Topical Application Bioassay to Quantify Insecticide Toxicity for Mosquitoes and Fruit Flies
In This Article
Summary
We describe the methodology and importance of the topical application bioassay to measure insecticide susceptibility in mosquitoes and fruit flies. The presented assay is high-throughput, utilizes insect mass-thus allowing for calculating a mass-relativized lethal dose instead of concentration-and likely has lower variability than other similar methods.
Abstract
The continued use of insecticides for public health and agriculture has led to widespread insecticide resistance and hampering of control methods. Insecticide resistance surveillance of mosquito populations is typically done through Centers for Disease Control and Prevention (CDC) bottle bioassays or World Health Organization (WHO) tube tests. However, these methods can result in a high degree of variability in mortality data due to variable insecticide contact with the insect, the relatively small numbers of organisms tested, extensive variation in mass between populations, and constantly changing environmental conditions, leading to variable outcomes. This paper presents the topical application bioassay, adapted as a high-throughput phenotypic bioassay for both mosquitoes and fruit flies, to test large numbers of insects along a range of insecticide concentrations.
This assay 1) ensures consistent treatment and insecticide contact with every organism, 2) produces highly specific dose-response curves that account for differences in average mass between strains and sexes (which is particularly important for field-collected organisms), and 3) allows for the calculation of statistically rigorous median lethal doses (LD50), which are necessary for resistance ratio comparisons-an alternative surveillance approach from diagnostic dose mortality, which is also used for larvicide resistance surveillance. This assay will be a complementary tool for accurately phenotyping mosquito populations and, as illustrated using fruit flies, is easily adaptable for use with other insects. We argue that this assay will help fill the gap between genotypic and phenotypic insecticide resistance in multiple insect species.
Introduction
Mosquitoes are responsible for over 700,000 deaths each year due to the diseases they transmit to humans, with over half of those deaths due to malaria alone1. The main preventive method against transmission of malaria and other vector-borne diseases is the use of insecticides, often in the form of long-lasting insecticide nets or indoor-residual spraying2. However, insecticide resistance is widespread among mosquitoes and other insect vectors, as well as agricultural pests3,4. To effectively manage resistance, surveillance is of key importance5. For this, highly accurate and high-throughput resistance detection methods are needed. Currently, the most widespread insecticide resistance surveillance tools for mosquitoes are the WHO tube test6 and the CDC bottle bioassay7. For fruit flies the residual contact application method (similar to the CDC bottle bioassay) is a commonly used insecticide bioassay8,9,10. However, variability in data from these methods is typically high, with measurements of the same laboratory mosquito strain ranging from ~20-70% mortality in CDC bottle assays and 0-50% in WHO tube tests when exposed to sublethal dosages11. Such variation is surprising because the limited genetic variation in most laboratory strains is expected to lead to limited insecticide susceptibility variation in the population. Nevertheless, there is still a high level of variation observed in the bioassay results.
Potential sources of this variation could be a result of heterogeneous insecticide exposure between specimens within the bioassay due to indirect insecticide exposure via the surface, heterogeneous environmental effects, normal biological variation between individuals of the same genotype, and variation in the mass of specimens of the same population12. An infrequently used method with higher replicability is the topical application bioassay. In this assay, the insecticide is directly applied to each insect13,14, removing the factor of heterogeneous exposure of different specimens within the same assay. However, due to the slow-throughput nature of this method, it is not routinely used as an insecticide susceptibility surveillance tool for mosquito populations. This paper presents a modified protocol for the topical application bioassay that allows for higher-throughput exposures while also correcting for variation in insect mass, a parameter that correlates to changes in insecticide susceptibility12. A reduction in noise and mass-associated variation in mortality data from variable insecticide exposure would allow for more accurate technical resistance surveillance11,15. Such data could be used to more accurately associate phenotypic resistance with genetic markers, fitness parameters, and/or vector competence. Additionally, we demonstrate how this assay could easily be adapted to other insect species by using the topical application bioassay on fruit flies, a smaller-bodied insect species.
The main limitation of the aforementioned residual contact applications is that insecticide exposure may vary from specimen to specimen within the same assay. In the case of CDC bottle bioassays and the contact method, insecticide exposure may vary between replicates of the same assay. The insects are exposed to insecticide that is either distributed on the inside of a glass bottle (CDC bottle bioassay and contact method) or on impregnated papers (WHO tube test). The concentration of insecticide on both surfaces (glass and paper) is known and predetermined by screening different species of known genotypes. However, the amount available to potentially be absorbed by the insect can greatly vary depending on the surface used, the insecticide mixture components, and how homogeneously the insecticide is distributed across the surface material16,17. In the CDC bottle bioassay, the insecticide coating on the inside of the bottle is dependent on procedures employed by each laboratory and user. In the WHO tube test, the insecticide-treated papers are centrally produced and thus most likely quite homogeneous across labs. However, in the WHO tube test, the exposure tube allows specimens to land and rest on non-insecticide-exposed metal mesh, leading to potential heterogeneous insecticide exposure among the specimens within each test. The actual amount of insecticide picked up and absorbed by specimens via each method still needs to be explored further18.
Additionally, the CDC bottle bioassay, WHO tube test, and contact method are most commonly used as threshold assays testing only one predetermined insecticide concentration. This approach can accurately detect the presence of resistance and is valuable for resistance surveillance (especially when resistance is spreading). However, threshold assays cannot quantify the strength of the resistance, which might be more predictive of the efficacy of intervention tools. If multiple insecticide concentrations are used with these methods, then they can be used as intensity assays. Intensity assays for the CDC bottle bioassay and the WHO tube test have been introduced by testing 5x and 10x the predetermined discriminating dosages to address this gap in surveillance6,19. While providing greater ability to differentiate between resistant populations, 3-5 (predetermined) dosages provide limited resolution to calculate lethal concentrations. Additionally, mosquitoes of various sizes are used in such assays. Yet, the mass is important to measure as larger specimens might need a higher dose to be killed as the effective dose per unit of mass will be much lower than that of a smaller organism12. Calculating a mass-relativized lethal dose (amount of insecticide per insect mass) would be a more useful metric than the more common lethal concentration (e.g., amount of insecticide per surface area) as it considers the variation of insect mass between sexes, populations, and genotypes. Such data would help fill the gap between genotypic and phenotypic resistance within the laboratory and the field and could also provide an easy way to calculate the needed application concentration to treat a population of insects of a known average mass.
