% peptone water to obtain ~107 CFU/mL.
- Transfer 500 µL of this dilution to a microcentrifuge tube and heat at 100 ± 1 °C for 10 min in a dry heat block.
- Cool to room temperature and store positive control DNA at -20 °C.
2. Preparation of primer mix (10x)
- Obtain commercially synthesized LAMP primers (Sal4-F3, Sal4-B3, Sal4-FIP, Sal4-BIP, Sal4-LF, and Sal4-LB) with standard desalting purification (Table 1).
| Primer name | Description | Sequence (5´-3´) | Length (bp) |
| Sal4-F3 | Forward outer primer | GAACGTGTCGCGGAAGTC | 18 |
| Sal4-B3 | Backward outer primer | CGGCAATAGCGTCACCTT | 18 |
| Sal4-FIP | Forward inner primer | GCGCGGCATCCGCATCAATA-TCTGGATGGTATGCCCGG | 38 |
| Sal4-BIP | Backward inner primer | GCGAACGGCGAAGCGTACTG-TCGCACCGTCAAAGGAAC | 38 |
| Sal4-LF | Loop forward primer | TCAAATCGGCATCAATACTCA-TCTG | 25 |
| Sal4-LB | Loop backward primer | AAAGGGAAAGCCAGCTTTACG | 21 |
Table 1: LAMP primers for screening Salmonella in animal food and confirming Salmonella from culture isolation. The primers are designed based on the Salmonella invA sequence (GenBank accession number M90846).
- Prepare stock solutions of each primer (100 µM) by rehydrating the primer with appropriate amount of sterile molecular grade water. Mix well by vortexing for 10 s and store at -20 °C (-80 °C for long-term storage).
- Prepare the primer mix (10x) according to a worksheet (Table 2). Add appropriate volumes of primer stock solutions and sterile molecular grade water into a microcentrifuge tube. Mix all reagents well by vortexing for 10 s.
| Component | Stock conc. (µM) | Primer mix conc. (µM) | Volume (µL) |
| Sal4-F3 primer | 100 | 1 | 10 |
| Sal4-B3 primer | 100 | 1 | 10 |
| Sal4-FIP primer | 100 | 18 | 180 |
| Sal4-BIP primer | 100 | 18 | 180 |
| Sal4-LF primer | 100 | 10 | 100 |
| Sal4-LB primer | 100 | 10 | 100 |
| Molecular grade water | N/A | N/A | 420 |
| Total | N/A | N/A | 1000 |
Table 2: Worksheet for preparing the LAMP primer mix (10x). The primers are listed in Table 1.
- Aliquot the 10x primer mix to 500 µL per microcentrifuge tube and store at -20 °C.
3. Assembly of a LAMP reaction
NOTE: To prevent cross-contamination, it is highly recommended to physically separate the areas used for preparing the LAMP master mix and adding DNA templates. Figure 1 is a LAMP diagram.
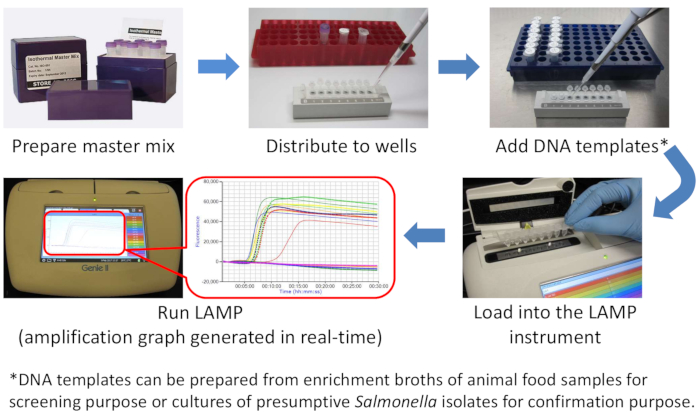
Figure 1: A schematic diagram of the Salmonella LAMP workflow. Please click here to view a larger version of this figure.
- Preparation and run setup
- Clean bench with isopropanol and a DNA- and DNase-degrading solution (Table of Materials). Clean pipettes and tube strip holders (Table of Materials) with the DNA- and DNase-degrading solution.
- Thaw the isothermal master mix, primer mix (10x), molecular grade water, positive control DNA, and DNA templates at room temperature.
