Murine Neural Plate Targeting by In Utero Nano-Injection (NEPTUNE) at Embryonic Day 7.5
In This Article
Summary
In this protocol, we describe how to inject the mouse amniotic cavity at E7.5 with lentivirus, leading to uniform transduction of the entire neural plate, with minimal detrimental effects on survival or embryonic development.
Abstract
Manipulating gene expression in the developing mouse brain in utero holds great potential for functional genetics studies. However, it has previously largely been restricted to the manipulation of embryonic stages post-neurulation. A protocol was developed to inject the amniotic cavity at embryonic day (E)7.5 and deliver lentivirus, encoding cDNA or shRNA, targeting >95% of the neural plate and neural crest cells, contributing to the future brain, spinal cord, and peripheral nervous system. This protocol describes the steps necessary to achieve successful transduction, including grinding of the glass capillary needles, pregnancy verification, developmental staging using ultrasound imaging, and optimal injection volumes matched to embryonic stages.
Following this protocol, it is possible to achieve transduction of >95% of the developing brain with high-titer lentivirus and thus perform whole-brain genetic manipulation. In contrast, it is possible to achieve mosaic transduction using lower viral titers, allowing for genetic screening or lineage tracing. Injection at E7.5 also targets ectoderm and neural crest contributing to distinct compartments of the eye, tongue, and peripheral nervous system. This technique thus offers the possibility to manipulate gene expression in mouse neural-plate- and ectoderm-derived tissues from preneurulation stages, with the benefit of reducing the number of mice used in experiments.
Introduction
The brain and spinal cord are among the first organs to initiate formation during embryogenesis1,2. Although the genes associated with neurodevelopmental disorders are being identified, the functional interrogation of genetic variants has lagged behind3,4. As the generation of conditional knockout mice can take months or years, an alternative technique to rapidly investigate gene function in the developing brain is of interest. In mouse embryos, neurulation - the morphogenetic process by which the neural plate transforms into the neural tube to give rise to the central nervous system (CNS) - occurs between days 8 and 10 post conception5. Prior to the onset of neurulation, the neural plate, as part of the ectoderm, consists of a single layer of columnar cells that will proliferate and differentiate into the numerous neuronal and glial cell types within the CNS6,7. Therefore, to experimentally induce long-lasting alterations to gene expression in the CNS, targeting the neural plate offers obvious advantages, including the accessibility of all progenitor cells.
In neuroscience, in ovo electroporation8,9 and viral transduction of mouse embryos have been used to manipulate embryonic CNS gene expression. The developing chick embryo has been a model of choice for studying gene function during spinal cord development due to the accessibility of the chick embryo in the egg and the resultant ease of manipulating gene expression. In particular, in ovo plasmid electroporation generates experimental and control conditions in each chick spinal cord. Electroporation causes cell membrane permeabilization and directs negatively charged DNA away from the (negative) cathode towards the (positive) anode by applying an electric pulse via two electrodes to the embryo. In mice, in utero electroporation has generally been limited to embryonic stages at which neurulation has completed, and the brain or spinal cord already consists of several cell layers, resulting in low electroporation efficiency10. Plasmid electroporation results in transient gene expression and generally targets few cells.
Ultrasound-guided in utero microinjection has been used to manipulate different embryonic structures such as the skin and brain11,12,13,14. However, injections targeting the developing murine CNS have shown low efficacy or have negatively impacted embryonic survival12,13,14. Therefore, an improved protocol was developed for the delivery of high-titer lentivirus into the amniotic cavity (AC) at E7.5, which was dubbed NEPTUNE for neural plate targeting with in utero nano-injection15. Injections resulted in a long-lasting targeting efficacy of >95% of the entire brain at E13.5. Furthermore, a staging step was introduced during ultrasound verification of pregnancy to sort females and pregnancies by developmental stage to minimize unnecessary procedures on research animals and maximize injection success. Injection efficiency and survival are tightly linked to the increase in AC size. Therefore, this paper describes how to measure AC size prior to injection to deliver a suitable volume to the AC that will not cause resorption of the embryo. NEPTUNE is a robust alternative to current in utero approaches and can be adapted for several uses, including, but not limited to, gain and loss of function studies, lineage tracing, or screening15,16.
Protocol
CD1 wild-type mice were housed according to European regulations, with a standard day and night cycle with food and water ad libitum. CD1 females were mated with CD1 males overnight, and vaginal plugs were checked in the morning (E0.3). Only pregnant females were used for the injection. Ethical approval for all experiments described here was granted by the Swedish Board of Agriculture (Jordbruksverket).
1. Preparation of glass needles: needle pulling and grinding
NOTE: Although preground needles can be bought, pulling needles in-house allows for easy adjustments of needle length, bore, and bevel angle.
- Mount a glass capillary into the micropipette/capillary puller. Use the following settings using the specified equipment (see the Table of Materials): heat 580 units; velocity 140 units; time 200 units; pressure 500-800 units.
NOTE: The units of different parameters are defined by the capillary puller. Units can vary for different equipment. - Press Pull to pull apart the capillary producing two glass capillaries with tapered ends.
NOTE: After pulling, the tips of the two glass capillaries are fused shut due to the high temperature of the micropipette puller. - Cut the tip with surgical scissors to obtain a needle length of ~7 mm (measured from where the needle begins to taper) (Figure 1A).
NOTE: For E7.5 injections, a long and fine needle is critical as the region of injection is very small and delicate. Short and wide-bore needles will result in embryonic deaths. - Grind the cut needle tip to create a sharp bevel (Figure 1C-F).
- Grind the needle at an angle of 20° at maximum speed for at least 30-45 min.
NOTE: Ultrapure water is added to the grinding plate to act as a lubricant, reduce friction/temperature, and wash away glass particles (Figure 1B). However, excess liquid can slow down the grinder. - Ensure that the needle tip (Figure 1C) touches the grinding plate surface (Figure 1D) but does not bend (Figure 1E).
NOTE: Bent grinding results in a long and fragile needle tip of an incorrect bevel angle, which can easily break during injection. Breaking needles can harm the embryo and must be replaced with an intact needle. A correctly ground tip is shown in Figure 1F,G. After grinding, the resulting needle bore is expected to have an inside diameter (ID) of ~15 µm and an outside diameter (OD) of ~35 µm (Figure 1G).
- Grind the needle at an angle of 20° at maximum speed for at least 30-45 min.
- Fill a 1 mL syringe with mineral oil and attach a 27 G needle.
- Remove the needle cap and insert the syringe needle into the newly ground glass capillary. Inject mineral oil until oil drips from the capillary tip. Keep injecting while withdrawing the 27 G needle until the capillary needle is filled with mineral oil, after which the syringe needle can be removed.
- Store ground and mineral oil-filled needles in a closed environment to prevent damage and dust accumulation. For storage, insert two rolls of modeling clay into a regular Petri dish to serve as holders (Figure 1H). Gently perch the needles on the clay and space them far apart for easy retrieval of the needles.
NOTE: As needle preparation is time-consuming, it is best to prepare needles at least one day prior to injections. Discard the needles after two litters maximum as they will become blunt. Always prepare backup needles in case of needle damage during preparation or injection.