The use of mass-relativized lethal dosages that kill 50% of the specimens (LD50) also incorporates several other benefits. Assessment of the toxicity of a specific compound in mg/kg (= ng/mg) is standard in human and veterinary toxicology14, and LD50 values are found on material safety data sheets. Lethal dosages also allow direct comparison of toxicity between different chemicals toward a particular species or the same chemical toward different species20, as well as high-quality evaluation of novel insecticides and chemicals13. Additionally, the LD50 can provide more meaningful and accurate resistance ratios than those derived from diagnostic dose mortality results, which can result in an overestimation of the resistance level present in a population. Therefore, this assay would be suitable for routine surveillance programs by providing more rigorous resistance monitoring based on mass-relativized lethal doses derived from more specimens than recommended for other bioassays21.
The topical application method has been used in insecticide susceptibility surveillance for mosquitoes and flies as an alternative for the standard insecticide susceptibility bioassays when resistance is already known or suspected22,23, as well as for surveillance in some pest insects24 to more accurately assess resistance profiles and insecticide intrinsic toxicity21. In topical application bioassays, the insecticide is applied to each organism, resulting in minimal variation in insecticide exposure. This paper presents a slightly adapted and improved method that allows for insecticide exposure to be applied to a large number of insects in a short period while also controlling for insect mass22. This higher-throughput method with good levels of replicability could be a useful additional tool for routine insecticide susceptibility surveillance.
Protocol
NOTE: Insecticides can cause human, animal, and environmental hazards25. Caution, training, and personal protective equipment are highly advised. Be sure to follow the material safety data sheets for all insecticides and solvents used.
1. Rear specimens
- Rear 3-5-day-old adult mosquitoes.
NOTE: The protocol below reflects conditions for Aedes aegypti rearing, closely following Food and Agriculture Organization of the United Nations guidelines26.- Rear mosquitoes of all life stages at 27 ± 1 °C and 75 ± 5% relative humidity with 12:12 h light and dark cycling.
- Hatch the mosquito eggs by submerging them in deionized water and adding yeast26, or place the submerged eggs inside a vacuum chamber for 30 min.
NOTE: Both methods decrease the oxygen content within the water and increase hatching27. - Feed the newly hatched larvae fish food (or an equivalent diet such as ground cat kibble) within trays and keep the larval density as similar as possible between trays as larval density impacts development12 (e.g., 200-250 larvae per tray containing a total of 1.5 L of water).
- Feed the larvae every other day until they reach the pupal stage (approximately 7-10 days), increasing the amount of food as needed.
NOTE: When fed too little, larval growth will be stunted, and the larvae may eat one another. When fed too much, the larvae may die, causing the water to go foul. - Once pupae develop, transfer them daily to a water bowl in adult mosquito cages and provide 10% sucrose solution ad libitum.
- Record the first day of adult emergence. Remove the remaining pupae from the cage 2 days after emergence starts.
NOTE: Male mosquitoes emerge faster. Note the emergence of males and females separately and ensure sufficient males and females are available for each test. - Wait for 3 days after removing the pupae to achieve 3-5-day-old mosquitoes for testing.
- Rear fruit flies (loosely following protocols of the University of Zurich28).
- Rear Drosophila strains in stock bottles at 23 ± 1 °C and 60 ± 5% relative humidity with 12:12 h light and dark cycling.
NOTE: Drosophila stock bottles should contain 75 mL of a standard fly medium, which is first poured as a liquid into the bottom of the bottles and then allowed to solidify overnight. - Transfer colonies to new stock bottles with fresh food every two weeks to prevent overpopulation and mold growth. To do this, knock down flies using a hand-held carbon dioxide (CO2) dispenser, transfer the anesthetized flies to a weighing paper on an ice pack or chill table, and brush the flies into a fresh stock bottle using a fine-tipped paintbrush. Be sure to keep the bottles on their sides during this process to prevent flies from falling into the food and drowning.
- Rear Drosophila strains in stock bottles at 23 ± 1 °C and 60 ± 5% relative humidity with 12:12 h light and dark cycling.
2. Prepare insecticide formulations using the gravimetric approach
- Make the first stock solution following the gravimetric approach using an analytical scale with 0.1 mg accuracy inside a fume hood.
NOTE: The gravimetric approach uses mass to measure the amounts of insecticide and solvent added. The standard practice (volumetric approach) will require an analytical scale to measure the amount of (solid) insecticide added when the first stock solution is prepared; however, the amount of solvent added and all following dilutions are measured by volume only. The gravimetric approach has a higher level of accuracy and is therefore preferred.- Determine the target insecticide concentration and target volume (maximum 10 mL is recommended if using 15 mL conical tubes to prevent spillage when storing in a freezer) for the first stock solution and calculate how much insecticide active ingredient (AI) to add using Eq (1):
 (1)
(1) - Prepare a storage tube (15 mL conical tube recommended for larger volumes, 1.5 mL microcentrifuge screw cap tubes recommended for volumes of 1 mL or less) and label with insecticide and solvent name, target concentration, and preparation date. Place the tube and lid on the scale within a rack or holder and tare the scale.
- Weigh the desired amount of solid or liquid insecticide AI determined from step 2.1.1. (e.g., deltamethrin used for the representative data) into the tube and record the mass.
- Tare the scale and add the desired volume of solvent (equivalent to the target volume) to the tube, close the lid immediately, and record the mass. Close the tube's lid immediately after adding the solvent (acetone used here) to avoid evaporation and mix the solution.
- Record the room temperature. Some solvents, such as acetone, can have significant changes in volume (and consequently density) depending on temperature.
- If storing immediately, wrap the tube's lid in parafilm (to reduce evaporation), place it in a tube rack/holder (to keep upright and prevent leaking), cover in foil (to prevent UV exposure), place it in a resealable plastic bag (to reduce evaporation), and place the bag in a -20 °C freezer. If not stored immediately, make sure the lid is secured and cover in foil or a light-protected container.
- Calculate the stock solution's actual concentration (mg/mL) by dividing the mass of insecticide AI added by the volume of solvent added (and the volume of insecticide added if in liquid form). To calculate the volume of added solvent (or liquid insecticide), divide the mass added by the known density that is appropriate for the recorded temperature.