- Turn on the LAMP instrument (Table of Materials) and tap the opening screen to access the home screen. Follow these steps to create a run.
NOTE: One model of the LAMP instrument has 2 blocks (A and B) with 8 samples in each block and another model has a single block that accommodates 8 samples (Table of Materials).- Tap LAMP+Anneal and select Edit to enter sample information.
NOTE: The default LAMP run profile consists of amplification at 65 °C for 30 min and an anneal phase from 98 °C to 80 °C with 0.05 °C decrement per sec. - Tap each sample row to activate the cursor and enter relevant sample information, using the AB block icon to switch between the two LAMP instrument blocks.
- Tap the Check icon when all sample information has been entered.
NOTE: Optionally, the run setup (termed “Profile,” which contains sample information along with the default LAMP run profile) may be saved for later use. Tap the Save icon and give the profile a unique name. When testing this same set of samples next time, a new run can be initiated using the saved profile. Tap the Folder icon at the bottom left of the home screen and select Profile to load saved profiles.
- Tap LAMP+Anneal and select Edit to enter sample information.
- LAMP reaction assembly
NOTE: When using both LAMP instrument blocks (A and B, a total of 16 samples), prepare the LAMP master mix for 18 samples. If using only one LAMP instrument block (8 samples total), prepare the LAMP master mix for 10 samples. For other sample numbers, adjust the volume accordingly to accommodate pipetting loss. Always include a positive control and an NTC in every LAMP run. Duplicate testing of each sample in independent LAMP runs is recommended.- Prepare the LAMP master mix according to a worksheet (Table 3). Add appropriate volumes of the isothermal master mix, primer mix, and molecular grade water into a microcentrifuge tube and vortex gently for 3 s. Centrifuge briefly.
- Place the tube strip in the strip holder and distribute 23 µL of the LAMP master mix to each well.
- Vortex all DNA templates and centrifuge briefly. Add 2 µL of DNA template to the appropriate well and cap tightly.
- Remove the tube strip from the holder and flick wrist to ensure all reagents have pooled at the bottom of the tube.
- Load the tube strip into the LAMP instrument block(s), ensuring caps are secure before closing the lid.
| Component | Working conc. | Final reaction conc. | Volume per sample (µL) | Volume for 18 samples (µL) | Volume for 10 samples (µL) |
| ISO-001 isothermal master mix | 1.67x | 1x | 15 | 270 | 150 |
| Primer mix | 10x | 1x | 2.5 | 45 | 25 |
| Molecular grade water | N/A | N/A | 5.5 | 99 | 55 |
| Master mix subtotal | N/A | N/A | 23 | 414 | 230 |
| DNA template | N/A | N/A | 2 | N/A | N/A |
Table 3: Worksheet for preparing the LAMP reaction mix. The primer mix (10x) is prepared according to Table 2 using stock solutions of primers listed in Table 1.
4. LAMP Run
NOTE: During a LAMP run, fluorescence readings are acquired using the FAM channel. The time-to-peak values (Tmax; min) are determined automatically by the instrument for the time point when fluorescence ratio reaches the maximum value of the amplification rate curve. The Tm (°C) is the melting/annealing temperature of the final amplified product.
- Click on the Run icon at the upper right of the screen and select the block(s) containing tube strip(s) to start the LAMP run.
- Optionally, while the reaction is in progress, tap the Temperature, Amplification, and Anneal tabs to see dynamic changes of various parameters during the LAMP run.
- Once the run is complete, tap the Amplification and Anneal tabs to see complete amplification and anneal curves and tap the Results tab to view the results.
- Optionally, for record keeping, record the run number located at the top left of the screen, using the format of “instrument serial number_run number,” e.g., “GEN2-2209_0030.”
5. Interpretation of LAMP Results
NOTE: LAMP results can be viewed on the LAMP instrument panel directly and/or using a LAMP software (Table of Materials).
- To interpret LAMP results on the instrument panel, follow these steps.
- Tap the Folder icon at the bottom left of the home screen and select Log to navigate to the file location to load the LAMP run of interest.
NOTE: The LAMP runs are organized by date, starting with year. - Observe the five tabs associated with each run: Profile, Temperature, Amplification, Anneal, and Results.