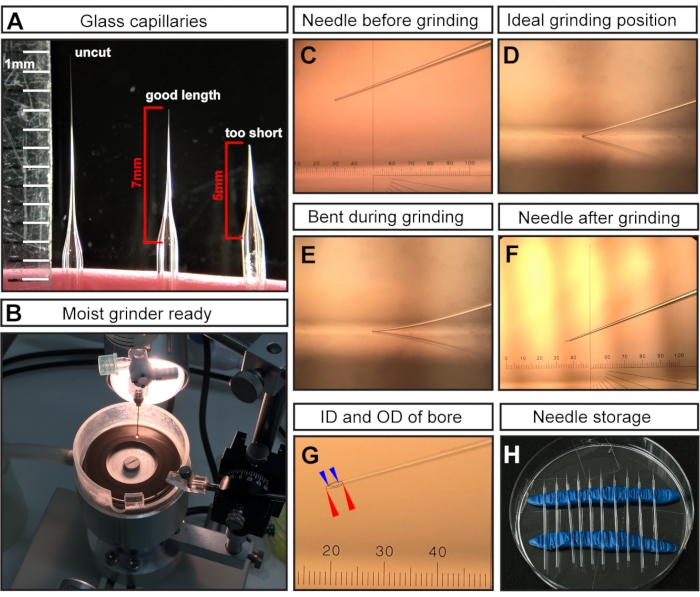
Figure 1: Needle preparation for E7.5 amniotic cavity injections. (A) Representative examples of a pulled but uncut glass capillary needle (left), a capillary needle cut at the optimal length for E7.5 injections (middle), and a capillary cut too short (right). (B) The grinder with uniform coverage of water, which is ready for needle grinding. (C-F) Representative examples of different needle tips. (C) Cut but unground needle tip mounted in grinder; (D) ideal grinding position with the needle tip just touching the grinder; (E) needle lowered too far and bending during the grinding process; (F) an ideal ground needle tip for E7.5 AC injections. (G) A ground needle tip showing the bore with an inside diameter of ~15 µm and an outside diameter of ~35 µm, which is suitable for E7.5 AC injection. The needle bore is shown as dashed lines. Outer diameter denoted with red arrowheads; inner diameter denoted with blue arrowheads. (H) Needle storage: Petri dish filled with pulled and ground needles. Two rows of modeling clay serve as holders. NOTE: For the ocular micrometer in C, F, G, 1 cm is divided into 100 pitches; the objective is 3x; therefore, 1 pitch= 10,000 µm/(100 × 3) ≈ 33.4 µm. Abbreviations: AC = amniotic cavity; ID = internal diameter; OD = outer diameter. Please click here to view a larger version of this figure.
2. Day before injection: prepare bench for ultrasound-verification of pregnancy
NOTE: All work should be carried out in a ventilated Biosafety Level 2 (BSL 2) bench when working with lentivirus. Ultrasound-verification of pregnancy can be performed on a ventilated bench.
- Switch on the ultrasound machine, heating table, and O2 supply for the isoflurane pump (can vary between equipment).
NOTE: Check the isoflurane system to ensure no isoflurane leakage into the air. - Place an empty waste bag (taped to the inside wall for easy access), hair removal cream, sterile packed cotton swabs, water, tissue paper, and surgical tape inside the BSL 2 bench.
- Prepare four pieces of surgical tape (~7 cm long) to secure the mouse limbs during the ultrasound check of pregnancy.
3. Ultrasound check to confirm pregnancy
NOTE: This step can be performed the day before E7.5 Injections, at E6.5. See the discussion for details about the check for gestational age.
- Place the time-mated female mouse in the induction chamber.
- Switch on the gas flow with an oxygen flow of ~2.1 LPM and an initial dose of 3-4% isoflurane to induce anesthesia.
- Verify that the female is completely anesthetized by checking the paw reflex. If the paw reflex is absent, lower isoflurane to 1.5-2%.
NOTE: It takes approximately 3 min to induce anesthesia. - Switch the gas flow from the induction chamber to the heating table nose cone.
- Place the anesthetized female in a supine position (abdomen up) on the heating table and place the snout in the attached nose cone to ensure maintenance of anesthesia during the ultrasound check of pregnancy.
- Fasten all four paws to the table with the prepared pieces of surgical tape without stretching the female's body or trapping the whiskers.
- Apply a pea-sized amount of hair removal cream on the lower abdomen. Using a cotton swab, distribute the cream over the lower abdomen (a ~ 3 x 3 cm square) and gently massage it in by rolling the cotton swab back and forth.
- Once the fur begins to detach from the skin, moisten a tissue paper and remove the cream and the fur. Clean the de-furred area with moist tissue paper until all the cream and hair are gone. Dry the skin.
- Apply a plum-sized amount of ultrasound gel to the shaved area and proceed to identify the uterus using ultrasound by any of the following three approaches.
- To do this manually, hold the ultrasound probe in the ultrasound gel and move the probe to find the uterus.
- For semimanual approach #1, position the ultrasound probe (attached to the railing system) above the female abdomen by loosening and moving part of the rail along the x-plane. Lower the ultrasound probe into the gel (z-plane) and scan through the lower abdomen (y-plane) (Figure 2A).
- For semimanual approach #2, lower the ultrasound probe (attached to the railing system) into the gel to image the lower abdomen. Keep the ultrasound probe stationary during the process, and move the heating table with the attached wheels along x and/or y- planes (Figure 2B).
NOTE: At these early embryonic stages, internal organs, e.g., the intestine, can appear similar to the uterus in ultrasound imaging. However, while embryos and decidua appear as a sequence of spheres (akin to beads along a necklace), the intestine has the appearance of a continuous tube. The connectivity of luminal spaces (separate spheres vs. a continuous tube) can be assessed by scanning back and forth through the structure of interest to determine whether the structure is a discrete sphere (embryo/decidua) (Figure 2C) or a continuous tube (intestine) (Figure 2D). At this stage, it is not necessary to record the number of embryos; it is sufficient to confirm their presence or absence.
- Once the pregnancy status is determined, lift the ultrasound probe away from the abdomen and wipe the lower abdomen clean with moist tissue paper to remove the ultrasound gel.
- Switch off the isoflurane pump and remove the surgical tape to release the paws.
- Place the female in the prone position (belly down) into a clean cage on a 40 °C heating plate. Keep the female under close observation until she regains consciousness, which takes 2-6 min.
NOTE: Ensure that the ultrasound check for one female is <10 min to minimize exposure to isoflurane. - Remove all materials and waste and clean all surfaces with 70% ethanol. Wipe off any leftover ultrasound gel from the ultrasound probe with a dry, soft, and lint-free paper tissue. Switch off the ultrasound machine, ventilated bench/ BSL2 bench, heating table, and O2 supply.
4. Ultrasound check for embryo staging
NOTE: This step is performed before the surgery and serves to stratify the pregnant females according to their AC sizes. This step is crucial at E7.5 when the aim is to target the developing CNS. At this early stage of development, a difference of a few hours in development significantly influences the size of the AC and the progression of neurulation.
- Anesthetize the first pregnant female mouse following the steps in steps 3.1-3.5.
- Place and fixate the female on the table as described in step 3.6.
- Apply a plum-sized amount of ultrasound gel to the shaved abdomen and lower the ultrasound probe to image the lower abdomen of the female.
NOTE: This step assumes the female was inspected by ultrasound on the previous day for pregnancy and has already had fur on the abdomen removed. If this is not the case, the fur must be removed before the addition of ultrasound gel following steps 3.7-3.8. At E7.5, the uterus and deciduas are larger and easier to distinguish from internal organs. In addition, the AC is larger and can be distinguished from the exocelomic cavity (ExC). - Scan through the left and right uterine horns as completely as possible.
NOTE: Some embryos lie deeper inside the body of the female and can be missed. - Record the number and stages of the embryos. Make a note of the number of ideal-sized amniotic cavities, acceptable cavities, and deciduas without a cavity (Figure 2E-G).
- Stage all the pregnancies and rank them accordingly.
- Inject females with a majority of ideal-size ACs immediately after the ultrasound check (step 4.4) following instructions in steps 4.5-4.7.
- For females with a majority of acceptable (in which the exocelomic and amniotic cavities are not yet clearly divided into two cavities) or absent cavities, postpone the injection and stage them again after a couple of hours. If most of the cavities are still too small, postpone the injections by 10-12 h.
