Use of an Integrated Low-Flow Anesthetic Vaporizer, Ventilator, and Physiological Monitoring System for Rodents
In This Article
Summary
Here, we present a protocol to safely and effectively administer anesthetic gas to mice using a digital, low flow anesthesia system with integrated ventilator and physiological monitoring modules.
Abstract
Low-flow digital vaporizers commonly utilize a syringe pump to directly administer volatile anesthetics into a stream of carrier gas. Per animal welfare recommendations, animals are warmed and monitored during procedures requiring anesthesia. Common anesthesia and physiological monitoring equipment include gas tanks, anesthetic vaporizers and stands, warming controllers and pads, mechanical ventilators, and pulse oximeters. A computer is also necessary for data collection and to run equipment software. In smaller spaces or when performing field work, it can be challenging to configure all this equipment in limited space.
The goal of this protocol is to demonstrate best practices for use of a low-flow digital vaporizer using both compressed oxygen and room air, along with an integrated mechanical ventilator, pulse oximeter, and far infrared warming as an all-inclusive anesthesia and physiological monitoring suite ideal for rodents.
Introduction
Research involving animal models often requires specialized data collection equipment. There are two common types of anesthetic vaporizer commonly used for small animal surgery. Traditional anesthetic vaporizers rely on the passive vaporization of volatile anesthetics based on atmospheric pressure and gas flow1,2,3,4,5,6,7,8,9,10. They are designed to operate at flow rates of 0.5 L/min to 10 L/min, making them ideal for large animal models11.
We recently demonstrated the effects of a low-flow digital vaporizer compared to a traditional vaporizer12,13. The low-flow digital anesthesia system can be used to maintain an animal on a nose cone at very low flow rates of 1.5-2.2 times the animal’s minute volume14,15,16.
There are numerous benefits to using a digital anesthesia system. It incorporates a built-in pump, which draws in ambient air to use as a carrier gas. This allows the user to administer anesthesia without the use of compressed gas. Recent studies17,18 have suggested that using air instead of oxygen as a carrier gas may be beneficial for many procedures.
Physiological monitoring and warming capabilities can also be installed into the digital low-flow anesthesia system. In most institutions, animal warming and physiological monitoring are required by Institutional Animal Care and Use Committees19,20,21,22. Studies comparing the physiological effects of anesthetic agents have shown a drastic depression of body temperature, cardiac function, and respiratory function23,24,25. Placing the animal on a warming pad to monitor and maintain a normal body temperature is often required. There are many methods of animal warming available, such as warm water heaters, electric heating pads, and heat lamps, but each of these have significant drawbacks. In studies comparing different methods of animal warming, far infrared warming has been found to be the most beneficial26. The digital vaporizer includes built in homeothermic far infrared warming to maintain a specific animal body temperature. This eliminates the needs for any additional warming pad controllers.
In addition to monitoring body temperature, pulse oximetry is a popular method of monitoring the animal’s heart rate and oxygen saturation. This noninvasive method is simple, accurate, and provides an overall assessment of the animal’s ability to regulate blood oxygenation levels. A paw sensor for pulse oximetry can be connected to the anesthesia system, as we have previously demonstrated2.
Mechanical ventilation is often required when the animal is under longer periods of anesthesia, or whenever the animal’s respiration pattern needs to be controlled. The low-flow digital vaporizer has the capability to deliver controlled breaths in either pressure- or volume-control. An integrated ventilator eliminates the need for an external ventilator and excess tubing setup requirements.
Because all these common monitors and features are combined into a single piece of equipment, the tubing setup is substantially simplified. The purpose of this protocol is to demonstrate the setup and use of an all-in-one digital anesthesia system.
Protocol
All animal studies were approved by the Purdue Animal Care and Use Committee.
1. Setup of the low-flow vaporizer
- Isoflurane or sevoflurane delivery
- Select a carrier gas source. To utilize the internal air pump, remove the red cap from the Inlet port on the back of the system, allowing the system to intake room air. To use compressed gas, use a pressure regulator or pressure reducer set to 15 PSI, and connect to the Compressed Gas port on the back of the system.
- Connect the charcoal canister to the exhaust port.
