A subscription to JoVE is required to view this content. Sign in or start your free trial.
Tools for the Real-Time Assessment of a Pseudomonas aeruginosa Infection Model
In This Article
Summary
Synthetic cystic fibrosis sputum medium (SCFM2) can be utilized in combination with both confocal laser scanning microscopy and fluorescence-activated cell sorting to observe bacterial aggregates at high resolution. This paper details methods to assess aggregate populations during antimicrobial treatment as a platform for future studies.
Abstract
Pseudomonas aeruginosa (Pa) is one of the most common opportunistic pathogens associated with cystic fibrosis (CF). Once Pa colonization is established, a large proportion of the infecting bacteria form biofilms within airway sputum. Pa biofilms isolated from CF sputum have been shown to grow in small, dense aggregates of ~10-1,000 cells that are spatially organized and exhibit clinically relevant phenotypes such as antimicrobial tolerance. One of the biggest challenges to studying how Pa aggregates respond to the changing sputum environment is the lack of nutritionally relevant and robust systems that promote aggregate formation. Using a synthetic CF sputum medium (SCFM2), the life history of Pa aggregates can be observed using confocal laser scanning microscopy (CLSM) and image analysis at the resolution of a single cell. This in vitro system allows the observation of thousands of aggregates of varying size in real time, three dimensions, and at the micron scale. At the individual and population levels, having the ability to group aggregates by phenotype and position facilitates the observation of aggregates at different developmental stages and their response to changes in the microenvironment, such as antibiotic treatment, to be differentiated with precision.
Introduction
Pseudomonas aeruginosa (Pa) is an opportunistic pathogen that establishes chronic infections in immune-compromised individuals. For those with the genetic disease cystic fibrosis (CF), these infections can span the course of a lifetime. CF causes the buildup of a viscous, nutrient-rich sputum in the airways, which becomes colonized by a variety of microbial pathogens over time. Pa is one of the most prevalent CF pathogens, colonizing the airways in early childhood and establishing difficult-to-treat infections1. Pa remains a significant clinical problem and is considered a leading cause of mortality in those with....
Protocol
1. Prepare synthetic cystic fibrosis medium (SCFM2)
NOTE: Preparation of SCFM2 comprises three main stages outlined below (Figure 2). For full details and references, see9,10,12.
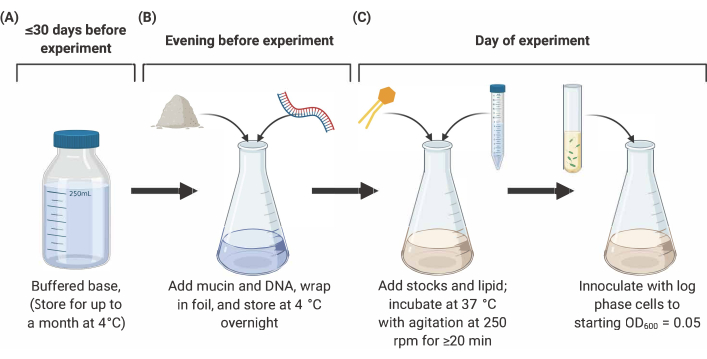
Figure 2.......
Representative Results
This work details methods to observe Pa aggregates at a high resolution and in an environment similar to that of chronic infection of the CF lung9,10,12. SCFM2 provides an in vitro system that promotes natural aggregation of Pa cells in sizes similar to those observed during actual infection10. The adaptability of SCFM2 as a defined medium can be leveraged to approach many resea.......
Discussion
This work has introduced methodologies that can be combined to study bacterial aggregate populations in the presence and absence of antibiotic treatment. High-resolution CLSM allows the visualization of changes in aggregate biomass and the structural orientation of aggregates over real time when exposed to antibiotics. In addition, physical and structural features of the biomass that remain after treatment with antibiotics can be quantified, with the goal to correlate these observations with future gene expression studie.......
Disclosures
The authors have no conflicts of interest.
Acknowledgements
S.E.D is supported by start-up funds provided by the Department of Molecular Medicine, The University of South Florida, as well as a CFF research grant (DARCH19G0) the N.I.H (5R21AI147654 - 02 (PI, Chen)) and the USF Institute on Microbiomes. We thank the Whiteley lab for ongoing collaboration involving data sets related to this manuscript. We thank Dr. Charles Szekeres for facilitating FACS sorting. Figures were created by A.D.G and S.E.D using Biorender.com.