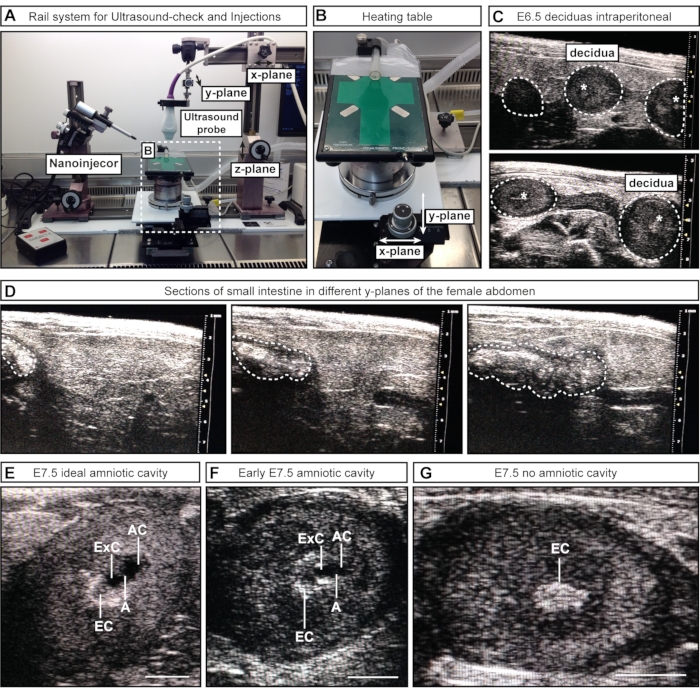
Figure 2: Inspection and staging of amniotic cavities during ultrasound-check. (A) Overview of the rail system with attached ultrasound probe, nanoinjector, and heating table. The ultrasound probe can be moved in x, y, and z-planes to achieve optimal alignment with the female abdomen or the ACs. (B) Heating table can be moved via two wheels in x- and/or y-planes to allow precise scanning and assessment of the ACs, while the ultrasound probe can remain static. (C) Representative ultrasound images of E6.5 deciduas inside the female abdomen during ultrasound check to confirm pregnancy (white dotted outlines). No cavities have formed at this point; however, sometimes, the ectoplacental cone (white asterisks) is visible. Deciduas can be recognized by their spherical shape and distinguished from the intestine, which appears as one continuous tube. (D) Representative image sequence of the small intestine (whited dotted outlines), which is continuous in scanning through the lower abdomen. (E-G) Representative ultrasound images during cavity staging prior to E7.5 injections. The amniotic and exocelomic cavities have formed and are separated by the amnion. The ectoplacental cone serves as the main blood supply and appears as a bright spot in the ultrasound. The AC is most distal from the ectoplacental cone. (E) Ideal-sized ACs appear larger than the exocelomic cavity, while medium-sized cavities appear smaller (F). If no cavities are visible (G), this means either that the embryo is resorbed or has not reached E7.5 yet. Scale bars = 1 mm. Abbreviations: A = Amnion; AC = Amniotic Cavity; ExC = Exocelomic Cavity; EC = Ectoplacental Cone. Please click here to view a larger version of this figure.
5. Day of injection: prepare the BSL2 bench for surgery
- Glue one piece of elastic membrane to the round, central opening of a commercially available, modified Petri dish. Ensure that the membrane is well attached to the Petri dish to prevent leakage.
NOTE: If the dish becomes leaky or is not well-glued, the edges of the elastic membrane can be further secured with duct tape. - Make a 1-1.5 cm long incision in the center of the elastic membrane.
- Switch on the ultrasound machine, BSL2 bench, heating plate, O2 supply for the isoflurane pump, and glass bead sterilizer (set to 300 °C).
- Inside the BSL2 bench, place one empty waste bag (taped to the inside wall for easy access); sterile packed cotton swabs (use a new one for each surgery/each female); surgical tools (scissors, tweezers, clips, suture), and surgical tape; one full bottle of room-temperature phosphate-buffered saline (PBS), one empty bottle for waste collection, and one 25 mL pipette; one Petri dish with elastic membrane for surgery; the lid of the Petri dish with a 2 x 2-3 x 3 cm piece of parafilm secured to the lid with a drop of water; modeling clay: 4 larger balls or cubes (~3 x 3 x 3 cm3 ) and one cylindrical piece (~4 x 1 cm); a syringe with analgesic (e.g., buprenorfine, 0.05-0.1 mg/kg body weight) and eye gelNOTE: The balls (or cubes) of modeling clay will serve as "feet" or stands/holders for the Petri dish once the dish is placed on top of the abdomen of the female. The cylindrical piece of clay secures the embryos inside the dish. If more than one solution is injected, or the needle needs to be refilled during injections, the same piece of parafilm can be used if solutions can be spaced apart.
6. Needle loading
- Wipe off any excess mineral oil with tissue paper for a better grip.
NOTE: Always clean away from the tip to prevent damage or injury. - Ensure that all components (one sealing O-ring, one spacer, and one front gasket-all housed under the collet, Figure 3A) are installed in the correct orientation (Figure 3B, collet removed for visualization) to hold the capillary needle in place and create an airtight connection, avoiding bubble formation when advancing or retracting the metal plunger inside the needle.
- Ensure that the metal plunger of the nanoinjector is fully retracted. Verify by pressing Fill and wait for a double beep that indicates that the plunger is fully retracted.
- With the collet attached but slightly loosened (unscrewed 45-90 degrees), slide the glass needle onto the metal plunger and push the capillary needle along with the metal plunger through the front gasket until it reaches the spacer (Figure 3C,D, collet removed for visualization).
NOTE: Some resistance appears when the plunger passes through the gasket and decreases when it reaches the spacer. Ensure that the capillary needle is firmly inserted into the spacer to create an airtight connection (Figure 3D). - Secure the needle by tightening the collet of the nanoinjector (screw back on securely).
NOTE: Do not overtighten as this can crush the needle. - Press Empty to push the plunger into the needle and expel the oil from the needle tip. If the glass needle moves, retract the plunger, remove the needle, and reload. If this causes air bubbles to form, remove the needle and refill with mineral oil.
NOTE: A properly secured needle should not move with the plunger. - Place a drop of the virus (~ 6 µL) or other injection solution on a piece of parafilm1 (Figure 3E; Evans blue dye is used for visualization purposes).
NOTE: The maximum volume of the nanoinjector is ~5 µL. - Press Fill to create an air bubble to serve as a divider between the oil and the solution (Figure 3F).
NOTE: This also serves as a positioning marker when filling or emptying the needle. - Lower the needle, immerse the tip into the solution, and press Fill (Figure 3F,G).
- Watch the fluid level as the solution is loaded after the air bubble. Push Empty to expel the clog and reload by pressing Fill if the air bubble becomes elongated without the fluid entering the needle. If this does not solve the problem, change the needle.
- When the needle is fully loaded (Figure 3H), lift the needle in the z-plane, and aspirate a small volume of air at the tip to prevent evaporation of the solution at the needle tip and clogging of the needle.
- Turn the nanoinjector with the attached needle away from the operator, towards the back of the bench, to prevent any accidental damage or injury.
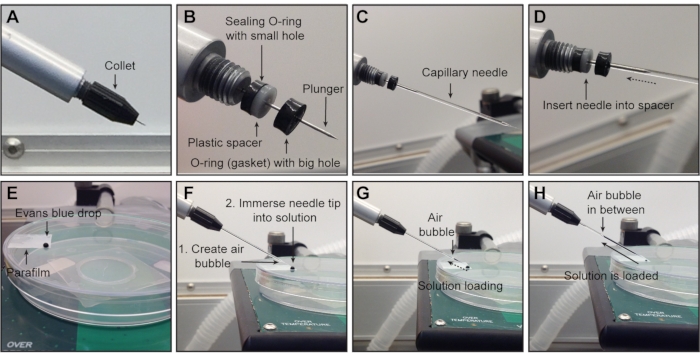
Figure 3: Attaching the needle to the nanoinjector and solution loading. (A) Starting position before the needle is mounted onto the nanoinjector: metal plunger completely retracted and collet attached. (B) Under the collet, all three components for holding and securing the needle are shown in correct order (from left to right): sealing O-ring (thin and black), spacer (white), O-ring (black) with big hole (that the needle must pass through). To ensure an airtight connection, the glass needle is slid over the metal plunger (C) and pushed through the opening of the front O-ring until it reaches the spacer (D). (E-H) Loading of the solution into the needle. (E) One drop of solution is placed on a piece of parafilm on a plate lid. (F) Create an air bubble by pressing Fill before loading the solution and immerse the needle tip in the solution. (G) Solution is loading into the needle. (H) Solution is loaded into the needle. NOTE: The collet has been removed in (B-D) for visualization but should remain attached during experiments. The final step of securing the needle is tightening the collet. Evans blue dye is used for visualization in E-H. Please click here to view a larger version of this figure.