NOTE: The Profile and Temperature tabs show programmed and actual temperatures, respectively, in the sample wells as the LAMP reaction proceeds. The Amplification and Anneal tabs show fluorescence readings and changes in fluorescence during the amplification and anneal phases, respectively. The Results tab shows a tabular view of the LAMP results. - Tap the Results tab to observe LAMP results for each well.
NOTE: There are three columns (Well, Amplification, and Anneal). The “Amplification” column shows the time-to-peak values (Tmax; min:sec) for each sample (“Well”) and the “Anneal” column shows the melting/annealing temperatures (Tm; °C) for any amplified product in that well. - Interpret the LAMP results and report final LAMP results as follows.
- Examine the control wells first. The NTC well should have blank Tmax while Tm can be either blank (both LAMP instrument models) or < 83 °C (only for the LAMP instrument model with two blocks). The positive control well should have Tmax between 5 and 10 min and Tm around 90 °C.
- Examine the sample wells. All samples with the correct Tm (approximately 90 °C) and Tmax (between 5−30 min) are considered positive for Salmonella.
- Report final LAMP results based on results from duplicate runs. If the duplicate runs have consistent results, final LAMP results can be reported. If duplicate runs are inconsistent, repeat both runs independently. If results are still inconsistent, the sample should be considered presumptive positive for Salmonella and will need to go through culture confirmation.
- Tap the Folder icon at the bottom left of the home screen and select Log to navigate to the file location to load the LAMP run of interest.
- To interpret LAMP results using the software, follow these steps.
- Click on the Computer icon on the left panel and navigate to the file location to load the LAMP run of interest.
NOTE: The computer with the software installed does not need to be connected to the LAMP instrument to analyze LAMP results, i.e., remote access is available. The LAMP runs are organized by date. - Observe the seven tabs associated with each run: Profile, Temperature, Amplification, Amplification Rate, Anneal, Anneal Derivative, and Result.
NOTE: Similar to the instrument panel view, the Profile and Temperature tabs show programmed and actual temperatures, respectively, in the sample wells as the LAMP reaction proceeds. The Amplification/Amplification Rate and Anneal/Anneal Derivative tabs show fluorescence readings or changes in fluorescence during the amplification and anneal phases, respectively. The Results tab shows a tabular view of the LAMP results which differ slightly from the instrument panel view. - Tap the Amplification Rate tab to view a graphic display of the fluorescence ratios by time. Click on the Setting icon at the top right of the screen and adjust the “Peak Detection Threshold Ratio” from 0.020 to 0.010.
NOTE: The adjustment is necessary to ensure that all valid peaks are identified, and the results obtained using the software match with those displayed on the instrument panel. - Tap the Result tab to observe LAMP results for each well.
NOTE: There are four columns (Graph Name, Well Number, Well Name, and Peak Value). The top portion of the “Peak Value” column shows “Amp Time” (Tmax; min:sec) for each sample (“Well Name”) while the bottom portion shows “Anneal Derivative” (Tm; °C) for any amplified product in that well. - Interpret the LAMP results and report final LAMP results following similar steps as when using the instrument panel with one exception that the NTC well and other negative samples should have blank Tm as the LAMP software settings eliminate those Tm < 83 °C results. Similarly, all samples with the correct Tm (approximately 90 °C) and Tmax (between 5−30 min) are considered positive for Salmonella.
- Click on the Computer icon on the left panel and navigate to the file location to load the LAMP run of interest.
Representative Results
Figure 2 and Figure 3 show representative LAMP graphs/tables displayed on both platforms. In this LAMP run, samples S1 to S6 are 10-fold serial dilutions of S. enterica serovar Infantis ATCC 51741 ranging from 1.1 x 106 CFU to 11 CFU per reaction. Positive control is S. enterica serovar Typhimurium ATCC 19585 (LT2) at 1.7 x 104 CFU per reaction and NTC is molecular grade water.
As shown in Figure 2E and Figure 3G, both NTC and PC wells are valid controls. The NTC well has blank Tmax while Tm is < 83 °C on the LAMP instrument panel and blank in the LAMP software, suggesting a negative result. The PC well has Tmax of 7 min 45 sec and Tm of ~ 90 °C on both platforms, suggesting a positive result. Samples S1 to S6 have Tmax between 6 min 30 sec and 12 min 15 sec, all being Salmonella-positive.