- Connect the Accessory Connector to the Inspiratory and Expiratory ports on the front of the system. Connect the induction chamber to branches with blue clips and the nose cone to branches with white clips (Figure 1).
- For mechanical ventilation
- Connect the intubation connector tubing to the yellow coded clips (Figure 2).
- Calibrate the ventilator by performing a deadspace calibration. From the Vent Run Screen, touch Setup, and then Calib & Tests. Select Deadspace Calibration and press Dial B.
- For pulse oximetry
- Connect the sensor to the port on the back of the system, labeled MouseSTAT.
- For warming
- Connect the warming pad to the ‘Pad Power’ port on the front of the system.
- Connect one sensor to the “Body Sensor’ port, and the other to the ‘Pad Sensor’ port. Secure the Pad Sensor to the warming pad.
2. Configure the settings
- For anesthesia
- Power on the anesthesia system. From the Anest Run Screen, touch Set Up.
- Choose the anesthetic agent. Touch Type Anest, and then turn Dial B to select Isoflurane or Sevoflurane.
- Set the syringe size. Touch Syringe Size, and then turn Dial B to select a size.
- Touch Back to return to the Anest Run Screen.
- Using the bottle top adapter, fill the syringe with anesthetic.
- Connect the syringe to the anesthesia system. Touch Remove to move the pusher block backwards if needed.
- Prime the syringe. Touch and hold Prime to move the pusher block forward until the pusher block touches the top of the syringe plunger. Turning B while holding the Prime button regulates the pusher block speed.
- For mechanical ventilation
- Touch the Vent Run Screen tab, and then Setup.
- Touch Body Weight and enter the weight of the animal.
- Touch Priority to choose volume or pressure-controlled ventilation. The Body Weight setting automatically sets appropriate respiratory rate and tidal volumes.
- For pulse oximetry
- Touch the Oxi Run Screen tab, and then Setup.
- Touch HR and turn Dial B to set the minimum allowed heart rate reading. Presets are available.
- For warming
- From the Warm Run Screen, touch Setup. Choose a warming method and target temperature setting.
3. Begin anesthesia delivery
- Anesthetize the mouse
- From the Anest Run Screen, touch Start Induction to begin airflow. The default Induction flow rate is 500 mL/min. Turning Dial A adjusts the flow rate as needed.
- Place the mouse in the induction chamber, closing the lid tightly. Adjust the Anesthetic Agent Concentration dial to 3% for isoflurane.
- Monitor until the mouse has reached the desired anesthetic plane, determined by a decrease in respiration rate and a loss of righting reflex when the chamber is tipped. Adjust the Anesthetic Agent Concentration dial as necessary.
- Once the animal has lost righting reflex and is sufficiently anesthetized, touch Stop Induction.
- If desired, touch Flush Chamber to empty the chamber of residual anesthetic gas.
- Open the clamps leading to the nose cone, and close the clamps leading to the chamber.
- Touch Start Nose Cone. The Body Weight setting determines the nose cone flow rate, though it can be manually adjusted by turning Dial A.
- Immediately fit the nose cone, and center the animal on the infrared warming pad.
- Insert the Animal Sensor as a rectal probe.
4. Begin mechanical ventilation
- Intubate the animal.
- Transfer the animal to the intubation stage while keeping the animal anesthetized.
- Suspend the animal from its upper incisors using a thread fixed onto the vertical intubation stage (Figure 3).
- Gently displace the animal’s tongue to the side and visualize the trachea using the lights provided in the intubation kit.
- Carefully insert tracheal tube and verify correct placement by connecting small air bladder to the tube and checking if the lungs inflate.
- Connect the endotracheal tube to the ventilation tubing.
- Touch Stop Nose Cone, and then touch Start Ventilator.
NOTE: The Body Weight setting automatically determines proper respiration rate and tidal volumes. To perform pressure-controlled ventilation, set the target inspiratory pressure between 15-18 cm H2O. Make adjustments to the ventilator settings as needed per surgical protocols.
5. Begin physiological monitoring
- Place the sensor over the hind paw of the animal (Figure 4). The Pulse Oximeter will begin reading HR and SpO2 automatically. Touch the Oxi Run Screen Tab to view pulse oximetry data.