7. Injections
NOTE: All instruments used in this procedure are sterilized before surgery and between each mouse.
- Anesthetize the first female with ideal-sized cavities (see section 4).
- Place and fixate the female on the table, as described in steps 3.1-3.6.
- Apply eye gel to the eyes to prevent corneal desiccation and inject pain killer subcutaneously.
NOTE: Recommended analgesics: Buprenorphine (0.05-0.1 mg/kg body weight) or Flunixin (2.5 mg/kg) or similar in accordance with local animal welfare regulations. Multimodal perioperative analgesia is maintained with injected analgesics and isoflurane. - Aseptically prepare the lower abdomen by wiping with a cloth soaked with 0.5 mg/ mL chlorhexidine solution (or similar) and dry the skin. Use surgical scissors to make a 1.5-2.0 cm vertical midline skin incision in the lower abdomen.
NOTE: Prepare the surgical area following locally approved disinfection routines. - Lift the skin gently and free the skin from the underlying muscle layer, approximately 1cm around the incision point, to facilitate suturing after the surgery.
- Make a 1 cm vertical midline incision into the muscle layer.
- With a pair of forceps, lift one side of the skin and muscle layer and with the other pair, look for the uterine horns.
NOTE: The deciduas are arranged like beads on a necklace, while the intestine is one long, continuous tube (Figure 4A). - With forceps, carefully pull out both uterine horns from the abdomen. Hold the tissue between embryos; do not directly squeeze them (Figure 4B).
NOTE: The uterus is attached to the body at the cervix, and the two ovaries/oviducts are attached via ligaments. Do not pull/detach the uterus from these anchor points. - Count and make a note of the number of embryos on the left and right sides.
- Number the embryos either from the ovary to the cervix or from the cervix to the ovary.
NOTE: This is especially important if injected embryos will be collected at embryonic stages. - With a moist cotton swab, gently push all embryos back into the abdominal cavity, except the first three to be injected.
- Place a drop of PBS onto the elastic in the Petri dish and hold it immediately above the embryos.
- Insert closed forceps into the incision of the elastic and release the forceps to open the elastic incision so the liquid drops onto the embryos.
NOTE: This ensures rehydration of the embryos and that the elastic membrane adheres to the wet skin of the female, preventing leakage of PBS in the next steps. - Pull a section of the uterus, corresponding to three embryos, through the elastic with forceps, and gently perch the Petri dish on the abdomen of the female.
- With the forceps and a moist cotton swab, adjust the uterus positioning and the elastic to ensure that the elastic is sealed to the skin of the female to prevent PBS leakage.
- Use the four clay feet to secure and fasten the Petri dish immediately above the abdomen, reducing pressure on the female and sensitivity of imaging to the breathing and heartbeat of the female.
- Press the modeling clay cylinder down to the right of the embryos/uterus to fixate the uterus (Figure 4C).
NOTE: This orientation instruction assumes that the needle is to the left of the female and that the embryos of the female's left uterine horn are exposed. The side of the uterus (left or right uterine horn) is crucial as the AC either faces the needle (left horn; Figure 4D) or faces the stabilizing clay cylinder (right horn; Figure 4E). - To inject embryos from the right uterine horn, place the clay cylinder on the left side of the embryos (Figure 4F) and turn the heating table 180° (Figure 4G) to achieve the correct orientation. If the needle can be moved instead, adapt as appropriate for the relevant setup.
- Add PBS to the Petri dish until the embryos and uterus is covered with PBS.
- Lower the ultrasound probe into the PBS and adjust the mouse/surgical table so that the first embryo is aligned with the ultrasound probe to facilitate the recording of the injections.
NOTE: "First" refers to the numbering of embryos from the ovary to the cervix. - Scan through all three embryos and inspect the ACs. Determine the injection volume as follows:
- Measure the diameter of the AC with the ultrasound machine. Inject 69 nL if the AC diameter is ≤0.2 mm; inject 2 x 69 nL (= 138 nL) if the AC diameter is >0.2 mm and ≤0.29 mm; and inject 3 x 69 nL (= 207 nL) if the AC diameter > 0.29 mm (Figure 4H).
NOTE: Previous experiments have shown that up to 207 nL injection volumes are well tolerated at E7.515. Successful injections with minimal impact on survival are achieved when the relative volume increase of the AC does not exceed 90%15. Variation in AC size between littermates or mouse strains is common and may require further optimization.
- Measure the diameter of the AC with the ultrasound machine. Inject 69 nL if the AC diameter is ≤0.2 mm; inject 2 x 69 nL (= 138 nL) if the AC diameter is >0.2 mm and ≤0.29 mm; and inject 3 x 69 nL (= 207 nL) if the AC diameter > 0.29 mm (Figure 4H).
- Set the injection volumes with the injection controller.
- Set the injection speed to slow with an injection rate of 23 nL/s.
NOTE: Different equipment models can result in different injection forces. - Lower the needle into the PBS using the main wheels on the rail system (x- and z-planes, Figure 4I) and press Empty on the nanoinjector controller until the liquid reaches the needle tip.
NOTE: If the needle has clogged, pressing Empty can dissolve the clog and/or expel the clog. - Lift the needle out of the PBS and press Inject. Verify that a drop of the approximate desired volume is discharged.
- Lower the needle into the PBS and align it with the ultrasound probe and embryo by moving the nanoinjector with the y-plane wheel on the micromanipulator (Figure 4J).
NOTE: The needle tip is perfectly aligned when it appears as a bright spot on the ultrasound image (Figure 4K,L). The needle can be moved in all three planes of direction with the micromanipulator. - Adjust the needle angle with the inject angle wheel to ensure a near-perpendicular angle of injection relative to the uterine wall. Insert the needle into the AC in one motion by using the inject wheel on the micromanipulator (Figure 4J-M). Keep an eye on the needle tip brightness. If the needle tip disappears from the ultrasound image, move the ultrasound probe forward or backward to bring the needle back in focus.
NOTE: Once the needle is inside the AC, minor modifications to the needle position and focus can be made without harming the embryo (adjustments within ~0.3 mm). Do not adjust the needle position more than this. - Press Inject (for 207 nL, set the volume at 69 nL and inject three times). After injection, wait for an additional 5-10 s before retracting the needle in one gentle movement.
