A subscription to JoVE is required to view this content. Sign in or start your free trial.
Endovascular Perforation Model for Subarachnoid Hemorrhage Combined with Magnetic Resonance Imaging (MRI)
* These authors contributed equally
In This Article
Summary
Here we present a standardized SAH mouse model, induced by endovascular filament perforation, combined with magnetic resonance imaging (MRI) 24 h after operation to ensure the correct bleeding site and exclude other relevant intracranial pathologies.
Abstract
The endovascular filament perforation model to mimic subarachnoid hemorrhage (SAH) is a commonly used model - however, the technique can cause a high mortality rate as well as an uncontrollable volume of SAH and other intracranial complications such as stroke or intracranial hemorrhage. In this protocol, a standardized SAH mouse model is presented, induced by endovascular filament perforation, combined with magnetic resonance imaging (MRI) 24 h after operation to ensure the correct bleeding site and exclude other relevant intracranial pathologies. Briefly, C57BL/6J mice are anesthetized with an intraperitoneal ketamine/xylazine (70 mg/16 mg/kg body weight) injection and placed in a supine position. After midline neck incision, the common carotid artery (CCA) and carotid bifurcation are exposed, and a 5-0 non-absorbable monofilament polypropylene suture is inserted in a retrograde fashion into the external carotid artery (ECA) and advanced into the common carotid artery. Then, the filament is invaginated into the internal carotid artery (ICA) and pushed forward to perforate the anterior cerebral artery (ACA). After recovery from surgery, mice undergo a 7.0 T MRI 24 h later. The volume of bleeding can be quantified and graded via postoperative MRI, enabling a robust experimental SAH group with the option to perform further subgroup analyses based on blood quantity.
Introduction
Subarachnoid hemorrhage (SAH) is caused by the rupture of an intracranial aneurysm and poses a life-threatening emergency, associated with substantial morbidity and mortality, accounting for approx. 5% of strokes1,2. SAH patients present with severe headaches, neurological dysfunction, and progressive disturbance of consciousness3. Around 30% of SAH patients die within the first 30 days after the initial bleeding event4. Clinically, 50% of patients experience delayed brain injury (DBI) after early brain injury. DBI is characterized by delayed cerebral ischemia and delayed neurological deficits. Current studies have shown that the synergistic effects of several different factors lead to the loss of neurological function, including the destruction of the blood-brain barrier, the contraction of small arteries, microcirculatory dysfunction, and thrombosis5,6.
One unique aspect of SAH is that the pathogenesis originates from an extraparenchymal location but then leads to detrimental cascades inside the parenchyma: the pathology begins with the accumulation of blood in the subarachnoid space, triggering a multitude of intraparenchymal effects, such as neuroinflammation, neuronal and endothelial cell apoptosis, cortical spreading depolarization, and brain edema formation7,8.
Clinical research is limited by several factors, making the animal model a critical element in consistently and accurately mimicking the pathomechanistic changes of the disease. Different SAH model protocols have been proposed, e.g., autologous blood injection into the cisterna magna (ACM). Also, a modified method with a double injection of autologous blood into the cisterna magna and optic chiasm cistern (APC) respectively9,10. While autologous blood injection is a simple way to simulate the pathological process of vasospasm and inflammatory reactions after subarachnoid hemorrhage, the following rise of intracranial pressure (ICP) is relatively slow, and no noteworthy changes in the permeability of the blood-brain barrier are induced11,12. Another method, the periarterial blood placement, usually used in large SAH models (e.g., monkeys and dogs), involves placing anticoagulated autologous blood or comparable blood products around the vessel. The diameter changes of the artery can be observed with a microscope, serving as an indicator for cerebral vasospasm after SAH13.
Barry et al. first described an endovascular perforation model in 1979 in which the basilar artery is exposed after removing the skull; the artery is then punctured with tungsten microelectrodes, using a microscopic stereotactic technique14. In 1995, Bederson and Veelken modified the Zea-Longa model of cerebral ischemia and established the endovascular perforation, which has been continuously improved ever since15,16. This method is based on the fact that mice and humans share a similar intracranial vascular network, known as the circle of Willis.