Following duplicate runs of the same set of samples, the final LAMP results are reported for these samples. This representative LAMP run shows that LAMP successfully detects Salmonella with a wide range of concentrations in the samples.
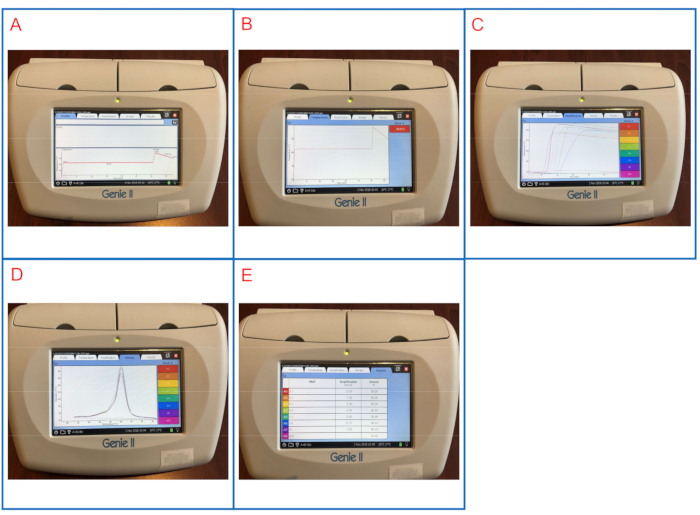
Figure 2: Representative LAMP results displayed on the LAMP instrument panel. (A) The Profile tab shows the programmed temperature profile. (B) The Temperature tab shows actual temperatures in the sample wells as LAMP reaction proceeds. (C) The Amplification tab shows fluorescence readings during LAMP amplification. (D) The Anneal tab shows changes in fluorescence (derivative) during the anneal phase. (E) The Results tab shows a tabular view of the LAMP results. Please click here to view a larger version of this figure.
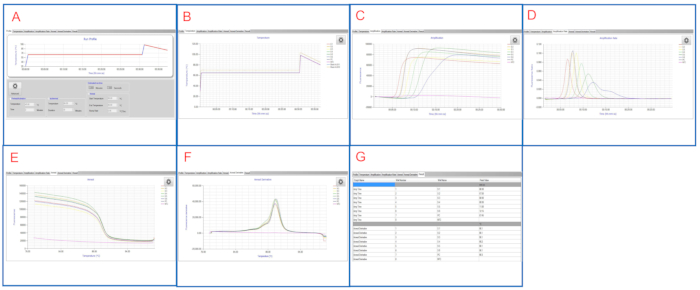
Figure 3: Representative LAMP results viewed in the LAMP software. (A) The Profile tab shows the programmed temperature profile. (B) The Temperature tab shows actual temperatures in the sample wells as LAMP reaction proceeds. (C) The Amplification tab shows fluorescence readings during LAMP amplification. (D) The Amplification Rate tab shows changes in fluorescence (fluorescence ratio) during LAMP amplification. (E) The Anneal tab shows fluorescence readings during the anneal phase. (F) The Anneal Derivative tab shows changes in fluorescence (derivative) during the anneal phase. (G) The Result tab shows a tabular view of the LAMP results. Please click here to view a larger version of this figure.
Discussion
We have presented here a simple, rapid, specific, and sensitive LAMP method for screening and confirming Salmonella in animal food and pure culture, respectively. With the convenience of an isothermal master mix that contains four key reagents, and a ready-to-use, in-house prepared primer mix, assembling a LAMP reaction requires only a few pipetting steps (Figure 1). The total run time including amplification and anneal phases is less than 38 min (Figure 2A,B and Figure 3A,B). Positive results are monitored via real-time fluorescence (Figure 2C and Figure 3C,D) and can be detected as early as 5 min26. The anneal phase serves as an extra confirmation of LAMP specificity since only samples with correct Tm (around 90 °C) are reported as positive (Figure 2D,E and Figure 3E−G). Sensitivities of 1 Salmonella cell in pure culture and < 1 CFU/25 g in animal food have been reported previously26.
As LAMP is quite effective and generates a large quantity of DNA1, it is critical that best laboratory practices are used to prevent cross-contamination, which may include physically separating the areas for preparing the LAMP master mix and adding DNA templates, avoiding generating aerosols, using filter pipette tips, changing gloves often, and refraining from opening LAMP reaction tubes post-amplification.
The specificity of this Salmonella LAMP method was previously tested using 300 bacterial strains (247 Salmonella of 185 serovars and 53 non-Salmonella) and demonstrated to be 100% specific26. Notably, significant differences in Tmax were observed between the two Salmonella species, S. enterica and Salmonella bongori, and among S. enterica subspecies, especially subsp. arizonae (IIIa)26. Nonetheless, these were still valid positive results per the rules for interpreting LAMP results. In our multi-laboratory collaborative study in dry dog food which involved 14 analysts19, samples having inconsistent results in duplicate LAMP runs were occasionally observed. These usually involved samples with delayed positive results (Tmax > 15 min). Repeating both runs independently usually resolved the issue. More rarely, we observed samples with correct Tm but no or irregular Tmax values (< 5 min). This was usually caused by air bubbles in the reaction tube.
Throughout the lifecycle of LAMP method development, evaluation, precollaborative study, and multi-laboratory validation, we have observed high tolerance of LAMP to inhibitors in various animal food or food matrices and culture media4,19,22,23,24, highlighting the robustness of the method and collaborating numerous other studies on a global scale8. This is superior compared to PCR or real-time PCR, which usually requires an internal amplification control to ensure that negative results are not due to matrix inhibition28. Further, LAMP demonstrated similar (or superior) specificity and sensitivity compared to PCR or real-time PCR in the vast majority of studies8. The cost of LAMP reagents is at about $1 per reaction. The LAMP instruments used in this protocol are small, low-maintenance, and portable. They can handle any isothermal amplification method that employs target detection by fluorescence measurement, LAMP included. Using the LAMP software, comprehensive reports can be generated in multiple format (pdf, text, and image).
Method validation is a critical step before a new method can be adopted for routine use. It is noteworthy that the LAMP protocol reported here has successfully completed multi-laboratory validation19. With the recent incorporation of this LAMP protocol into the U.S. FDA’s BAM Chapter 5 Salmonella27, it is expected that the method will gain much wider use, both as a rapid screening method in animal food and as a reliable confirmation method for presumptive Salmonella isolates from all food categories.
Acknowledgements
The authors thank members of the FDA’s Microbiology Methods Validation Subcommittee (MMVS) and Bacteriological Analytical Manual (BAM) Council for critically reviewing Salmonella LAMP method validation studies.
Materials
| Name | Company | Catalog Number | Comments |
| Brain heart infusion (BHI) broth | BD Diagnostic Systems, Sparks, MD | 299070 | Liquid growth medium used in the cultivation of Salmonella. |
| Buffered peptone water (BPW) | BD Diagnostic Systems, Sparks, MD | 218105 | Preenrichment medium for the recovery of Salmonella from animal food samples. |
| DNA AWAY | Thermo Fisher Scientific, Waltham, MA | 7010 | Eliminates unwanted DNA and DNase from laboratory bench, glassware, and plasticware without affecting subsequent DNA samples. |
| Genie Explorer software | OptiGene Ltd., West Sussex, United Kingdom | Version 2.0.6.3 | Supports remote operation of Genie instruments including LAMP runs and data analysis. |
| Genie II or Genie III (LAMP instrument) | OptiGene Ltd., West Sussex, United Kingdom | GEN2-02 or GEN3-02 | A small instrument capable of temperature control up to 100 °C with ± 0.1 °C accuracy and simultaneous fluorescence detection via the FAM channel. Genie II has 2 blocks (A and B) with 8 samples in each block. Genie III has a single block that accommodates 8 samples. |