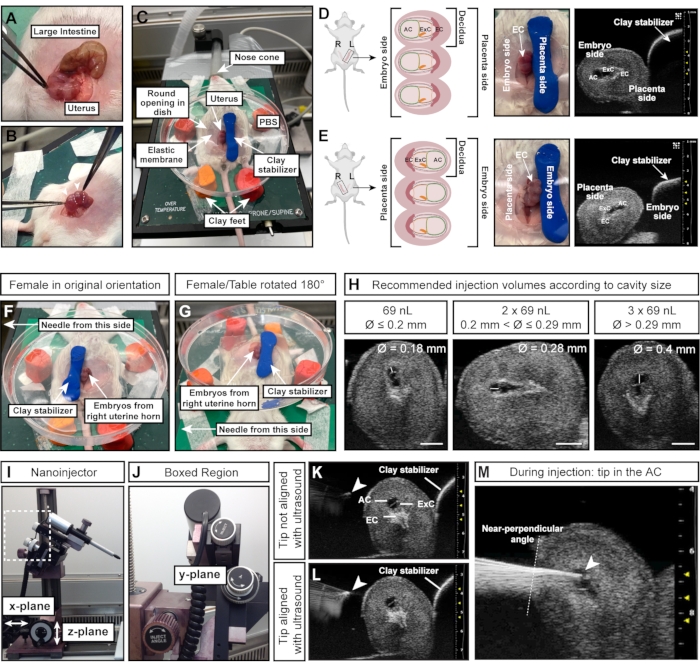
Figure 4: Optimal size and orientation of the amniotic cavity for successful injections. (A) Uterine horn with multiple E7.5 deciduas, shaped like a string of spheres (bottom) compared to large intestine (top). (B) Grasp uterine tissue (white dotted lines) between deciduas. Avoid squeezing the deciduas (white arrowheads) directly with forceps as deciduas and developing embryos are fragile at this early stage and prone to resorption upon excessive external force. (C) Female in supine position with deciduas exposed in a Petri dish filled with PBS and mounted on four feet of modeling clay. The deciduas are stabilized by an additional piece of modeling clay, shaped like a cylinder. (D, E) Orientation of the AC is influenced by the side of the uterine horn that is exposed. If deciduas from the left uterine horn are used, the AC will face away from the clay stabilizer and will be easily accessible to the needle on the left (D). However, if the right uterine horn is used, the ectoplacental cone will instead face the needle, making it more difficult to access the AC (E). Therefore, when injecting into the right uterine horn, the clay stabilizer is placed towards the needle-facing side (F), and the entire heating table is rotated 180° (G). (H) Recommended injection volumes according to AC sizes. In general, cavities with a diameter ≤ 0.2 mm can be injected with a maximum of 69 nL. Diameters > 0.2 mm and ≤ 0.29 mm tolerate volumes up to 2 x 69 nL (138 nL) and cavities > 0.29 mm can be injected with 3 x 69 nL (207 nL). Scale bars = 1 mm. (I, J) Nanoinjector is attached to the rail system and can be moved in x- and/or z-planes. The angle of the needle can be adjusted with the inject angle wheel. (K, L) Needle tip (white arrowhead) is aligned with the AC when appearing brightest in the ultrasound (L). (M) Ultrasound image showing the injection process, in which the needle tip is in the AC and well aligned (white arrowhead). Abbreviations: A = Amnion; AC = Amniotic Cavity; ExC = Exocelomic Cavity; EC = Ectoplacental Cone. Please click here to view a larger version of this figure.
- Move the stage to the next embryo and repeat steps 7.22-7.26 for the other two embryos if they have suitable AC sizes.
- Lift the ultrasound probe and needle out of the PBS using the micromanipulator. Turn the needle away from the operator to avoid damage and injury.
- With forceps, direct the 1st and 2nd embryos back into the abdomen of the female by gently pushing them through the elastic. Gently grasp the tissue adjacent to the 3rd embryo and pull the uterus towards the upper end of the elastic incision. Gently pull up the 4th-6th embryos with forceps and allow the 3rd embryo to re-enter the abdomen.
NOTE: This step can be done without changing the PBS or removal of the Petri dish. - Repeat as needed until all embryos with optimal ACs have been injected or the time limit has been reached.
- Gently push the embryos/uterus back into the abdomen of the female.
- Aspirate the PBS and remove the Petri dish. If a virus has been used, handle as infectious waste.
- Sew together the muscular layer with Vicryl (USP 6-0, needle length 13 mm, 3/8 Circle) in simple continuous or interrupted sutures and close the skin with 1-2 clips (see the Table of Materials).
- Switch off the isoflurane pump.
- Remove the surgical tape and place the female in the prone position (belly down) in a clean cage on a 40 °C heating plate.
NOTE: The female is expected to regain consciousness and be mobile within 10 min. Ensure that the procedure is completed within 30 min. The genetic background, age, and weight of the female may influence sensitivity to anesthesia. Monitor the mice during surgery for signs of slow breathing (slow breathing means anesthesia is too deep) or movement (anesthesia is too light). Buprenorphine (0.05-0.1 mg/kg body weight) or Flunixin (2.5 mg/kg) or similar in accordance with local animal welfare regulations, can be provided 8 hours after the first injection if needed. - If another female is to be injected, prepare the surgical area while monitoring the awakening of the first one.
- Wipe the surgical tools with 70% ethanol to remove any residual blood or tissue and sterilize them in the preheated glass bead sterilizer for 10 s. Discard the cotton swabs and used tissue paper. Wipe the Petri dish and clay pieces dry with tissue paper.
- Repeat steps 7.1-7.35 until all females have been injected.
- Empty and discard the needle.
- If there is a virus or injection solution left in the needle, press Empty on the nanoinjector and empty the needle's content on tissue paper.
- Retract the metal plunger completely by pressing Fill until there is a double-beep from the controller, which indicates that the plunger is completely retracted.
- Loosen the collet and slide the needle off the metal plunger. Discard the needle into the sharps waste container.
- Clean the BSL-2 bench.
- If a virus was used, spray the entire surgical field with disinfectant (see the Table of Materials). After 15 min, wipe off the disinfectant and clean the entire surgical field with 70% ethanol. If no virus was used, clean all surfaces with 70% ethanol.
- Wipe off any remaining PBS from the ultrasound probe with dry, soft, and lint-free tissue paper.
- Discard waste according to biosafety guidelines.
- Switch off the ultrasound machine, heating table, BSL2 bench, and O2 supply.
Representative Results
Embryos injected at E7.5 with hPGK-H2B-GFP lentivirus11,12 were collected at E13.5 and examined under the fluorescent dissection microscope (Figure 5A). Successful transduction of the neural plate results in embryos with strong expression of a fluorescent reporter in the brain mainly and in other ectoderm-derived tissues, e.g., the skin (Figure 5A,B). Injection of an excessively high volume (greater than the volumes recommended here, e.g., ≥500 nL) increases the pressure in the AC and can result in either complete resorption (data not shown) or neural tube defects such as exencephaly (Figure 5A). Successful injections at E7.5 result in uniform transduction from the forebrain to the hindbrain (Figure 5C-J).
Lentiviral titers around 2 × 1010 infectious units (IFU) achieve over 95% targeting, while titers of ~1 × 109 IFU achieve 15% targeting efficiency15. In addition, structures that have previously been difficult to target using electroporation, such as the choroid plexus17,18, are also targeted (Figure 5 E,F). Transduction efficacy can be modified by adjusting the viral titer delivered into the AC. Injections of low titer result in the transduction of single-cell clones (Figure 5C, Figure 5E, and Figure 5G,H) while usage of high-titer virus transduces nearly 100% of the entire brain (Figure 5D, Figure 5F, Figure 5H, and Figure 5J). Therefore, NEPTUNE can be used for either clonal transduction, lineage tracing, and genetic screening approaches or studying the global effects of gene overexpression or downregulation in the entire brain.
Mammalian eye development is the result of well-organized communication between three derivatives of the embryonic ectoderm: the neural retina (NR) and retinal pigment epithelium (RPE) are derived from the neuroepithelium of the ventral forebrain, while the surface ectoderm gives rise to the future lens and corneal epithelium. However, the central stroma and the posterior endothelium, the other two layers of the cornea, are derived from neural crest cells of the periocular mesenchyme19,20. Coronal sections through E13.5 embryos, injected at E7.5 with high-titer lentivirus showed, similar to the brain, high and uniform transduction of the neural tissue of the eye, as well as the lens, cornea, and mesenchyme (Figure 5K). As neurulation progresses, injections at E8.5 and E9.5 result in the continued targeting of the lens and corneal epithelium (Figure 5L and Figure 5N), while transduction of the neuroectoderm-derived tissues of the eye is less efficient at E8.5 (Figure 5L,M) or not targeted at E9.5 (Figure 5N).