For postoperative evaluation and grading of SAH in the mouse model, different approaches have been proposed. Sugawara et al. developed a grading scale that has been widely used since 200817. This method assesses the severity of SAH based on morphological changes. However, for this method, the mouse's brain tissue morphology must be examined under direct vision, and therefore, the mouse must be sacrificed for assessment. Furthermore, several methods for determining SAH severity in vivo have been established. Approaches range from simple neurological scoring to monitoring of intracranial pressure (ICP) to various radiological imaging techniques. Furthermore, MRI grading has been shown as a new, non-invasive tool to grade SAH severity, correlating to neurological score18,19.
Here, a protocol for an SAH model caused by endovascular perforation is presented, combined with postoperative MRI. In an attempt to establish a system to objectify the amount of bleeding in an in vivo setting, we also developed a system for SAH grading and quantification of total blood volume based on 7.0 T high-resolution T2-weighted MRI. This approach ensures the correct induction of SAH and exclusion of other pathologies such as stroke, hydrocephalus, or intracerebral hemorrhage (ICH) and complications.
Protocol
The experiments were performed in accordance with the guidelines and regulations set forth by Landesamt fuer Gesundheit und Soziales (LaGeSo), Berlin, Germany (G0063/18). In this study, C57Bl/6J male (8-12 weeks old) mice with a weight of 25 ± 0.286 g (average ± s.e.m.) were used.
1. Animal preparation
- Induce anesthesia by injecting ketamine (70 mg/kg) and xylazine (16 mg/kg) intraperitoneally. Maintain normal body temperature, contributing to quick induction of deep anesthesia. Test for adequate sedation with a pain stimulus, such as a toe pinch, and verify the absence of a reaction.
- Carefully shave the neck hair of the mouse with a razor, clean it with 70% ethanol followed by betadine/chlorhexidine, and apply 1% lidocaine on the skin surface for local pain control.
- Place the mouse in a supine position. Use tape to fix the limbs and tail, gently stretching the skin of the neck to the opposite side of the surgery. Simultaneously, elevate the neck slightly.
- Use ophthalmic ointment (e.g., 5% dexpanthenol) to prevent dehydration of the eyes during the operation.
2. SAH induction
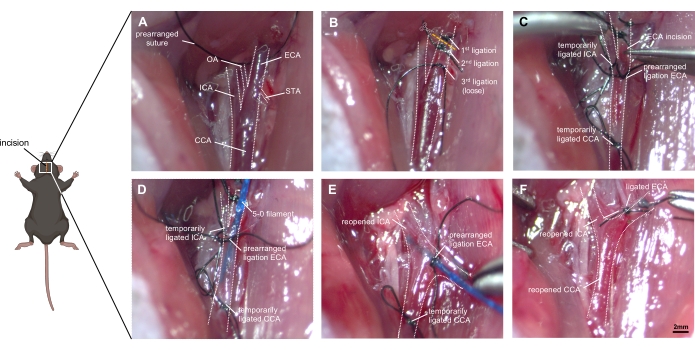
Figure 1: Step-by-step images of surgical technique. (A) Depiction of the exposed right carotid artery anatomy: the CCA and its bifurcation into ICA and ECA are identified, as well as the small branches of the ECA (OA and STA). (B) The ECA is mobilized from the surrounding tissue and ligated with two sutures before cutting it. A third ligation needs to be placed loosely near the bifurcation without occluding it. (C) The ICA and CCA are occluded temporarily (with either ligation or clips) to prevent excessive bleeding when the ECA is carefully incised. (D) The filament is inserted into the ECA and advanced into the CCA. The prearranged ligation must be tightened carefully so that no blood effusion occurs but advancing the filament remains possible. (E) The ICA and CCA are reopened, and the ECA stump needs to be adjusted to a cranial direction. By pushing the filament ~9 mm forward into the ICA, the ACA-MCA bifurcation will be reached, and the vessel is then perforated by pushing the filament ~3mm further. (F) The filament is withdrawn after ensuring a temporal re-ligation of the CCA. The prearranged ligation of the ECA is quickly occluded, and the CCA is reopened to allow reperfusion. Abbreviations: ACA = anterior cerebral artery, CCA = common carotid artery, ECA = external carotid artery, MCA = middle cerebral artery, ICA = internal carotid artery, OA = occipital artery, PPA = pterygopalatine artery, STA = superior thyroid artery. Scale bar = 2 mm. Please click here to view a larger version of this figure.
- Open the neck skin with a sterile scalpel, from the chin to the upper edge of the breastbone (1.5 cm), and bluntly separate salivary glands from their surrounding connective tissue.
- Separate the muscle group along one side [in this case, the right side] of the trachea, exposing the common carotid artery (CCA) sheath covered with nourishing blood vessels and venules. The CCA and the vagal nerve are located in close proximity to each other.