- Calculate the density (g/mL) of the stock solution by dividing the total mass added (insecticide and solvent) by the total volume added (solvent and insecticide, if in liquid form). See step 2.1.7 for converting liquid mass to volume.
- Determine the target insecticide concentration and target volume (maximum 10 mL is recommended if using 15 mL conical tubes to prevent spillage when storing in a freezer) for the first stock solution and calculate how much insecticide active ingredient (AI) to add using Eq (1):
- Serially dilute the initial stock solution via 10% dilutions. If needed, use these serial dilutions to create an initial dose-response curve to identify the target range of insecticide concentrations for the bioassay.
- Calculate the volume of insecticide stock solution and the solvent to add to each tube (e.g., 1 mL of insecticide stock solution diluted in 9 mL of solvent for a 10 mL dilution of 10% of the previous concentration).
- Vortex the stock solution for 10 s. Tare a prelabeled first dilution tube on the scale. Add the required volume of stock solution to the first dilution tube using a pipette. Immediately close the lid of both tubes and record the mass in the first dilution tube.
- Tare the first dilution tube again and add the required volume of solvent. Close the lid immediately, record the mass of the added solvent, and vortex the first dilution for 10 s.
- Repeat steps 2.2.2 and 2.2.3 for the remaining dilutions.
- Store all dilutions as described above in step 2.1.6.
- Calculate the actual concentrations of the dilutions by following step 2.1.7.
- Calculate the density of each insecticide dilution by dividing the total mass added (insecticide solution and solvent) by the total volume added (insecticide solution and solvent). For each serial dilution, use the previous insecticide stock dilution's density to calculate the new dilution's density following Eq (2):
 (2)
(2)
- Optional: Create insecticide dilutions with smaller increments by serial dilution.
- Select the concentrations and volumes of each new solution to make with the aid of a dose-response curve of the initial serial dilutions, previous trials, or published literature.
NOTE: Chosen concentrations should result in a mortality range of 0-100%, with a minimum of three concentrations from this range to allow for Probit analysis. - Use the serial dilutions as stock solutions to make each new dilution and follow step 2.2 to create the new dilutions between the 10-fold dilutions.
- Select the concentrations and volumes of each new solution to make with the aid of a dose-response curve of the initial serial dilutions, previous trials, or published literature.
- Optional: Aliquot the insecticide solution. If larger volumes of the insecticide solutions are made, aliquot the solutions into 1.5 mL screw-cap tubes to avoid contamination, evaporation, and degradation of the stock solutions from frequent handling and exposure to light.
- Aliquot the solutions, starting from the lowest concentration and work towards the highest concentration to reduce potential contamination. Mix each stock solution by vortexing for 10 s before opening and pipetting the desired volume (e.g., 0.5 mL) into a prelabeled screw cap tube.
- Store the aliquots in a light-resistant container in a -20 °C freezer.
NOTE: It is recommended to regularly (monthly) replace aliquots with small new aliquots taken directly from the stock pesticide dilutions. This will limit the potential for contamination to be carried over into other experiments or changes due to evaporation or UV degradation while the samples are used on the bench. The protocol can be paused here and restarted even years later, as long as the insecticide solutions are stored properly (see step 2.1.6) and kept in the -20 °C freezer.
- Use a permanent marker pen to mark the meniscus before storing to monitor solvent evaporation. When removing insecticide solution to make aliquots, mark the meniscus every time the solution is removed.
3. Prepare topical application bioassay workspace
NOTE: It is recommended to work in a benchtop insect handling tent for easier capture of escaping mosquitoes or flies. See Supplemental Figure S1 for images of an insect handling tent.
- Remove the needed insecticide solutions from the freezer, vortex immediately, and place them in a light-resistant container at room temperature to let the insecticides warm to room temperature before using.
NOTE: Insecticide AIs can separate from the solvent at cooler temperatures. Additionally, acetone volume changes with temperature, which can alter the applied insecticide dose. Mixing the solutions and allowing them to warm to room temperature helps ensure consistency when using the insecticide solutions. - Set out all needed tools and materials for the topical application assay in the insect handling tent as referenced in the Table of Materials.
- Clean the syringe barrel and needle with analytical grade acetone by completing 5 washes per acetone aliquot. Complete this with 5 separate aliquots for a total of 25 washes. See Supplemental Figure S2 for syringe and repeater pipettor parts.
- Set out 5 microcentrifuge tubes with 0.5 mL of acetone each.
- Fill the syringe barrel with 0.025 mL of acetone from the first tube and then expel the acetone into a waste container by swiftly pushing down on the plunger. Repeat four more times to complete a total of five acetone washes from the same acetone aliquot. Then, fill the syringe barrel completely with air and expel the air and potential acetone remnants into the waste container. Repeat two more times to complete three "washes" with air.
- Repeat step 3.3.2 for the remaining 4 tubes of acetone.
- Create an air pocket within the barrel between the syringe plunger and the top of the needle by pulling up the plunger slightly into the barrel (~5 mm).
NOTE: This air pocket protects the plunger from contacting the insecticide solutions and reduces insecticide carryover. - Set the syringe aside until ready to use for topical application.
- Create a key containing the doses to be applied and assign random IDs following random number or letter generators (see Supplemental File 1).
- Label the plastic holding cups with the random ID for blind mortality assessment.
NOTE: If needed, the protocol can be paused here and restarted at a later day and time. If more than a few hours pass while pausing, it is encouraged to repeat step 3.3 to ensure the syringe is clean and to place the insecticide solutions back in the freezer until about an hour before dosing the insects and then repeat step 3.1.
4. Prepare specimens for the topical bioassay. See Figure 1 for a procedural overview
- Sort and weigh the mosquitoes
- Using an aspirator powered by suction from inhalation, aspirate the desired number of 3-5-day-old adult mosquitoes needed for the assay, including an excess to account for damaged individuals. Transfer the mosquitoes into a conical tube (up to 100 mosquitoes per tube) by placing the tip of the aspirator into the tube with cotton wrapped around the tip and gently exhale and tap the aspirator. Use the cotton to plug the tube when the aspirator tip is removed and then cap with the lid. Avoid filling the aspirator and tubes with too many mosquitoes at once, as this adds additional stress on the mosquitoes and can cause death.
- Briefly knock down the mosquitoes in the tubes by placing them for a minimum of 10 min at 4 °C or burying them under ice in an ice tray.