While most viral particles infect the exposed tissues upon injection, some particles transduce non-ectodermal derived tissues that develop later (Figure 6A). Salivary glands and ducts develop around E11.5 from the oral epithelium21 and are targeted with NEPTUNE (Figure 6B). Following injections at E9.5, the lingual epithelium of the tongue is well transduced; however, the underlying mesenchyme is negative (Figure 6C; brackets denote transduced cells; asterisks denote autofluorescence signal not transduced cells). In addition, there are positive clusters within the lingual epithelium, separated by negative sections (Figure 6C, white arrowheads), suggesting transduction of the papilla surface. Neural crest cells have been described in the underlying tongue mesenchyme and within the lingual epithelium, where they are involved in the development of taste papillae and taste buds22. Indeed, injections at E7.5 result in widespread transduction of the tongue mesenchyme at E13.5 (Figure 6D), suggesting that early injections target neural crest cells, contributing to mesenchyme in the tongue.
In vertebrates, the dorsal root ganglia (DRGs) are a central component of the peripheral nervous system (PNS), as all somatosensory input from the body's periphery (temperature, pain, pressure) is transmitted to the brain via activation of the DRG neurons23. Both neurons and glial cells of the DRG are derived from trunk neural crest cells24. Lentiviral injection, in which the ubiquitous hPGK promoter controls expression of the fluorescent reporter, leads to widespread targeting of the central and peripheral nervous system (Figure 7A), transducing both neurons and progenitors in the spinal cord (Figure 7B), as well as the DRG (Figure 7C). Using a MiniPromoter for doublecortin25 makes it possible to limit GFP expression to neurons only (15 and Figure 7D,E).
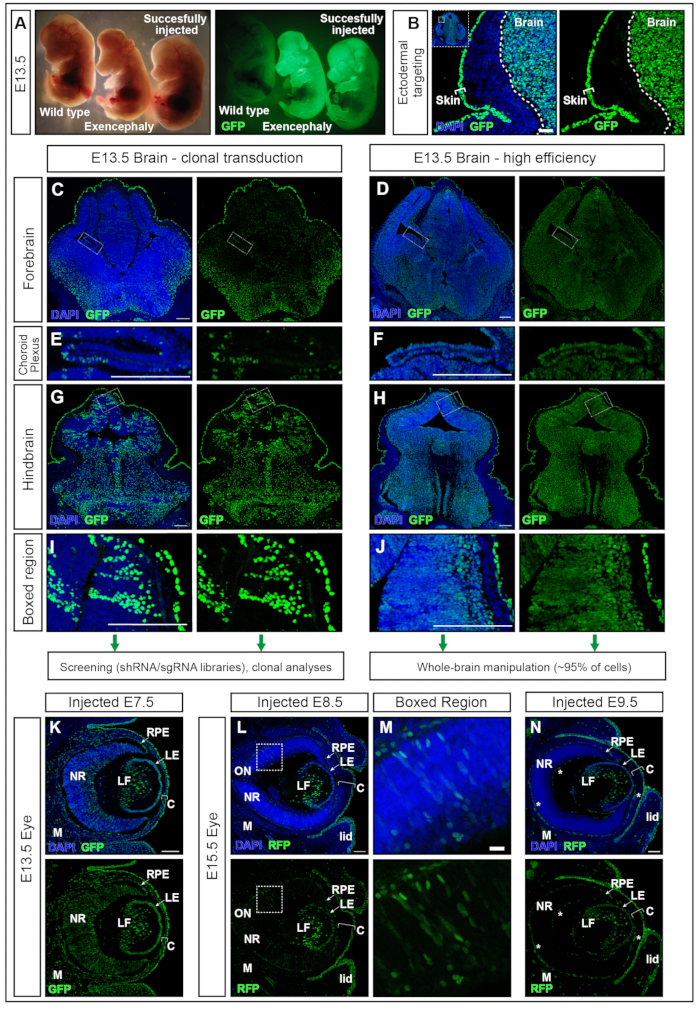
Figure 5: High efficiency or clonal transduction with NEPTUNE. (A) E13.5 embryos under a dissection microscope illuminated with standard lighting (left panel) and same embryos illuminated for GFP (right panel). Uninjected embryo on the far left, successfully injected embryo on the far right, yielding positive signal in the brain (rightmost embryo). Exencephalic embryo due to excess volume injected (middle embryo). (B) Skin and brain targeting with E7.5 injection. Scale bar = 50 µm. (C, D) E13.5 confocal images of forebrain with low clonal transduction (C) or high-efficiency transduction (D). (C, E, G, I) Clonal transduction of different regions in brain. (D, F, H, J) High-efficiency transduction of different regions in brain. Scale bars = 200 µm. Different transduction efficacies are representative of other areas of the CNS. (E, F) Choroid Plexus targeting, clonally (E) or with high efficiency (F). Scale bars = 200 µm. (G, H) Clonal targeting (G) and high-efficiency (H) targeting of hindbrain, magnified in (I, J). Scale bars = 200 µm. (K) GFP reporter expression in eye at E13.5 after in utero injection at E7.5 (L, M) GFP reporter expression in eye at E15.5 after in utero injection at E8.5 (L), boxed region magnified in (M), or at E9.5 (N). Autofluorescent blood vessels are marked with white stars. Scale bars = 100 µm. Abbreviations: NEPTUNE = neural plate targeting with in utero nano-injection; C = Cornea; LE = Lens Epithelium; LF = Lens fibers; M = Mesenchyme; NR = Neural Retina; ON = Optic Nerve; RPE = Retinal Pigment Epithelium; GFP = green fluorescent protein; CNS = central nervous system. Please click here to view a larger version of this figure.
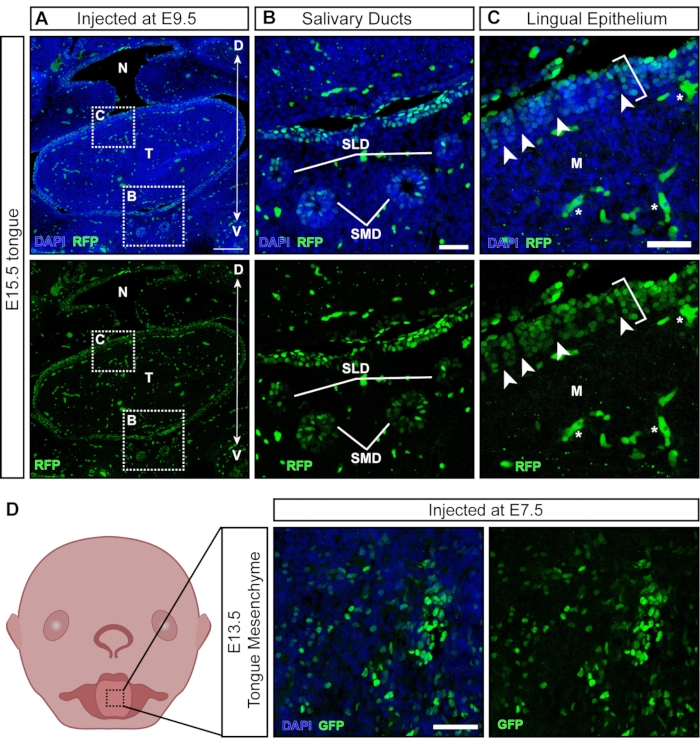
Figure 6: In utero transduction of non-neural tissues. (A) Confocal image of E15.5 oral cavity. Embryo was injected with fluorescent reporter lentivirus at E9.5. Scale bar = 200 µm. (B, C) (B) Magnification of inset panel; salivary duct epithelium transduced with virus. (C) Magnification of inset panel; dorsal lingual epithelium transduced with GFP reporter virus (white bracket). The underlying mesenchyme derived from neural crest cells is negative. White arrowheads indicate papillae with negative neural crest cells surrounded by virus-transduced epithelial cells (autofluorescent blood cells/vessels are marked with white stars). Scale bars = 50 µm. (D) Schematic and confocal image of E13.5 tongue mesenchyme after injections with fluorescent reporter virus at E7.5. Scale bar = 50 µm. Abbreviations: D = Dorsal; M = Mesenchyme; N = Nasopharynx; SLD = Sublingual Duct; SMD = Submandibular Duct; T = Tongue; V = Ventral; GFP = green fluorescent protein. Please click here to view a larger version of this figure.