- Dissociate the CCA and leave a free 8-0 silk suture around the CCA without ligating it in advance. Pay attention to the protection of the vagal nerve, as it is easily damaged (Figure 1A).
- A triple bifurcation of the CCA, the ICA, and the ECA is visible along the lower posterior third of the diastasis. Dissect the distal end of the ECA and ligate the vessel twice as far distaally as possible.
- Disconnect the ECA at the midpoint of the twice ligated segment, creating a vessel stump.
- Prearrange one ligation for the filament around the ECA stump, do not close it until successful filament insertion.
- Use a suture or micro clip to occlude the ICA and CCA temporarily (Figure 1B).
- Make a small incision (approximately half of the ECA diameter) in the ECA using microvascular scissors. Insert a 5-0 (alternatively 4-0) prolene filament into the ECA and advance it into the CCA.
- Close the ligature on the ECA slightly while loosening the micro clip on the ICA and CCA (Figure 1C).
- Gently pull back on the filament and adjust the ECA stump in the cranial direction, invaginating the filament through the bifurcation into the ICA (Figure 1D).
- Point the filament tip medially at an angle of ~30° to the tracheal midline and ~30° to the horizontal plane. Push the filament forward inside the ICA. After reaching the ACA-MCA bifurcation, resistance is encountered (~9 mm).
- Advance the filament 3 mm further, perforating the right ACA. Promptly withdraw the filament to the ECA stump, allowing blood flow into the subarachnoid space.
- Keep the filament in this position for about 10 s (Figure 1E). The presence of muscle tremors, ipsilateral miosis, gasping for breath, altered heart rhythm, and urinary incontinence can be supporting evidence of successful surgery.
- Temporarily close the CCA to avoid excess blood loss. Pull out the filament instantly and ligate the ECA with the prearranged suture. Reopen the CCA and allow reperfusion and further effusion of blood into the subarachnoid space (Figure 1F).
- After checking for bleeding leakage, disinfect the skin surrounding the wound to prevent postoperative skin infections, and suture the wound with a non-absorbable 4-0 polyester fiber suture.
- Place the mouse in a thermal box until consciousness is regained. Wait until the animal is fully awake and ensure it has regained sufficient consciousness to maintain sternal recumbency. Do not return animals to the company of other mice until fully recovered.
- Administer 200-300 mg/kg body weight paracetamol for postoperative pain relief.
- Check on the mice daily after surgery.
3. MRI measurement
- 24 h after surgery, perform MRI using a rodent scanner (Table of Materials) and a dedicated mouse head resonator- here, a 20 mm transmit/receive quadrature volume resonator was used.
- Place the mouse on a heated circulating water blanket to ensure a constant body temperature of ~37 °C. Induce anesthesia with 2.5 % isoflurane in an O2/N2O mixture (30%/70%) and maintain with 1.5-2 % isoflurane via facemask under continuous ventilation monitoring.
- First perform a fast reference scan acquiring 3 orthogonal slice packages (Tri-Pilot-Multi, FLASH with repetition time TR/echo time TE = 200 ms/3 ms, 1 average, flip angle FA = 30°, field of view FOV = 28 mm x 28 mm, matrix MTX = 256 x 256, slice thickness 1 mm, total acquisition time TA =30 s).
- Then use a high resolution T2-weighted 2D turbo spin-echo sequence for imaging (imaging parameters TR/TE = 5505 ms/36 ms, RARE factor 8, 6 averages, 46 contiguous axial slices with a slice thickness of 0.35 mm to cover the whole brain, FOV = 25.6 mm x 25.6 mm, MTX = 256 x 256, TA = 13 min).
- If the result is unclear, use an additional respiration triggered T2*weighted gradient echo sequence with the same isodistance as the T2w scan (2D FLASH, TR/TE = 600 ms/6.3 ms, FA = 30°, 1 average, 20 axial slices with 0.35 mm thickness, FOV and MTX identical to T2w, TA = 5-10 min depending on the respiration rate).
- Transfer the data into the DICOM image format and use ImageJ software for SAH grading and volumetry of blood clots. Details on the quantification are listed as a step-by-step guide in the supplementary material (Supplementary Figure 1).