Representative Results
Ten week old, male, wild type C57Bl6j mice weighing 25.41 ± 0.8 g were used for this study. The mice were anesthetized and maintained on a nose cone or intubated and maintained on an integrated mechanical ventilator with 1.5-2.5% isoflurane while heart rate and oxygen saturation were monitored. The animals were group-housed in microisolation caging and provided free-access to standard rodent chow and water by bottle.
Heart rate and SpO2 were monitored during maintenance via pulse oximetry (Figure 5, Figure 6, and Figure 7,). Body temperature was maintained at 36.5-37.5 °C via an infrared heating pad and heat lamp. Ventilated animals received continuous delivery of isoflurane during the intubation procedure via intubation stand with integrated nose cone. Each mouse was successfully ventilated or maintained on a nose cone at low flow rates not exceeding 141 mL/min of room air (RA) or oxygen (O2) for 15 minutes. The animals’ heart rates and blood oxygen saturation remained stable with few significant changes in either measurement for all groups. SpO2 remained between 82-99% for all groups, while body temperature was maintained between 36.5-37.5 °C. We observed that both position of the pulse-oximeter and body temperature influenced SpO2 measurements. If we observed an invalid reading from the pulse-oximeter, we adjusted the placement of the sensor and heating level to keep core body temperature stable.
A two-way ANOVA with a Bonferroni correction was performed to determine significance of data in Figure 5, Figure 6 and Figure 7. A p-value less than 0.05 was considered significant.
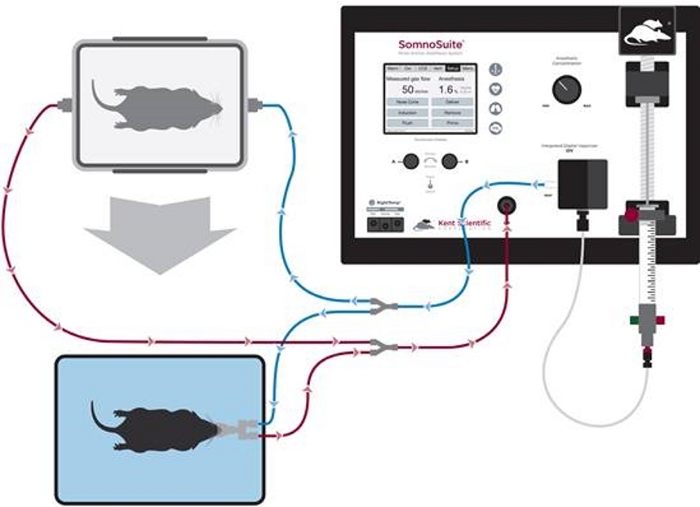
Figure 1: Diagram of tubing setup for anesthetic induction and nose cone maintenance. Please click here to view a larger version of this figure.
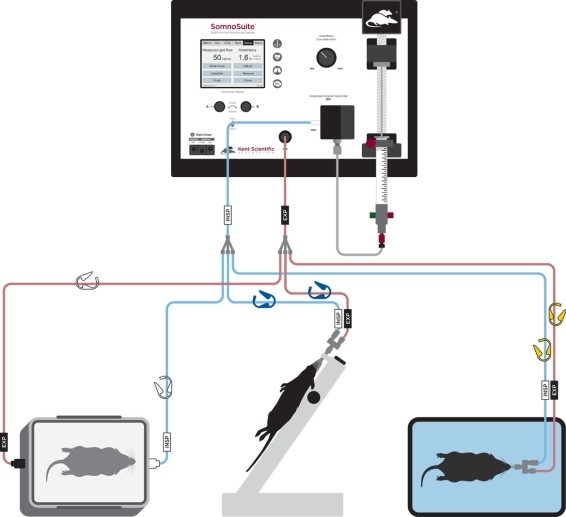
Figure 2: Diagram of tubing setup for anesthetic induction, intubation, and ventilation. Please click here to view a larger version of this figure.
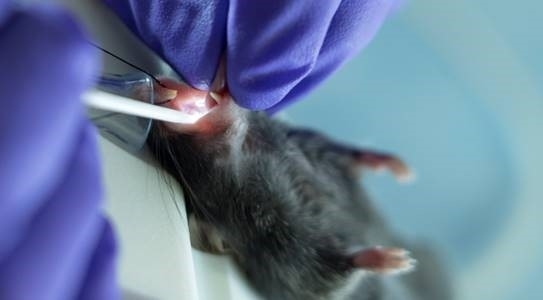
Figure 3: Mice received continuous delivery of isoflurane during the intubation procedure via an intubation stand with an integrated nose cone. Please click here to view a larger version of this figure.