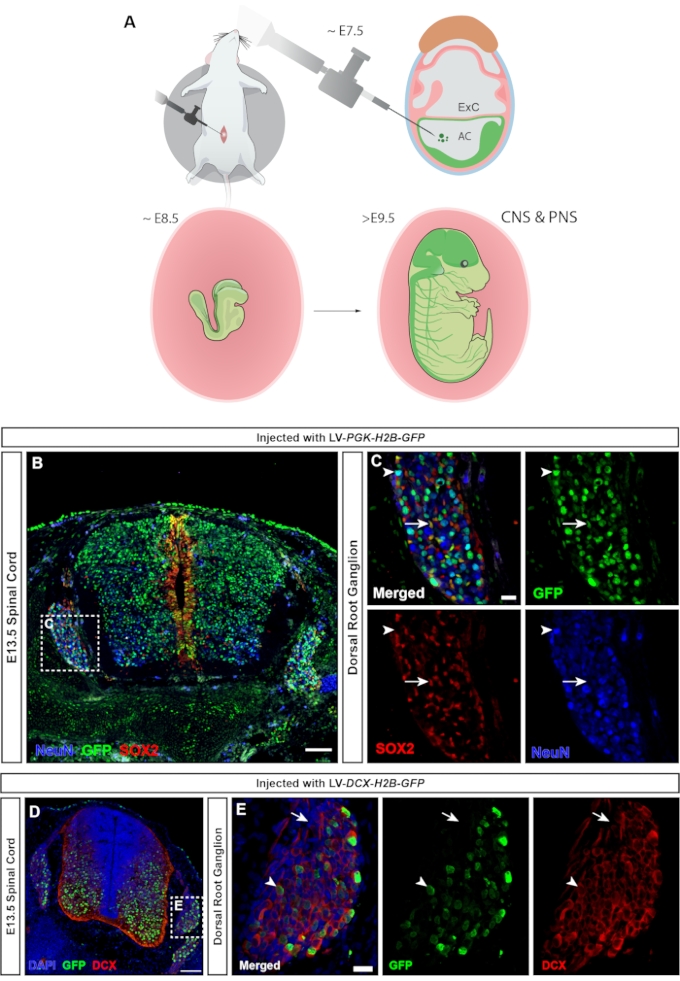
Figure 7: Transduction of neural crest-derived dorsal root ganglion cells. (A) NEPTUNE targeting at E7.5 allows targeting of both CNS and PNS. (B) Confocal image of E13.5 spinal cord and DRGs injected with hPGK-H2B-GFP reporter lentivirus at E7.5 shows transduction of both SOX2+ neural progenitors and NeuN+ neurons. Scale bar = 100 µm. (C) Boxed region of B showing the DRG with GFP expression in SOX2+ (white arrows) and NeuN+ (white arrowheads) cell populations. Scale bar = 20 µm. (D) Confocal image of E13.5 spinal cord and DRG injected with DCX-H2B-GFP lentivirus at E7.5, targeting only DCX+ cells. Scale bar = 100 µm. (E) Boxed region of D showing DRG with GFP expression restricted to DCX+ neurons (white arrowheads). DCX- cells are negative for GFP (white arrows). Scale bar = 20 µm. Abbreviations: NEPTUNE = neural plate targeting with in utero nano-injection; CNS = central nervous system; PNS = peripheral nervous system; DRGs = dorsal root ganglia; GFP = green fluorescent protein. Please click here to view a larger version of this figure.
Discussion
There are several steps in this protocol that influence embryonic survival, the quality of the injections, and the readout. The gestational age of embryos is defined as E0.5 at noon the day of the vaginal plug after overnight mating. Performing the ultrasound check for pregnancy at E6.5 in the late afternoon/evening ensures that the embryos are developed enough to be identified by ultrasound. The check (1) allows for prescreening of how many plug-positive mice are actually pregnant, (2) ensures no virus is thawed unnecessarily and wasted in the event of plug-positive mice not being pregnant, and (3) reduces unnecessary interventions on mice (avoids surgery on non-pregnant females).
At E7.5, embryos are sensitive to external forces and should be handled with care. For example, pulling on the uterine horns or squeezing the deciduas can lead to embryo resorption. The uterine tissue should always be kept moist when outside the female abdomen to prevent the tissue from drying. The majority of the deciduas should remain inside the female abdomen, with only 3-4 exposed for injections. Needle sharpness is another crucial determinant for successful injections. Blunt or broken needle tips result in repeated poking of deciduas or compression against the modeling clay before entering the AC, which can increase the resorption rate. Therefore, well-ground and sharp needles should always be stored safely and replaced after a maximum of 2 females.
This protocol describes how to target the neural plate with one single injection of lentivirus. Furthermore, it shows how transduction efficacy can be adapted from single-cell clones to the entire brain. However, other non-neural tissues, including the skin and oral epithelium, are targeted as well. In addition, all cell types (progenitors and differentiated cells) are transduced, making this approach efficient but nonspecific. The use of MiniPromoters in the viral construct leads to the specific expression of the transgene in neurons or astrocytes15. This has the advantage of avoiding the use of dedicated transgenic Cre animals and therefore reduces the amount of labor (strain maintenance and genotyping) and costs.
The limitations of NEPTUNE include its technical difficulty, challenges in obtaining pregnant females at a predictable and consistent rate, and the costs of acquiring specialized instrumentation. Furthermore, nonselective targeting of cells by lentivirus can be seen as both a benefit and a limitation of the technique. Injection of larger volumes into the AC results in exencephaly13, although brain malformations and exencephaly are avoided with the volumes described here15. A negative impact on brain development is thus a risk with in utero nano-injections that must be carefully avoided by injecting correct volumes adapted to the embryonic stage and AC size.
Future adaptations of the technique may focus on viral tropism. Adeno-associated viruses (AAVs) have different serotypes, which have been shown to robustly target different cell types in the CNS17,26. However, AAVs do not integrate into the host cell genome and therefore may be lost in cells with a high division rate. Although there are several ways to increase the specificity of NEPTUNE, transgenic animals are still the gold standard when it comes to gene manipulation in vivo. Cas9 mice and sgRNA-encoding lentivirus have been used for genetic screening in the embryonic epidermis27 and may also be adapted to the developing CNS.
Injections into the AC at E7.5 efficiently target cells of the neuroectoderm before initiation of neurulation and targets the developing brain more efficiently than in utero electroporation. This allows the study of genetic cues important for brain development from an earlier time point. In contrast to classical genetic mouse models, NEPTUNE offers a flexible approach to perform functional gene analysis. Phenotypes following overexpression or gene deletion can be studied within days to weeks compared to months or years. Injections of multiple viral constructs allow for the manipulation of several genes within one embryo and avoid the generation of double or triple knockout animals. Therefore, NEPTUNE not only saves time but also can reduce the number of animals used in research.
Acknowledgements
We thank Bettina Semsch and Jia Sun (Infinigene) for expert care of mice; Florian Salomons and Göran Månsson from Biomedicum Imaging Core (BIC) for assistance with image acquisition and consultation. Funding: We thank the following funders for their support of this project: The Swedish Research Council, Karolinska Institutet (KI Foundations, Career Development Grant, Ph.D. student KID funding, and SFO StratNeuro funding, the Center of Innovative Medicine), The Ollie and Elof Ericssons Foundation, the Tornspiran Foundation, the Jeansssons Foundation, Sven and Ebba-Christina Hagbergs Prize and research Grant, Knut and Alice Wallenberg Project Grant, Fredrik and Ingrid Thurings Foundation, Lars Hiertas Minne, The Childhood Cancer Foundation (Barncancerfonden), The Åhlen Foundation, Åke Wibergs Foundation, Tore Nilssons Foundation, and the Swedish Foundations Starting Grant to ERA. Figure 4D,E were created with BioRender.com.