Results
Mortality
For this study, a total of 92 male C57Bl/6J mice aged between 8-12 weeks were subjected to SAH operation; in these, we observed an overall mortality rate of 11.9% (n = 12). Mortality occurred exclusively within the first 6-24 h after surgery, suggesting perioperative mortality as well as SAH bleeding itself as the most likely contributing factors.
SAH bleeding grade
A total of 50 mice received MRI 24 h postoperatively to confirm SAH and e...
Discussion
In summary, a standardized SAH mouse model induced by endovascular filament perforation operation is presented with minor invasion, short operative time, and acceptable mortality rates. MRI is conducted 24 h postoperatively to ensure the correct bleeding site and the exclusion of other relevant intracranial pathologies. Furthermore, we classified different SAH bleeding grades and measured bleeding volumes, allowing further subgroup analyses based on bleeding grade.
Adequate positioning of the ...
Disclosures
No conflicts of interest
Acknowledgements
SL was supported by the Chinese Scholarship Council. KT was supported by the BIH-MD scholarship of the Berlin Institute of Health and the Sonnenfeld-Stiftung. RX is supported by the BIH-Charité Clinician Scientist Program, funded by the Charité -Universitätsmedizin Berlin and the Berlin Institute of Health. We acknowledge support from the German Research Foundation (DFG) and the Open Access Publication Fund of Charité - Universitätsmedizin Berlin.
Materials
| Name | Company | Catalog Number | Comments |
| Eye cream | Bayer | 815529836 | Bepanthen |
| Images analysis software | ImageJ | Bundled with Java 1.8.0_172 | |
| Ligation suture (5-0) | SMI | Silk black USP | |
| Light source for microscope | Zeiss | CL 6000 LED | |
| Ketamine | CP-pharma | 797-037 | 100 mg/mL |
| MRI | Bruker | Pharmascan 70/16 | 7 Tesla |
| MRI images acquired software | Bruker | Bruker Paravision 5.1 | |
| Paracetamol (40 mg/mL) | bene Arzneimittel | 4993736 | |
| Prolene filament (5-0) | Erhicon | EH7255 | |
| Razor | Wella | HS61 | |
| Surgical instrument (Fine Scissors) | FST | 14060-09 | |
| Surgical instrument (forceps#1) | AESCULAP | FM001R | |
| Surgical instrument (forceps#2) | AESCULAP | FD2855R | |
| Surgical instrument (forceps#3) | Hammacher | HCS 082-12 | |
| Surgical instrument (Needle holder) | FST | 91201-13 | |
| Surgical instrument (Vannas Spring Scissors) | FST | 15000-08 | |
| Surgical microscope | Zeiss | Stemi 2000 C | |
| Ventilation monitoring | Stony Brook | Small Animal Monitoring & Gating System | |
| Wounding suture(4-0) | Erhicon | CB84D | |
| Xylavet | CP-pharma | 797-062 | 20 mg/mL |
References
- Macdonald, R. L., Schweizer, T. A. Spontaneous subarachnoid haemorrhage. The Lancet. 389 (10069), 655-666 (2017).
- van Gijn, J., Kerr, R. S., Rinkel, G. J. Subarachnoid haemorrhage. The Lancet. 369 (9558), 306-318 (2007).
- Abraham, M. K., Chang, W. -. T. W. Subarachnoid hemorrhage. Emergency Medicine Clinics of North America. 34 (4), 901-916 (2016).
- Schertz, M., Mehdaoui, H., Hamlat, A., Piotin, M., Banydeen, R., Mejdoubi, M. Incidence and mortality of spontaneous subarachnoid hemorrhage in martinique. PLOS ONE. 11 (5), 0155945 (2016).
- Okazaki, T., Kuroda, Y. Aneurysmal subarachnoid hemorrhage: intensive care for improving neurological outcome. Journal of Intensive Care. 6 (1), 28 (2018).
- Kilbourn, K. J., Levy, S., Staff, I., Kureshi, I., McCullough, L. Clinical characteristics and outcomes of neurogenic stress cadiomyopathy in aneurysmal subarachnoid hemorrhage. Clinical Neurology and Neurosurgery. 115 (7), 909-914 (2013).
- de Oliveira Manoel, A. L., et al. The critical care management of spontaneous intracranial hemorrhage: a contemporary review. Critical Care. 20 (1), 272 (2016).
- Schneider, U. C., et al. Microglia inflict delayed brain injury after subarachnoid hemorrhage. Acta Neuropathologica. 130 (2), 215-231 (2015).