Figure 4: Integrated pulse oximeter sensor placement over the hind paw. Please click here to view a larger version of this figure.
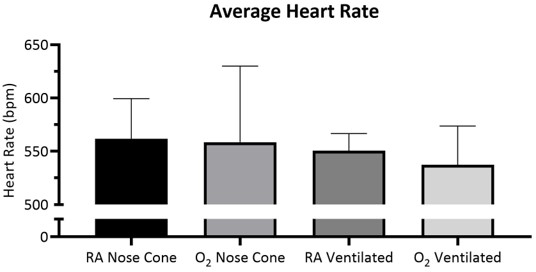
Figure 5: Average heart rate over 15 minutes ± SD with room air (RA) or 100% oxygen (O2) delivered through nose cone or ventilated through tracheal tube (n=5/group). No significant difference was observed between groups. Please click here to view a larger version of this figure.
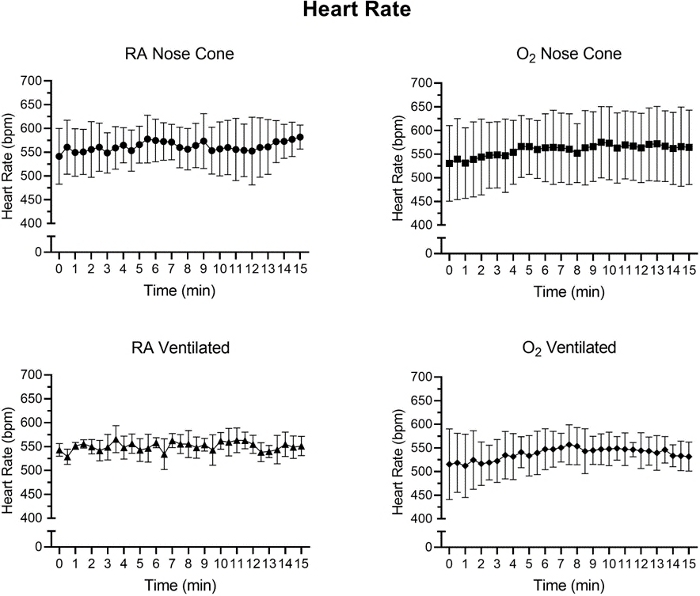
Figure 6: Heart rate values (bpm) recorded after initial anesthetic induction with the low flow anesthesia system. Average heart rate values calculated from 30-second time intervals over a 15-minute period. Each data point represents mean ± SD of all animals in each group (n=5). No significant changes in heart rate were observed over the 15-minute period in any group. Please click here to view a larger version of this figure.
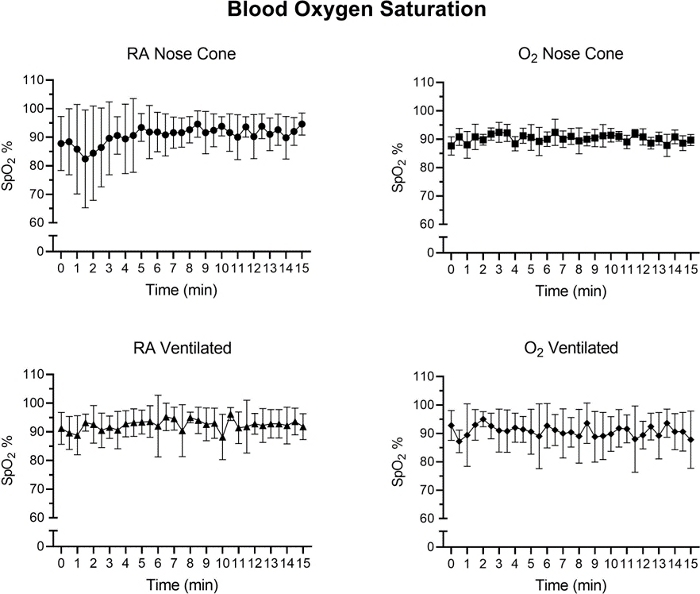
Figure 7: The tissue oxygen saturation levels (%) after initial anesthetic induction with the low flow anesthesia system. Average SpO2 values calculated from 30-second time intervals over a 15-minute period. Each data point represents mean ± SD of all animals in each group (n=5). No significant changes in SpO2 were observed over the 15-minute period in any group. Please click here to view a larger version of this figure.