NOTE: Mosquitoes can be held at 2 °C for several hours with minimal mortality29; however, it is best to minimize the duration for which the mosquitoes are on ice to reduce potential negative effects. - Transfer the knocked down mosquitoes to the insect handling tent and carefully tip the mosquitoes out onto a plastic tray (e.g., Petri dish) placed on the ice. Pour only about 50 mosquitoes at a time to ensure each touches the cool tray beneath it and stays knocked down.
- Sort the mosquitoes by sex by gently picking them up by the leg(s) (or wings) with forceps and place each sex into a separate holding cup. Count the number of mosquitoes of each sex while sorting and stop when the desired number is reached. While sorting, remove any mosquitoes that are injured (e.g., missing legs) or are extra-large (e.g., abnormally enlarged abdomen) or small (easily distinguished with the naked eye as smaller than the average mosquito size of that population).
NOTE: Handling the mosquitoes by the appendages reduces structural damage to their soft primary bodies (e.g., abdomen). - Record the weight of each cup of mosquitos using an analytical scale with 0.1 mg precision.
- Place an empty cup with a Petri dish as a lid on the scale and tare the scale. Pour the mosquitoes into the container, place the lid on top, and place the container on the scale.
- Record the combined weight and number of specimens on the score sheet (see Supplemental File 2). Immediately place the cup of specimens back on ice to keep them immobilized.
- Repeat steps 4.1.5.1-4.1.5.2 until all cups of specimens are weighed.
- Divide the prepared mosquitoes into groups of 20-25 in separate cups placed on ice labeled with the random IDs. When transferring mosquitoes, aim to reduce stress and physical damage caused by the forceps. Ideally, pick the mosquitoes up using forceps 1-2 times only: once for sorting/weighing and a potential second time for transfer to the experimental cups.
NOTE: An ideal number of mosquitoes per cup is 20-25, which is enough for a replicate, is reasonable to assess the mortality, and should not result in density-induced stress/death in the cup.
- Sort and weigh the fruit flies
- Anesthetize the flies using CO2 for 7 s.
NOTE: If flies are exposed to CO2 for more than 7 s, they may have trouble crawling and flying when they awaken30. - Pour the flies onto an ice pack wrapped in bench paper and use a fine-tipped paintbrush to separate and count the males and females.
- Use the paintbrush to gently pick up the chosen flies and place them into a clean, empty stock bottle. Choose equal numbers of male and female fruit flies (e.g., 15 males and 15 females) and label the stock bottles with the strain name and fruit fly total (e.g., Canton-S, 30 flies).
NOTE: It is important to have equal numbers of female and male fruit flies because male fruit flies can experience heightened aggression towards each other after being removed from the presence of females31. Therefore, to avoid non-insecticide mortality or injuries, it is best to have equal numbers of males and females (or omit male fruit flies completely). - Record the weight of each bottle of fruit flies using an analytical scale.
- Place an empty vial (labeled with a random ID, refer to step 3.4) with a Petri dish as a lid on the scale and tare the scale.
NOTE: Glass vials are recommended for use with fruit flies as they significantly reduce the static. - Anesthetize the bottle of fruit flies corresponding to the vial's random ID using CO2 for 7 s.
- Pour the fruit flies onto weighing paper and use the paper as a funnel to introduce the flies into the vial. Place the Petri dish lid on top of the vial of fruit flies and place it on the scale.
- Record the combined weight and number of specimens on the score sheet and then immediately place the vial of fruit flies in a tray of ice, with the lid still on top to prevent the flies from escaping.
- Repeat steps 4.2.4.1-4.2.4.4 for each bottle of fruit flies.
- Place an empty vial (labeled with a random ID, refer to step 3.4) with a Petri dish as a lid on the scale and tare the scale.
- Anesthetize the flies using CO2 for 7 s.
- When the above steps are complete, immediately move on to the next section.
5. Dose specimens
- Load the syringe with the proper insecticide concentration. Start with the least concentrated dose and work towards the most concentrated dose with each group of organisms. To prevent waste, only load the syringe with the needed volume of insecticide plus a recommended extra 2 µL.
- Tip the specimens onto weighing paper(s) placed atop a tray on the ice. Separate the specimens that are close together using a clean, insecticide-free paintbrush or cotton swab to allow easy access to each specimen for dosing. For mosquitoes, use the paintbrush also to ensure that each specimen is laying on their dorsum and their ventral surface is facing up.
- Using the syringe, apply one droplet of insecticide solution (or acetone for the control) to the ventral thorax and abdomen area for mosquitoes and the dorsum for fruit flies. Apply a 0.2 µL droplet (which requires a 10 µL syringe) for smaller sized insects such as fruit flies and a 0.5 µL droplet (which requires a 25 µL syringe) for mosquitoes.
NOTE: Insecticide sensitivity does not significantly differ between primary body parts (such as the head, thorax, and abdomen) compared to appendages (such as wings, legs, or proboscis)32. Therefore, the application site does not have to be exact as long as the dose droplet is applied to the primary body. The ventral thorax and abdomen area are chosen for mosquitoes because they often lay on their dorsal side when knocked down, whereas the dorsum is chosen for fruit flies because they often lay on their ventral side when knocked down. This decreased specificity of the application site helps increase the throughput of this method. - Immediately pour the specimens back into the labeled plastic cup and cover the cup with netting and a rubber band. Place the cup into a holding tray and note on the cup any specimens that were killed, damaged, or escaped in this process (to exclude them in the final count of specimens in that cup). For the first cup, record the time when dosing is completed.
- Replace the weighing paper(s) on which the specimens are placed to avoid insecticide contamination between doses.
- Repeat dosing for each cup until all specimens have been dosed with the proper insecticide concentrations and record the ending time when all specimens have been dosed.
- Provide 10% sucrose solution to each cup via a soaked cotton ball and set the cups aside until mortality is assessed the following day. Store the mosquitoes at 27 ± 1 °C with 75 ± 5% relative humidity5 and the fruit flies at 23 ± 1 °C with 60 ± 5% relative humidity.
NOTE: Be careful while squeezing the cotton balls to avoid oversaturation or undersaturation. The cotton balls should be moist but not dripping. Dripping sugar water in the cup can lead to mortality of the specimens and thus impact the mortality assessment of the insecticide.