- Delgado, T. J., Brismar, J., Svendgaard, N. A. Subarachnoid haemorrhage in the rat: angiography and fluorescence microscopy of the major cerebral arteries. Stroke. 16 (4), 595-602 (1985).
- Piepgras, A., Thomé, C., Schmiedek, P. Characterization of an anterior circulation rat subarachnoid hemorrhage model. Stroke. 26 (12), 2347-2352 (1995).
- Suzuki, H., et al. Heme oxygenase-1 gene induction as an intrinsic regulation against delayed cerebral vasospasm in rats. Journal of Clinical Investigation. 104 (1), 59-66 (1999).
- Dudhani, R. V., Kyle, M., Dedeo, C., Riordan, M., Deshaies, E. M. A Low mortality rat model to assess delayed cerebral vasospasm after experimental subarachnoid hemorrhage. Journal of Visualized Experiments: JoVE. (71), e4157 (2013).
- Iuliano, B. A., Pluta, R. M., Jung, C., Oldfield, E. H. Endothelial dysfunction in a primate model of cerebral vasospasm. Journal of Neurosurgery. 100 (2), 287-294 (2004).
- Barry, K. J., Gogjian, M. A., Stein, B. M. Small animal model for investigation of subarachnoid hemorrhage and cerebral vasospasm. Stroke. 10 (5), 538-541 (1979).
- Bederson, J. B., Germano, I. M., Guarino, L. Cortical blood flow and cerebral perfusion pressure in a new noncraniotomy model of subarachnoid hemorrhage in the rat. Stroke. 26 (6), 1086-1092 (1995).
- Veelken, J. A., Laing, R. J. C., Jakubowski, J. The Sheffield model of subarachnoid hemorrhage in rats. Stroke. 26 (7), 1279-1284 (1995).
- Sugawara, T., Ayer, R., Jadhav, V., Zhang, J. H. A new grading system evaluating bleeding scale in filament perforation subarachnoid hemorrhage rat model. Journal of Neuroscience Methods. 167 (2), 327-334 (2008).
- Egashira, Y., Shishido, H., Hua, Y., Keep, R. F., Xi, G. New grading system based on magnetic resonance imaging in a mouse model of subarachnoid hemorrhage. Stroke. 46 (2), 582-584 (2015).
- Mutoh, T., Mutoh, T., Sasaki, K., Nakamura, K., Taki, Y., Ishikawa, T. Value of three-dimensional maximum intensity projection display to assist in magnetic resonance imaging (MRI)-based grading in a mouse model of subarachnoid hemorrhage. Medical Science Monitor. 22, 2050-2055 (2016).
- Kothari, R. U., et al. The ABCs of measuring intracerebral hemorrhage volumes. Stroke. 27 (8), 1304-1305 (1996).
- Leclerc, J. L., et al. A comparison of pathophysiology in humans and rodent models of subarachnoid hemorrhage. Frontiers in Molecular Neuroscience. 11, 71 (2018).
- Titova, E., Ostrowski, R. P., Zhang, J. H., Tang, J. Experimental models of subarachnoid hemorrhage for studies of cerebral vasospasm. Neurological Research. 31 (6), 568-581 (2009).
- Marbacher, S., et al. Systematic review of in vivo animal models of subarachnoid hemorrhage: Species, standard parameters, and outcomes. Translational Stroke Research. 10 (3), 250-258 (2019).
- Marbacher, S., Fandino, J., Kitchen, N. D. Standard intracranial in vivo animal models of delayed cerebral vasospasm. British Journal of Neurosurgery. 24 (4), 415-434 (2010).
- Thompson, J. W., et al. In vivo cerebral aneurysm models. Neurosurgical Focus. 47 (1), 1-8 (2019).
- Frontera, J. A., et al. Prediction of symptomatic vasospasm after subarachnoid hemorrhage: The modified fisher scale. Neurosurgery. 59 (1), 21-26 (2006).
- Fisher, C. M., Kistler, J. P., Davis, J. M. Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by computerized tomographic scanning. Neurosurgery. 6 (1), 1-9 (1980).
- Wilson, D. A., et al. A simple and quantitative method to predict symptomatic vasospasm after subarachnoid hemorrhage based on computed tomography: Beyond the fisher scale. Neurosurgery. 71 (4), 869-875 (2012).
- Schüller, K., Bühler, D., Plesnila, N. A murine model of subarachnoid hemorrhage. Journal of Visualized Experiments: JoVE. (81), e50845 (2013).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved