A subscription to JoVE is required to view this content. Sign in or start your free trial.
An Epithelial Abrasion Model for Studying Corneal Wound Healing
In This Article
Summary
Here, a protocol for creating a central corneal epithelial abrasion wound in the mouse using a trephine and a blunt golf club spud is described. This corneal wound healing model is highly reproducible and is now being used to evaluate compromised corneal wound healing in the context of diseases.
Abstract
The cornea is critical for vision, accounting for about two-thirds of the refractive power of the eye. Crucial to the role of the cornea in vision is its transparency. However, due to its external position, the cornea is highly susceptible to a wide variety of injuries that can lead to the loss of corneal transparency and eventual blindness. Efficient corneal wound healing in response to these injuries is pivotal for maintaining corneal homeostasis and preservation of corneal transparency and refractive capabilities. In events of compromised corneal wound healing, the cornea becomes vulnerable to infections, ulcerations, and scarring. Given the fundamental importance of corneal wound healing to the preservation of corneal transparency and vision, a better understanding of the normal corneal wound healing process is a prerequisite to understanding impaired corneal wound healing associated with infection and disease. Toward this goal, murine models of corneal wounding have proven useful in furthering our understanding of the corneal wound healing mechanisms operating under normal physiological conditions. Here, a protocol for creating a central corneal epithelial abrasion in mouse using a trephine and a blunt golf club spud is described. In this model, a 2 mm diameter circular trephine, centered over the cornea, is used to demarcate the wound area. The golf club spud is used with care to debride the epithelium and create a circular wound without damaging the corneal epithelial basement membrane. The resulting inflammatory response proceeds as a well-characterized cascade of cellular and molecular events that are critical for efficient wound healing. This simple corneal wound healing model is highly reproducible and well-published and is now being used to evaluate compromised corneal wound healing in the context of disease.
Introduction
The cornea is the transparent anterior one-third of the eye. The cornea serves several functions including protecting the inner structures of the eye and forming a structural barrier that protects the eye against infections1. More importantly, the cornea is critical for vision, providing about two-thirds of the refractive power of the eye2,3. Crucial to the role of the cornea in vision is its transparency. However, due to its outward position, the cornea is exposed to a wide variety of injuries on a day-to-day basis that can lead to disruption of its barrier function, loss of transparen....
Protocol
All animal protocols were approved by the Institutional Animal Care and Use Committees at the University of Houston and Baylor College of Medicine. The guidelines outlined in the Association for Research in Vision and Ophthalmology (ARVO) statement on the use of animals in vision and ophthalmic research were followed in handling and using the mice.
1. Preparation
- Preparation of fluorescein solution
- Prepare 1% fluorescein solution by dissolving 10 mg of .......
Representative Results
Figure 3 shows a transmission electron micrograph of a corneal wound created with the blunt golf club spud, demonstrating that the epithelial basement membrane is indeed intact after injury.
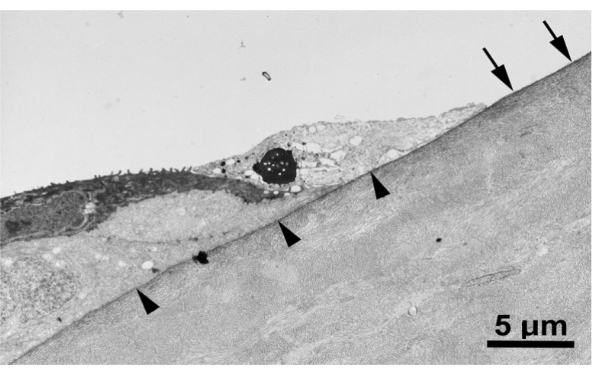
Figure 3: Epithelial basement membrane remains intact after corneal abrasion. Transmission electron mi.......
Discussion
The purpose of this methods paper was to describe a protocol for creating a central corneal epithelial abrasion wound in the mouse using a trephine and a blunt golf club spud. This murine model has been used to study corneal inflammation and its contribution to wound healing. This type of model can be used to study corneal wound healing mechanisms under normal physiological conditions and in pathologies17,28,29,
Acknowledgements
Funding: Supported by: NIH EY018239 (A.R.B., C.W.S., and R.E.R.), P30EY007551 (A.R.B.), and Sigma Xi Grant in Aid of Research (P.K.A.). The content is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health, or Sigma Xi.
....Materials
| Name | Company | Catalog Number | Comments |
| Anti-CD31 antibody | BD Bioscience, Pharmingen | 550274 | |
| Anti-CD41 antibody | BD Bioscience, Pharmingen | 553847 | |
| Anti-Ly6G antibody | BD Bioscience, Pharmingen | 551459 | |
| Bovine serum albumin (BSA) | ThermoFisher scientific | B14 | |
| C57BL/6 mice | Jackson Laboratories | 664 | |
| DAPI | Sigma Aldrich | D8417 | |
| DeltaVision wide-field deconvolution fluorescence microscope | GE Life Sciences | ||
| Dissecting microscope | Leica microsystems | ||
| Electronic Toploading Balances (Weighing scale) | Fisher Scientific | ||
| Ethanol | ThermoFisher scientific | T038181000CS | |
| Golf-club spud | Stephens instruments | S2-1135 | |
| Iris curve scissors | Fisher Scientific | 31212 | |
| Isoflurane | Patterson veterinary | 07-893-1389 | |
| Ketamine | Patterson veterinary | 07-890-8598 | |
| Phospate buffered saline (PBS) | ThermoFisher scientific | AM9624 | |
| Sodium fluorescein salt | Sigma Aldrich | 46970 | |
| Surgical blade (scapel blade) | Fine Science tools | 10022-00 | |
| Trephine | Integra Miltex | 33-31 | |
| TritonX -100 | Fisher Scientific | 50-295-34 | |
| Forcep | Fine Science tools | 11923-13 | |
| Xylazine | Patterson veterinary | 07-808-1947 |
References
- DelMonte, D. W., Kim, T. Anatomy and physiology of the cornea. Journal of Cataract and Refractive Surgery. 37 (3), 588-598 (2011).
- Meek, K. M., Knupp, C. Corneal structure and transparency. Progress in Retinal and Eye Research. 49, 1-....
This article has been published
Video Coming Soon
ABOUT JoVE
Copyright © 2024 MyJoVE Corporation. All rights reserved