....Materials
| Name | Company | Catalog Number | Comments |
| Amino acids | |||
| Alanine | Acr s Organics s Organics | 56-41-7 | |
| Arginine HCl | MP | 1119-34-2 | |
| Asparagine | Acr s Organics s Organics | 56-84-8 | Prepared in 0.5 M NaOH |
| Cystine HCl | Alfa Aesar | L06328 | |
| Glutamic acid HCl | Acr s Organics s Organics | 138-15-8 | |
| Glycine | Acr s Organics s Organics | 56-40-6 | |
| Histidine HCl H2O | Alfa Aesar | A17627 | |
| Isoleucine | Acr s Organics s Organics | 73-32-5 | |
| Leucine | Alfa Aesar | A12311 | |
| Lysine HCl | Alfa Aesar | J62099 | |
| Methionine | Acr s Organics s Organics | 63-68-3 | |
| Ornithine HCl | Alfa Aesar | A12111 | |
| Phenylalanine | Acr s Organics s Organics | 63-91-2 | |
| Proline | Alfa Aesar | A10199 | |
| Serine | Alfa Aesar | A11179 | |
| Threonine | Acr s Organics s Organics | 72-19-5 | |
| Tryptophan | Acr s Organics s Organics | 73-22-3 | Prepared in 0.2 M NaOH |
| Tyrosine | Alfa Aesar | A11141 | Prepared in 1.0 M NaOH |
| Valine | Acr s Organics s Organics | 72-18-4 | |
| Antibiotic | |||
| Carbenicillin | Alfa Aesar | J6194903 | |
| Day-of Stocks | |||
| CaCl2 * 2H2O | Fisher Chemical | C79-500 | |
| Dextrose (D-glucose) | Fisher Chemical | 50-99-7 | |
| 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) | Fisher (Avanti Polar Lipids) | 4235-95-4 | shake 15-20 min at 37 °C to evaporate chloroform |
| FeSO4 * 7H2O | Acr s Organics s Organics | 7782-63-0 | this stock equals 1 mg/mL, MUST make fresh |
| L-lactic acid | Alfa Aesar | L13242 | pH stock to 7 with NaOH |
| MgCl2 * 6H2O | Acr s Organics s Organics | 7791-18-6 | |
| N-acetylglucosamine | TCI | A0092 | |
| Prepared solids | |||
| Porcine mucin | Sigma | M1778-100G | UV-sterilize |
| Salmon sperm DNA | Invitrogen | 15632-011 | |
| Stain | |||
| Propidium iodide | Alfa Aesar | J66764MC | |
| Salts | |||
| K2SO4 | Alfa Aesar | A13975 | |
| KCl | Alfa Aesar | J64189 | add solid directly to buffered base |
| KNO3 | Acr s Organics s Organics | 7757-79-1 | |
| MOPS | Alfa Aesar | A12914 | add solid directly to buffered base |
| NaCl | Fisher Chemical | S271-500 | add solid directly to buffered base |
| Na2HPO4 | RPI | S23100-500.0 | |
| NaH2PO4 | RPI | S23120-500.0 | |
| NH4Cl | Acr s Organics s Organics | 12125-02-9 | add solid directly to buffered base |
| Consumables | |||
| Conical tubes (15 mL) | Olympus plastics | 28-101 | |
| Conical tubes (50 mL) | Olympus plastics | 28-106 | |
| Culture tubes w/air flow cap | Olympus plastics | 21-129 | |
| 35 mm four chamber glass-bottom dish | CellVis | NC0600518 | |
| Luria Bertani (LB) broth | Genessee Scientific | 11-118 | |
| Phosphate-buffered saline (PBS) | Fisher Bioreagents | BP2944100 | |
| Pipet tips (p200) | Olympus plastics | 23-150RL | |
| Pipet tips (p1000) | Olympus plastics | 23-165RL | |
| Serological pipets (5 mL) | Olympus plastics | 12-102 | |
| Serological pipets (25 mL) | Olympus plastics | 12-106 | |
| Serological pipets (50 mL) | Olympus plastics | 12-107 | |
| Ultrapure water (RNAse/DNAse free); nanopure water | Genessee Scientific | 18-194 | Nanopure water used for preparation of solutions in Table 1 |
| Syringes (10 mL) | BD | 794412 | |
| Syringes (50 mL) | BD | 309653 | |
| 0.22 mm PES syringe filter | Olympus plastics | 25-244 | |
| PS cuvette semi-mico | Olympus plastics | 91-408 | |
| Software | |||
| Biorender | To prepare the figures | ||
| FacsDiva6.1.3 | Becton Dickinson, San Jose, CA | ||
| Imaris | Bitplane | version 9.6 | |
| Zen Black | |||
| Equipment | |||
| FacsAriallu | Becton Dickinson, San Jose, CA | ||
| LSM 880 confocal laser scanning microscope | Zeiss |
References
- Ramsay, K. A., et al. The changing prevalence of pulmonary infection in with fibrosis: A longitudinal analysis. Journal of Cystic Fibrosis. 16 (1), 70-77 (2017).
- Bessonova, L., et al.
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
ABOUT JoVE
Copyright © 2025 MyJoVE Corporation. All rights reserved