6. Assess mortality
- Record specimen mortality at 24 h after the start of insecticide exposure. Classify mosquitoes as alive if they can fly and hold themselves upright; as dead if they are immobile or ataxic (unable to stand or take off for flight), as described by the WHO6. Follow the same mortality assessment for fruit flies8,33.
NOTE: To assess delayed mortality, mortality can additionally be assessed after 48 and 72 h with daily sugar water changes. - After mortality is recorded, place all the cups of specimens in a contained bag in a freezer for at least 1 h to ensure all specimens are dead before disposal or subsequent use (e.g., molecular or chemical analysis).
7. Perform replicates
- Repeat steps 3-6 on a new set of specimens, taking care to perform replicates at the same time each day, as insecticide susceptibility can change depending on the time of day34.
- Ensure a minimum of 3 replicates for each concentration for accurate estimation of the lethal dose that kills 50% of the specimens (LD50). Include more replicates if a high level of variability is observed.
- Complete the analysis after all data are collected.
8. Analyze the results
- Record data in a spreadsheet program and use the random ID key to unmask the data (reference step 3.4). Save the data as a text file (see example data in Supplemental File 3) for analysis in the statistical program R35 (see example R code in Supplemental File 4) or other software of choice36.
- Within the software program, complete the following analysis. See Supplemental File 4 for an example R code.
- Calculate the dose of insecticide (ng) per specimen mass (mg) following Eq (3) below:
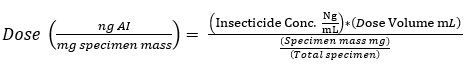 (3)
(3) - Calculate mortality and apply Abbott's formula37 to correct mortality relative to the mortality observed in each control37. Alternatively, use the Schneider-Orelli (1947) formula to correct mortality38. With either formula, apply the correction to all data regardless of mortality in each control, as previously described37 and implemented39, unless the control data are unusually high (see discussion below).
NOTE: Abbott's formula and equivalent alternatives, such as the Schneider-Orelli formula, adjust mortality values proportionately to the extent of mortality not observed in the controls and will not cause a decrease in mortality for cups that had 100% mortality. For more information, see the cited references for these formulas. - Transform corrected mortality data into probit (probability unit) values40 and perform linear regression between the insecticide dose and transformed mortality data. Use a chi-square test to assess the fit of the linear model(s).
NOTE: Mortality values of 0 (0% mortality) or 1 (100% mortality) are removed from the data before completing the probit transformation. This is necessary due to the nature of the probit transformation. As such, the graphed data will not include positive or negative controls or any other data that resulted in 0% or 100% mortality (after Abbott's correction has been applied). - Calculate the LD50 and 95% confidence intervals (CIs) per specimen strain, population, and/or sex following previously published methods39,41,42.
- NOTE: If the 95% CIs of two strains do not overlap, the strains have significantly different dose responses.
- If applicable, calculate resistance ratios (RRs) by dividing the LD50 of the strain of interest by the LD50 of the reference/control strain.
- Calculate the dose of insecticide (ng) per specimen mass (mg) following Eq (3) below:
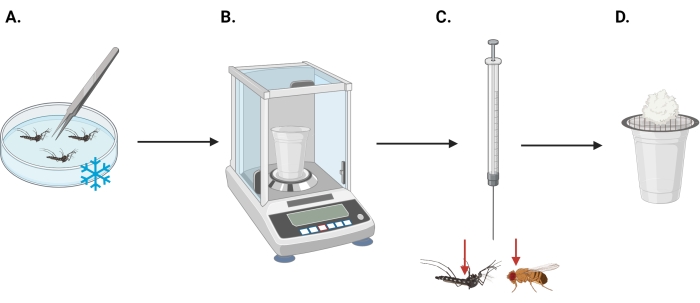
Figure 1: Topical application assay protocol diagram. Topical application assay protocol begins with (A) sorting specimens on ice, followed by (B) weighing specimens on an analytical scale, (C) dosing specimens with insecticide solution(s), and (D) 24 h waiting period post insecticide exposure with access to 10% sucrose solution ad libitum (via a soaked cotton ball), followed by mortality assessment. Red arrows indicate target insecticide application location for mosquitoes (left) and fruit flies (right). Note that the image is not to scale. Please click here to view a larger version of this figure.
Representative Results
These representative results feature two different strains of Ae. aegypti, Rockefeller (ROCK), and an isolated field strain from Florida with known knockdown resistance mutations F1534C and V1016I (IICC genotype). Additionally, Drosophila melanogaster (Canton: S strain) is featured.
Figure 2 and Figure 3 illustrate the dose response of each organism by strain and sex tested following the above protocol. As no differences were observed between the dose-response curves of male and female mosquitoes within each strain (t = 1.70, p = 0.098 for ROCK and t = 0.64, p = 0.527 for IICC), data from both sexes within each mosquito strain were pooled. The mass-relativized LD50 for ROCK and IICC are 0.008 ng/mg (95% CI: 0-0.104) and 0.336 ng/mg (95% CI: 0.235-0.438), respectively. The 95% CIs of these values do not overlap, indicating significantly different dose responses of the strains. The RR of the IICC strain (relative to the ROCK strain) is 41.7, which according to the WHO, is considered highly resistant5. For the Canton-S fruit flies, the mass-relativized LD50 is 0.213 ng/mg (95% CI: 0-0.490).