| Genie strip | OptiGene Ltd., West Sussex, United Kingdom | OP-0008 | 8-well microtube strips with integral locking caps and a working volume of 10 to 150 µl. |
| Genie strip holder | OptiGene Ltd., West Sussex, United Kingdom | GBLOCK | Used to hold Genie strips when setting up a LAMP reaction, the aluminum holder can also be used as a cool block. |
| Hydrochloric acid (HCl) solution, 1 N | Thermo Fisher Scientific, Waltham, MA | SA48-500 | Adjusts pH of animal food samples after adding BPW and prior to overnight enrichment. |
| Heat block | Thermo Fisher Scientific, Waltham, MA | 88-860-022 | Heats samples at 100 ± 1 oC for DNA extraction. |
| Incubator | Thermo Fisher Scientific, Waltham, MA | 3960 | Standard laboratory incubator. |
| ISO-001 isothermal master mix | OptiGene Ltd., West Sussex, United Kingdom | ISO-001 | An optimized master mix to simplify the assembly of a LAMP reaction, containing a strand-displacing GspSSD DNA polymerase large fragment from Geobacillus spp., thermostable inorganic pyrophosphatase, reaction buffer, MgSO4, dNTPs, and a double-stranded DNA binding dye (FAM detection channel). |
| Isopropanol | Thermo Fisher Scientific, Waltham, MA | A416 | Disinfects work surfaces. |
| LAMP primers | Integrated DNA Technologies Inc., Coralville, IA | Custom | LAMP primers with detailed information in Table 1. |
| Microcentrifuge | Eppendorf North America, Hauppauge, NY | 22620207 | MiniSpin plus personal microcentrifuge. |
| Microcentrifuge tubes | Thermo Fisher Scientific, Waltham, MA | 05-408-129 | Standard microcentrifuge tubes. |
| Molecular grade water | Thermo Fisher Scientific, Waltham, MA | AM9938 | Used in making primer stocks, primer mix, and LAMP reaction mix. |
| Sodium hydroxide (NaOH) solution, 1 N | Thermo Fisher Scientific, Waltham, MA | SS266-1 | Adjusts pH of animal food samples after adding BPW and prior to overnight enrichment. |
| Nonselective agar (e.g., blood agar, nutrient agar, and trypticase soy agar) | Thermo Fisher Scientific, Waltham, MA | R01202 | Solid growth medium used in the cultivation of Salmonella. |
| Peptone water | BD Diagnostic Systems, Sparks, MD | 218071 | Dilutes overnight Salmonella cultures to make positive control DNA. |
| Pipettes and tips | Mettler-Toledo Rainin LLC, Oakland CA | Pipet Lite LTS series | Standard laboratory pipettes and tips. |
| PrepMan Ultra sample preparation reagent | Thermo Fisher Scientific, Waltham, MA | 4318930 | A simple kit used for the rapid preparation of DNA templates for use in a LAMP reaction. |
| Salmonella reference strain LT2 | ATCC, Manassas, VA | 700720 | Salmonella reference strain used as positive control. |
| Trypticase soy broth (TSB) | BD Diagnostic Systems, Sparks, MD | 211768 | Liquid growth medium used in the cultivation of Salmonella. |
| Vortex mixer | Scientific Industries, Inc., Bohemia, NY | SI-0236 | Standard laboratory vortex mixer. |
| Whirl-pak filter bag | Nasco Sampling Brand, Fort Atkinson, WI | B01318 | Filter bags to hold animal food samples for preenrichment. |
References
- Notomi, T., et al. Loop-mediated isothermal amplification of DNA. Nucleic Acids Research. 28 (12), 63 (2000).
- Kaneko, H., Kawana, T., Fukushima, E., Suzutani, T. Tolerance of loop-mediated isothermal amplification to a culture medium and biological substances. Journal of Biochemical and Biophysical Methods. 70 (3), 499-501 (2007).
- Francois, P., et al. Robustness of a loop-mediated isothermal amplification reaction for diagnostic applications. FEMS Immunology and Medical Microbiology. 62 (1), 41-48 (2011).
- Yang, Q., Wang, F., Prinyawiwatkul, W., Ge, B. Robustness of Salmonella loop-mediated isothermal amplification assays for food applications. Journal of Applied Microbiology. 116 (1), 81-88 (2014).
- Nagamine, K., Watanabe, K., Ohtsuka, K., Hase, T., Notomi, T. Loop-mediated isothermal amplification reaction using a nondenatured template. Clinical Chemistry. 47 (9), 1742-1743 (2001).
- Zhang, X., Lowe, S. B., Gooding, J. J. Brief review of monitoring methods for loop-mediated isothermal amplification (LAMP). Biosensors & Bioelectronics. 61, 491-499 (2014).