Discussion
This digital low-flow anesthesia system integrates anesthesia, ventilation, warming, and physiological monitoring systems into a single piece of equipment. Additionally, the system contains an internal pump, allowing it to draw in ambient air for use as a carrier gas, eliminating the need for a source of compressed gas.
In this procedure, the system is used as a sole piece of equipment to replace an anesthetic vaporizer, mechanical ventilator, pulse oximeter, and warming pad. We previously demonstrated anesthetic delivery at a flow rate of 100mL/min2. The flow rate settings are critical for this anesthetic delivery technique, as the flow rate directly controls the volume of liquid anesthetic used. We also previously demonstrated how using low flow rates save anesthetic liquid1,2. When a traditional vaporizer is connected to a mechanical ventilator, the vaporizer must run continuously while the ventilator inlet samples from the gas stream. In the case of the digital vaporizer with integrated ventilator, only the gas necessary for ventilation is output by the ventilator. This reduces the costs associated with anesthetic liquid, carrier gases, and charcoal filters.
Though there are many advantages to using a low-flow digital vaporizer, there are limitations as well. This system is designed to operate at low flow rates ideal for rodents and other small mammals, but does not deliver anesthesia above flow rates of 1000 mL/min. This particular system is therefore only suitable for small animal species. The integrated pulse oximeter includes a sensor for paw use only. The sensor is not recommended for use on the tail, which may be a limitation for certain surgical procedures. Further, while respiration rate can be monitored through this system via the paw sensor, it can be difficult to obtaining consistent respiratory recordings over an extended period of time. Finally, unlike a traditional vaporizer, this digital system requires electricity. Batteries are available for use in instances where electrical power is unavailable or in the event of a power outage, and can power the system through several hours of usage.
This setup and protocol demonstrate safe and effective use of a digital, low flow anesthesia system with integrated ventilator and physiological monitoring modules. This setup will be useful for any laboratories with limited bench spaces, or where it is not feasible to house multiple pieces of equipment and tubing near a surgical field. There are numerous benefits to an all-in-one system, including the elimination of compressed gas tanks and separate physiological monitoring equipment. Overall, this integrated system could be considered by groups where use of a traditional vaporizer is not ideal.
Acknowledgements
The authors have no acknowledgments.
Materials
| Name | Company | Catalog Number | Comments |
| Intubation Kit | Kent Scientific Corporation | ETM-MSE | Includes intubation stage, intubation tube, LED light |
| Isoflurane Liquid Inhalation 99.9% | Henry Schein, Inc. | 1182097 | Glass bottle 250mL |
| MouseSTAT Pulse Oximeter | Kent Scientific Corporation | SS-03 | Integrated into SomnoSuite |
| Oxygen Tank | Indiana Oxygen Company | 23-160246 | Medical Grade O2 99% |
| RoVent Automatic Ventilator | Kent Scientific Corporation | SS-04 | Integrated into SomnoSuite |
| SomnoSuite Low Flow Digital Anesthesia System | Kent Scientific Corporation | SS-01 | Includes RightTemp Homeothermic Warming control, pad, and temperature sensors |
| SomnoSuite Mouse Starter Kit | Kent Scientific Corporation | SOMNO-MSEKIT | Includes nose cone, syringes, induction chamber, and charcoal canister |
References
- El-Attar, A. M. Guided isoflurane injection in a totally closed circuit. Anaesthesia. 46 (12), 1059-1063 (1991).
- Lockwood, G., Chakrabarti, M. K., Whitwam, J. G. A computer-controller closed anaesthetic breathing system. Anaesthesia. 48 (8), 690-693 (1993).
- Lowe, H. J., Cupic, M. Dose-regulated automated anesthesia (Abstract). British Journal of Clinical Pharmacologyl. 12 (2), 281-282 (1971).