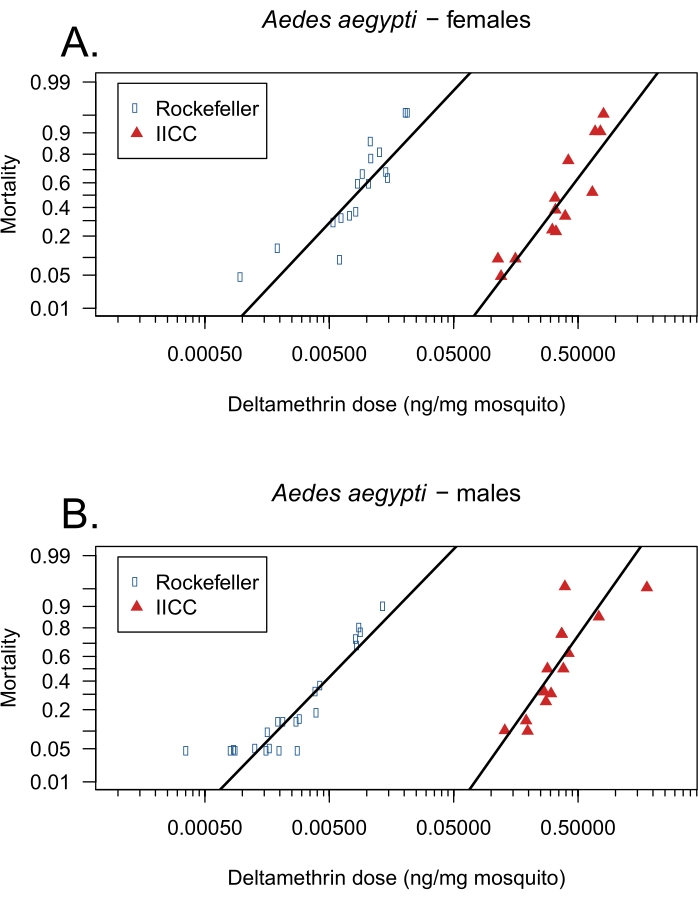
Figure 2: Representative data of mosquitoes using topical application bioassay. Representative dose-response data from topical application bioassay following the above protocol using deltamethrin and mosquitoes: (A) female Ae. aegypti ROCK (n = 880) and IICC (n = 550) strains, (B) male Ae. aegypti ROCK (n = 880) and IICC (n = 569) strains. Deltamethrin testing concentrations ranged from 0.00075 ng/µL to 9.68705 ng/µL, and the dose of deltamethrin applied (ng) per average mosquito mass (mg) is reflected on the x-axis. Mortality is shown as a proportion on the y-axis. The black line through each data point cluster represents the strain and sex-specific linear regression. Please click here to view a larger version of this figure.
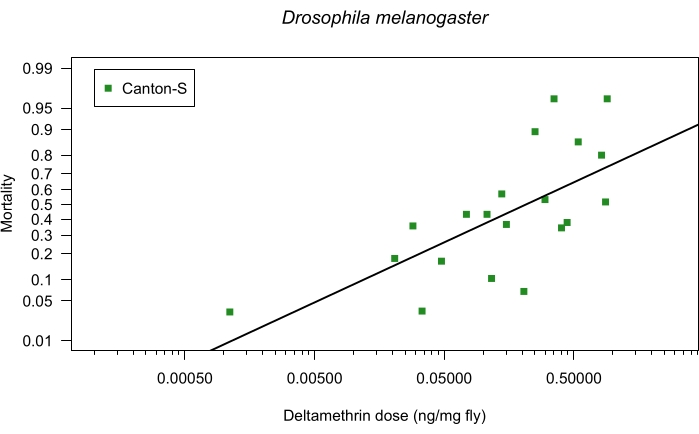
Figure 3: Representative data of fruit flies using topical application bioassay. Representative dose-response data from topical application bioassay following the above protocol using deltamethrin and fruit flies: D. melanogaster Canton-S strain (n = 1014). Deltamethrin testing concentrations ranged from 0.00499 to 5.02876 ng/µL, and the dose of deltamethrin applied (ng) per average fruit fly mass (mg) is reflected on the x-axis. Mortality is shown as a proportion on the y-axis. The black line represents the linear regression. Please click here to view a larger version of this figure.
Supplemental Figure S1: Benchtop insect handling tent. Benchtop insect handling tent is used for easier capture of escaping mosquitoes or flies during the topical application assay. Structure is closed in A and open in B. This structure was built with PVC pipe and fine-mesh fabric. Please click here to download this File.
Supplemental Figure S2: Syringe and repeater applicator unit. Syringe and repeater applicator unit used for dosing insects. Main parts include 1) needle, 2) syringe barrel, 3) plunger, 4) repeater, and 5) repeater button. Please click here to download this File.
Supplemental File 1: Randomization script: Randomization script to create non-biased labels for all cups of each experiment. Please click here to download this File.
Supplemental File 2: Mortality score sheet: Mortality score sheet to assist mortality assessment. Sheet also includes places to record all other important information to record, as referenced in the protocol, such as the insecticide application start and end times. Please click here to download this File.
Supplemental File 3: Example mortality data: Example data file used to create Figure 2. The column heading descriptions are as follows: "id" = identification code of each data point; "species" = species name (e.g., Aedes aegypti); "insecticide" = name of insecticide topically applied (e.g., Deltamethrin); "strain" = name of mosquito strain (e.g., ROCK); "date" = start date topical application; "sex" = sex of mosquitoes; "age" = age of mosquitoes (young = 3-5-day-old; old = 4 weeks old); "total.mosq" = total number of mosquitoes weighed in batch; "weight" = weight (mg) of all mosquitoes within batch; "concentration" = concentration of insecticide (µg/mL); "syringe" = droplet volume (mL) of syringe; "dose" = amount of insecticide active ingredient applied to each mosquito (ng); "total" = number of mosquitoes in each cup; "dead" = number of dead mosquitoes in each cup. Please click here to download this File.
Supplemental File 4: R analysis code: Example R code that can be used to complete the Probit analysis (as described in step 8 of the protocol). The representative results (accessible via the supplemental example data file) can be used with this R code. Please click here to download this File.
Discussion
This paper presents an adapted protocol for the topical application assay for mosquitoes and fruit flies. This procedure could be easily adapted to be used in the field and with other organisms as it requires minimal specialized equipment. Addressed below are this protocol's critical steps, potential modifications, troubleshooting advice, limitations of the method, and significance of this method.
Critical steps in the protocol: There are three critical steps in the protocol that, if completed incorrectly, can drastically impact the results of the bioassay: insecticide concentration accuracy, specimen knockdown, and mortality assessment.
Insecticide concentration accuracy:
It is extremely important to have accurate insecticide solutions to obtain replicable dose-response curves and meaningful results. The volumetric approach to insecticide solution preparation is more common within the literature for both CDC bottle bioassay7 and topical applications13,14,43. However, the gravimetric approach described here is inherently more accurate due to the consideration of temperature through the inclusion of (temperature-specific) density, leading to more accurate formulation preparation.