Materials
| Name | Company | Catalog Number | Comments |
| 1 mL Syringe | BD Bioscience | 309628 | |
| 27 G Needle | BD Bioscience | 300635 | |
| 3.5 inches capillaries | Drummond Scientific | 3000203G/X | Were used to pull in house needles |
| 70 MHz MS Series transducer | Visual Sonics | MS700 | |
| Aquasonic clear ultrasound gel | Parker Laboratories | Mar-50 | |
| Autoclip Applier 9 mm | Angthos | 12020-09 | |
| CD1 mice | Charles River, Germany | Crl:CD1(ICR) | Females: from age of 8 weeks old Males: from the age of 12 weeks old |
| Cotton Swab | OneMed Sverige AB | 120788 | |
| DPBS | Gibco | 14190094 | |
| Dressing forceps delicate straight 13 cm | Agnthos | 08-032-130 | |
| EG-400 Narishige Micropipette Grinder | Narishige | NA | |
| EZ clips 9 mm | Angthos | 59027 | clips |
| Iris Scissors, Super Cut, straight, 9 cm | Agnthos | 307-336-090 | |
| Isofluorane | Baxter Medical AB | EAN: 50085412586613 | Purchased from Swedish Pharmacy |
| Kimwipes | Kimberly Clarke | 7557 | |
| Membrane Tape | Visual Sonics | SA-11053 | |
| Micropipette Puller | Sutter Instrument | P-97 | |
| Modeling Clay | Sense AB | 10209 | |
| Mouse Handling Table | Visual Sonics | 50249 | |
| Nanoject II Auto Injector Kit | Drummond | 3-000-205A | |
| Parafilm | Bemis | HS234526C | |
| Petri dish with central opening (low wall) | Visual Sonics | SA-11620 | |
| Petri dish, (ØxH): 92 x 16 mm | Sarstedt | 82.1472.001 | |
| Rely+On Virkon | DuPont | 130000132037 | disinfectant |
| Silicone membrane | Visual Sonics | SA-11054 | |
| Steri 250, hot bead sterilizer | Angthos | 31100 | |
| Surgical Tape (1.25 cm x 9.14 m) | Medicarrier | 67034 | |
| Vevo Compact Dual (Med. Air & O2) Anesthesia System | Visual Sonics | VS-12055 | |
| Vevo Imaging Station 2 | Visual Sonics | VS-11983 | |
| Vevo2100 | Visual Sonics | VS-20047 | |
| Vicryl 6-0; C-3 needle, 45 cm purple filament | Agnthos | J384H |
References
- Theiler, K. . The House mouse. Atlas of embryonic development. , (1989).
- Barresi, M. J. F., Gilbert, S. F. . Developmental biology. , (2016).
- Taylor, J. C., et al. Factors influencing success of clinical genome sequencing across a broad spectrum of disorders. Nature Genetics. 47 (7), 717-726 (2015).
- Pizzo, L., et al. Rare variants in the genetic background modulate cognitive and developmental phenotypes in individuals carrying disease-associated variants. Genetics in Medicine. 21 (4), 816-825 (2019).
- Sakai, Y. Neurulation in the mouse: Manner and timing of neural tube closure. The Anatomical Record. 223 (2), 194-203 (1989).
- Schoenwolf, G. C. Shaping and bending of the avian neuroepithelium: Morphometric analyses. Developmental Biology. 109 (1), 127-139 (1985).
- Smith, J. L., Schoenwolf, G. C., Quan, J. Quantitative analyses of neuroepithelial cell shapes during bending of the mouse neural plate. Journal of Comparative Neurology. 342 (1), 144-151 (1994).
- Katahira, T., Nakamura, H. Gene silencing in chick embryos with a vector-based small interfering RNA system. Development Growth and Differentiation. 45 (4), 361-367 (2003).
- Muramatsu, T., Mizutani, Y., Ohmori, Y., Okumura, J. I. Comparison of three nonviral transfection methods for foreign gene expression in early chicken embryos in ovo. Biochemical and Biophysical Research Communications. 230 (2), 376-380 (1997).
- Saito, T., Nakatsuji, N. Efficient gene transfer into the embryonic mouse brain using in vivo electroporation. Developmental Biology. 240 (1), 237-246 (2001).
- Beronja, S., Livshits, G., Williams, S., Fuchs, E. Rapid functional dissection of genetic networks via tissue-specific transduction and RNAi in mouse embryos. Nature Medicine. 16 (7), 821-827 (2010).
- Pierfelice, T. J., Gaiano, N. Ultrasound-guided microinjection into the mouse forebrain in utero at E9.5. Journal of Visualized Experiments JoVE. (45), e2047 (2010).
- Gaiano, N., Kohtz, J. D., Turnbull, D. H., Fishell, G. A method for rapid gain-of-function studies in the mouse embryonic nervous system. Nature Neuroscience. 2 (9), 812-819 (1999).
- Slevin, J. C., et al. High resolution ultrasound-guided microinjection for interventional studies of early embryonic and placental development in vivo in mice. BMC Developmental Biology. 6, 10 (2006).
- Mangold, K., et al. Highly efficient manipulation of nervous system gene expression with NEPTUNE. Cell Reports Methods. 1, 100043 (2021).
- Kameneva, P., et al. Single-cell transcriptomics of human embryos identifies multiple sympathoblast lineages with potential implications for neuroblastoma origin. Nature Genetics. 53 (5), 694-706 (2021).
- Kaiser, K., et al. MEIS-WNT5A axis regulates development of fourth ventricle choroid plexus. Development. 148 (10), (2021).
- Haddad, M. R., Donsante, A., Zerfas, P., Kaler, S. G. Fetal brain-directed AAV gene therapy results in rapid, robust, and persistent transduction of mouse choroid plexus epithelia. Molecular Therapy. Nucleic Acids. 2 (6), 101 (2013).
- Heavner, W., Pevny, L. Eye development and retinogenesis. Cold Spring Harbor Perspectives in Biology. 4 (12), 008391 (2012).
- Swamynathan, S. K. Ocular surface development and gene expression. Journal of Ophthalmology. 2013, 103947 (2013).
- Amano, O., Mizobe, K., Bando, Y., Sakiyama, K. Anatomy and histology of rodent and human major salivary glands: -overview of the Japan Salivary Gland Society-sponsored workshop. Acta Histochemica et Cytochemica. 45 (5), 241-250 (2012).
- Liu, H. X., Komatsu, Y., Mishina, Y., Mistretta, C. M. Neural crest contribution to lingual mesenchyme, epithelium and developing taste papillae and taste buds. Developmental Biology. 368 (2), 294-303 (2012).
- Marmigère, F., Ernfors, P. Specification and connectivity of neuronal subtypes in the sensory lineage. Nature Reviews. Neuroscience. 8 (2), 114-127 (2007).
- Zirlinger, M., Lo, L., McMahon, J., McMahon, A. P., Anderson, D. J. Transient expression of the bHLH factor neurogenin-2 marks a subpopulation of neural crest cells biased for a sensory but not a neuronal fate. Proceedings of the National Academy of Sciences of the United States of America. 99 (12), 8084-8089 (2002).
- Portales-Casamar, E., et al. A regulatory toolbox of MiniPromoters to drive selective expression in the brain. Proceedings of the National Academy of Sciences of the United States of America. 107 (38), 16589-16594 (2010).
- Tervo, D. G. R., et al. A designer AAV variant permits efficient retrograde access to projection neurons. Neuron. 92 (2), 372-382 (2016).
- Loganathan, S. K., et al. Rare driver mutations in head and neck squamous cell carcinomas converge on NOTCH signaling. Science. 367 (6483), 1264-1269 (2020).
Explore More Articles
This article has been published
Video Coming Soon
ABOUT JoVE
Copyright © 2024 MyJoVE Corporation. All rights reserved