- Nagamine, K., Hase, T., Notomi, T. Accelerated reaction by loop-mediated isothermal amplification using loop primers. Molecular and Cellular Probes. 16 (3), 223-229 (2002).
- Yang, Q., Domesle, K. J., Ge, B. Loop-mediated isothermal amplification for Salmonella detection in food and feed: Current applications and future directions. Foodborne Pathogens and Disease. 15 (6), 309-331 (2018).
- Mori, Y., Notomi, T. Loop-mediated isothermal amplification (LAMP): Expansion of its practical application as a tool to achieve universal health coverage. Journal of Infection and Chemotherapy. 26 (1), 13-17 (2020).
- Mansour, S. M., Ali, H., Chase, C. C., Cepica, A. Loop-mediated isothermal amplification for diagnosis of 18 World Organization for Animal Health (OIE) notifiable viral diseases of ruminants, swine and poultry. Animal Health Research Reviews. 16 (2), 89-106 (2015).
- Kumar, Y., Bansal, S., Jaiswal, P. Loop-mediated isothermal amplification (LAMP): A rapid and sensitive tool for quality assessment of meat products. Comprehensive Reviews in Food Science and Food Safety. 16 (6), 1359-1378 (2017).
- Kundapur, R. R., Nema, V. Loop-mediated isothermal amplification: Beyond microbial identification. Cogent Biology. 2, 1137110 (2016).
- . Compliance Policy Guide Sec. 690.800 Salmonella in Food for Animals Available from: https://www.fda.gov/downloads/iceci/compliancemanuals/compliancepolicyguidancemanual/ucm361105.pdf (2013)
- Bird, P., et al. Evaluation of the 3M molecular detection assay (MDA) 2 - Salmonella for the detection of Salmonella spp. in select foods and environmental surfaces: collaborative study, first action 2016.01. Journal of AOAC International. 99 (4), 980-997 (2016).
- D'Agostino, M., et al. Validation of a loop-mediated amplification/ISO 6579-based method for analysing soya meal for the presence of Salmonella enterica. Food Analytical Methods. 9 (11), 2979-2985 (2016).
- Ge, B., et al. Multi-laboratory validation of a loop-mediated isothermal amplification method for screening Salmonella in animal food. Frontiers in Microbiology. 10, 562 (2019).
- D'Agostino, M., Diez-Valcarce, M., Robles, S., Losilla-Garcia, B., Cook, N. A loop-mediated isothermal amplification-based method for analysing animal feed for the presence of Salmonella. Food Analytical Methods. 8 (10), 2409-2416 (2015).
- Galan, J. E., Ginocchio, C., Costeas, P. Molecular and functional characterization of the Salmonella invasion gene invA: homology of InvA to members of a new protein family. Journal of Bacteriology. 174 (13), 4338-4349 (1992).
- Chen, S., Wang, F., Beaulieu, J. C., Stein, R. E., Ge, B. Rapid detection of viable salmonellae in produce by coupling propidium monoazide with loop-mediated isothermal amplification. Applied and Environmental Microbiology. 77 (12), 4008-4016 (2011).
- Yang, Q., Chen, S., Ge, B. Detecting Salmonella serovars in shell eggs by loop-mediated isothermal amplification. Journal of Food Protection. 76 (10), 1790-1796 (2013).
- Yang, Q., et al. Evaluation of loop-mediated isothermal amplification for the rapid, reliable, and robust detection of Salmonella in produce. Food Microbiology. 46, 485-493 (2015).
- Yang, Q., Domesle, K. J., Wang, F., Ge, B. Rapid detection of Salmonella in food and feed by coupling loop-mediated isothermal amplification with bioluminescent assay in real-time. BMC Microbiology. 16 (1), 112 (2016).
- Domesle, K. J., Yang, Q., Hammack, T. S., Ge, B. Validation of a Salmonella loop-mediated isothermal amplification assay in animal food. International Journal of Food Microbiology. 264, 63-76 (2018).
- Bustin, S. A., et al. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clinical Chemistry. 55 (4), 611-622 (2009).
This article has been published
Video Coming Soon
ABOUT JoVE
Copyright © 2024 MyJoVE Corporation. All rights reserved