Specimen knockdown:
Knocking down the specimens is a critical component of this method and allows for the accurate administration of the insecticide and weight measurements. However, knocking down organisms inevitably contains the risk of physical stress and damage, as previously demonstrated30. Therefore, be cautious and mindful when knocking down the specimens to ensure i) each specimen is knocked down for a similar duration, ii) the length of knockdown is kept to a minimum, and iii) the method of knockdown is kept consistent across all specimens. Additionally, it is advised to test the knockdown method separately, prior to insecticide application, to ensure the method is successful and does not induce control mortality greater than 10%. The initial test may take longer for an inexperienced user, leading to longer knockdown times. Therefore, be cautious when interpreting results from the first assays.
Mortality assessment:
Assessing mortality can be challenging, especially when the insecticide does not completely kill but only knocks down or maims the mosquito or fly. Therefore, it is important to be aware of how the insecticide impacts the target organism and have a clear definition for "dead" (or knocked down) organisms before starting. Additionally, it is recommended to have the same person assess mortality between doses and replicates to reduce variation.
Protocol modifications: Several modifications described below can be applied to this protocol to improve its versatility and accessibility.
Adapting the assay to smaller or larger-sized insects:
When using smaller or larger specimens, it is advised to apply a smaller or larger dose volume of insecticide, respectively. As an example, we adapted the mosquito protocol to fruit flies by reducing the 0.5 µL dose to a 0.2 µL dose. Ensure the correct syringe size is chosen for the chosen dose volume.
Adapting the assay to field insects:
When using field insects, there may be more variation in insect size. Therefore, weighing the insects in smaller groups (e.g., per cup) would be recommended instead of as a large group (e.g., all insects used for one experiment). This can help capture the potential variation in insecticide susceptibility associated with the differences in field insect mass.
Equipment modifications:
Insect handling tent: Dosing of the specimen can be completed under an insect handling tent that is simply constructed with PVC pipe and mosquito netting. This can be an alternative to an enclosed room (e.g., insectary) and help eliminate potential insecticide contamination in areas where insect rearing might occur. This insect handling tent is easy to construct and low-cost (~$70). Alternatively, an insect handling cage could be purchased (~$425).
Chill table: Ice packs or trays of ice can be used for knocking down the specimen and/or keeping the specimen knocked down.
Incubator: Incubators are recommended for rearing the specimen and holding the specimen for 24 h after insecticide treatment. If an incubator is not available, it can be constructed. Equipment needed to build the incubator includes an insulated container, humidifier, heat cables, humidity and temperature controller, and a light, which should add up to a total cost of ~$170, following and expanding upon previous methods44.
Holding cups: Although plastic cups are used to sort and hold the treated specimen, wax-lined paper cups or glass containers would be suitable alternatives.
Organism and life stage modification:
This method is very adaptable for use with other vectors, insects, and/or arthropods such as Culex quinquefasciatus mosquitoes32, house flies32, and cockroaches45, as well as non-adult life stages, such as mosquito larvae46.
Topical application location modification:
This method describes applying the insecticide to the ventral thorax and abdomen region for mosquitoes (and the dorsum for fruit flies). However, other application locations can be used as long as the exposure site is consistent. Consistency is important because insecticide sensitivity can vary based on application location32.
Troubleshooting advice: This method has several steps that are initially challenging. Described below are some of the most common issues one might encounter.
Leaking/evaporating insecticide solutions:
Insecticides are commonly dissolved in acetone, a highly volatile compound. This means acetone evaporates quickly at room temperature, increasing the insecticide concentrations over time. If the insecticide solutions appear to be leaking or evaporating, remake the solutions, ensure the tube's lid is on tightly, and double-check that the storage protocols are being properly followed (e.g., parafilm is being used, and the tubes are stored upright). If leaking persists, try filling the tubes with a lower volume to allow more room for the change in volume the acetone experiences at different temperatures. Additionally, if using acetone as the solvent, ensure the tubes are rated for acetone storage (e.g., FEP, TFE, and PFA plastics). If using hydrophobic insecticides, store the solutions in glass vials (as hydrophobic insecticides adhere to glass less than plastic). It is also good practice to mark the meniscus of the solution prior to storing to monitor evaporation.
Weight drifting on microbalance when weighing organisms:
If the weight reading on the scale is drifting (slowly going up or down), this could be due to static. Drift most often occurs when weighing organisms in plastic items, as plastic can easily hold a static charge. To avoid this, a weighing paper can be placed underneath the plastic container being weighed, or a non-plastic container such as glass can be used.
Abnormal mortality results:
There are many ways by which the mortality results may seem abnormal, such as observing high mortality in the controls or high/low mortality throughout all insecticide doses. Review the following cases for troubleshooting each scenario.
High control mortality
If there is high mortality in the control group (10% or greater), evaluate the knockdown method and length of time the specimens are knocked down. If possible, shorten the length of time for which the specimens are knocked down. Other potential factors to consider for high mortality in the controls include i) checking if the incubator settings are correct—abnormal temperatures and/or humidity could lead to increased mortality. Temperature and humidity should be checked with an independent data logger. ii) Assessing insect handling. Handling insects too much or too roughly could lead to high mortality. iii) Checking if there is no insecticide contamination in the 100% acetone used to treat the control group or on the instrumentation. Replace acetone and clean all instruments with acetone or ethanol. Avoid contamination by frequently replacing gloves, preventing spillage, and cleaning instruments. Note that in Supplemental File 3, a maximum of two mosquitoes died within the control (acetone-only) cups. This level of mortality is not considered high (it is less than 10%), and therefore, there was no cause for concern.
High mortality in all exposed groups (but not in control groups)
Use lower insecticide concentrations or smaller dose volumes for testing. The dosages used might be above the minimum dose that will not induce mortality. Use several 10-fold dilutions to identify the correct dose range, and rule out contamination. To avoid contamination, start dosing with the lowest concentration and work towards the highest concentration. Additionally, make sure all equipment used is regularly cleaned with acetone and/or ethanol, the doses applied to the specimen are very small, and even the slightest cross-contamination could impact the results.
Low mortality in all exposed groups
Use higher insecticide concentrations. The dosages used might all be too low to cause mortality in the population. To identify the correct dose range, expose specimens to several more 10-fold concentrated dosages. Ensure the insecticide solutions have not expired or degraded (potentially due to high temperature or light exposure). If the solutions have expired or are suspected of having degraded, remake the solutions and ensure proper storage conditions are followed.