- Walker, T. J., Chackrabarti, M. K., Lockwood, G. G. Uptake of desflurane during anaesthesia. Anaesthesia. 51 (1), 33-36 (1996).
- Weingarten, M., Lowe, H. J. A new circuit injection technic for syringe-measured administration of methoxyflurane: a new dimension in anesthesia. Anesthesia & Analgesia. 52 (4), 634-642 (1973).
- Enlund, M., Wiklund, L., Lambert, H. A new device to reduce the consumption of a halogenated anaesthetic agent. Anaesthesia. 56 (5), 429-432 (2001).
- Kelly, J. M., Kong, K. L. Accuracy of ten isoflurane vaporisers in current clinical use. Anaesthesia. 66 (8), 682-688 (2011).
- Matsuda, Y., et al. NARCOBIT - A newly developed inhalational anesthesia system for mice. Experimental Animals. 56 (2), 131-137 (2007).
- Soro, M., et al. The accuracy of the anesthetic conserving device (Anaconda) as an alternative to the classical vaporizer in anesthesia. Anesthesia & Analgesia. 111 (5), 1176-1179 (2010).
- Ward, C. S. . Physical principles and maintenance. Anaesthetic equipment. , (1985).
- Ambrisko, T. D., Klide, A. M. Evaluation of isoflurane and Sevoflurane vaporizers over a wide range of oxygen flow rates. American Journal of Veterinary Research. 67 (6), 936-940 (2006).
- Damen, F. W., Adelsperger, A. R., Wilson, K. E., Goergen, C. J. Comparison of traditional and integrated digital anesthetic vaporizers. Journal of the American Association for Laboratory Animal Science. 54 (6), 756-762 (2015).
- Adelsperger, A. R., Bigiarelli-Nogas, K. J., Toore, I., Goergen, C. J. Use of a Low-flow Digital Anesthesia System for Mice and Rats. Journal of Visualized Experiments. (115), e54436 (2016).
- Flecknell, P. . Laboratory animal anaesthesia. , (2009).
- Mapleson, W. W. The elimination of rebreathing in various semiclosed anaesthetic systems. British Journal of Anaesthesia. 26 (5), 323-332 (1954).
- Chakravarti, S., Basu, S. Modern Anaesthesia Vapourisers. Indian Journal of Anaesthesia. 57 (5), 464-471 (2013).
- Mullin, L., et al. Effect of anesthesia carrier gas on in vivo circulation times of ultrasound microbubble contrast agents in rats. Contrast Media & Molecular Imaging. 6 (3), 126-131 (2011).
- Flores, J. E., et al. The effects of anesthetic agent and carrier gas on blood glucose and tissue uptake in mice undergoing dynamic FDG-PET imaging: sevoflurane and isoflurane compared in air and in oxygen. Molecular Imaging and Biology. 10 (4), 192 (2008).
- Carroll, G. . Small Animal Anesthesia and Analgesia. , (2008).
- Thomas, J., Lerche, P. . Anesthesia and Analgesia for Veterinary Technicians, 4th ed. 335, (2011).
- McKelvey, D. H. . Veterinary Anesthesia and Analgesia. , (2003).
- Tranquilli, W. J., Thurmon, J. C., Grimm, K. A. . Lumb and Jones' veterinary anesthesia and analgesia. , 23-86 (2013).
- Matsuda, Y., et al. Comparison of newly developed inhalation anesthesia system and intraperitoneal anesthesia on the hemodynamic state in mice. Biological and Pharmaceutical Bulletin. 30 (9), 1716-1720 (2007).
- Garber, J., et al. . Guide for the Care and Use of Laboratory Animals. 8th edn. , (2011).
- Zarndt, B. S., et al. Use of a far-infrared active warming device in Guinea pigs (Cavia porcellus). Journal of the American Association for Laboratory Animal Science. 54 (6), 779-782 (2015).
- Wolforth, J., Dyson, M. C. Flushing induction chambers used for rodent anesthesia to reduce waste anesthetic gas. Lab Animal. 40 (3), 76-83 (2011).
This article has been published
Video Coming Soon
ABOUT JoVE
Copyright © 2024 MyJoVE Corporation. All rights reserved