Inconsistent mortality between replicates/days
The time of the day when insects are exposed to the insecticide could affect the level of resistance expressed, especially for metabolic resistance34. Repeat this protocol during the same window of time each day to avoid time-of-day as a potential variable contributing to changes in mortality. Other potential factors contributing to inconsistent mortality between replicates include i) specimens being differentially reared between experiments. Ensure all specimens are of the same age range, reared at the same temperature and similar densities and food availability. ii) insecticide concentrations degrading over time or becoming more concentrated due to acetone evaporation. Remake the solutions and ensure proper storage conditions. iii) Inconsistent mortality scoring. Ensure the same person scores mortality or develop a clear protocol to be used consistently across the team. Use blind scoring to reduce bias in mortality scoring.
Insects sticking to the surface of the sorting tray:
Acetone reacts to plastics used in this protocol, such as Petri dishes. The specimen will likely adhere to the surface if using acetone on Petri dishes or similar plastic surfaces. This adhesion can be avoided by lining the sorting tray with weighing paper or using a non-plastic sorting tray. Additionally, condensation on the surface of plastic in the sorting tray or holding cups can lead to insects adhering to the condensation, or the specimen may be too cold and potentially freeze to the surface. Adjust the knockdown method to reduce condensation while preventing the specimens becoming too cold/frozen (e.g., place weighing paper between the specimens and the plastic sorting tray).
R analysis errors:
Once the mortality data are collected, a variety of complications may occur during analysis. The most common reason an R code cannot complete the actions for the data file is that the data format does not match the code (e.g., column headings and/or empty cells). If more serious complications arise, refer to the R help pages built into Rstudio35.
Limitations of the above-described topical application method:
Insecticide absorption via topical application method does not mimic natural exposure:
Topical application on the primary body is not the natural way of insecticide absorption. In the field, insects mostly absorb insecticides through their legs over the length of time they are in contact with the insecticide-treated surface or on their wings through small aerosol particles47,48, rather than a rapid exposure on the ventral surface. However, the direct application of a known insecticide dose will accurately establish a phenotypic response to insecticides, needed for genetic and evolutionary studies or comparisons of insecticide susceptibility across space or time. Therefore, this approach is beneficial for testing technical resistance but will not directly measure practical resistance (the efficacy of the actual intervention tool in a field setting15). However, it is important to note that the current standard methods (e.g., WHO tube tests and CDC bottle bioassays) also cannot capture or mimic aerosol (i.e., by fogging) insecticide exposure in the field.
Topical application assays can only assess contact absorption insecticides:
This method is intended for insecticides that work through contact and absorption of the insecticide and not for use with oral insecticides, such as boric acid commonly used in attractive toxic sugar baits49.
Significance of the method:
The topical application method expands on well-established standards for insecticide bioassays by calculating the lethal dose (not concentration) and measuring technical (not practical) resistance15. Given below are the advantages and disadvantages of this method over existing insecticide susceptibility assays.
Lethal dose calculation:
This method determines the lethal dose of the insecticide, rather than the lethal concentration that the CDC and WHO bioassays use to establish the discriminating dose11. The lethal dose is more meaningful because it is a quantified amount of insecticide known to elicit mortality. In contrast, the lethal concentration does not consider how much insecticide the organism actually acquires. When using the lethal dose calculation, differences between sex- or size-dependent susceptibility profiles can be more accurately observed and quantified, making this measurement even more versatile.
Technical resistance:
This method assesses technical resistance, which is resistance as measured under standardized, controlled environments. Such measurements are suitable for surveillance of the spread of insecticide resistance and linking phenotypic resistance with potential markers15. Because of the decreased variation in mortality resulting from the topical application bioassay, it allows for better identification of new resistance markers. However, due to the unnatural exposure of insecticides to the mosquito, this assay is not suitable for the estimation of efficacy of a specific intervention in a specific population. Other assays are needed for measurements of such practical resistance15.
Specimen adaptability:
This method can be practiced on other important arthropods such as crop pests (e.g., Colorado potato beetle), house pests (e.g., cockroaches and bed bugs), or pollinators (e.g., bees) with simple changes to the knockdown approach and/or insecticide dose, volume, and/or concentration (as described above). The ease of adaptability can help analogize insecticide resistance research across different research fields. The use of an LD50 value instead of a lethal concentration that kills 50% of the specimens (LC50) allows accurate comparison across species.
Cost:
Similar to CDC bottle bioassays and WHO tube tests, costs to run the topical application assay are minimal (see the Table of Materials). The essential pieces of equipment are the syringe (approximately $70) and the dispenser (approximately $100), which are reusable across assays.
Number of specimens needed:
A minimum of 20-25 specimens should be used per topical application assay cup. A minimum of five insecticide concentrations is recommended to be tested per experiment, with a minimum of three replicates recommended for the procedure. Overall, this results in a minimum of 300-375 specimens needed for a complete test, comparable to the number of specimens needed to perform resistance intensity tests using WHO tube tests or CDC bottle bioassays. However, if reduced variability is achieved with the topical application bioassay, the same number of specimens may lead to more statistical power to compare susceptibility data across space or time.
Disclosures
The authors declare no conflicts of interest.
Acknowledgements
This research was supported by a CAREER award by the National Science Foundation to SH under award number 2047572. We thank Damien Rivera for his assistance in fruit fly rearing and preparation for topical application assay, Dr. Ganetzky at the University of Wisconsin-Madison for sharing his Canton-S fruit fly strain, the Centers for Disease Control and Prevention for sharing the Rockefeller strain, and the United States Department of Agriculture Center for Medical Agricultural and Veterinary Entomology for sharing the IICC isoline strain. Figure 1 was created with BioRender.com.
Materials
| Name | Company | Catalog Number | Comments |
| 1.5 mL microcentrifuge tubes | Thomas Scientific | 20A00L068 | Acetone aliquot storage |
| 1.5 mL screw cap tubes | Thomas Scientific | 1182K23 | Insecticide dilution storage |
| 15 mL conical tubes | VWR | 339651 | Insecticide dilution storage |
| 20 mL glass scintillation vials | Fisher Scientific | 0334125D | Fruit fly weighing |
| 25 μL syringe | Fisher Scientific | 14815288 | Topical applicator |
| Acetone | Fisher Scientific | AC423240040 | ACS 99.6%, 4 L |
| Aedes aegypti (IICC strain) | USDA CMAVE | NA | Insecticide resistant |
| Aedes aegypti (Rockefeller strain) | CDC | NA | Insecticide susceptible |
| Analytical scale | Fisher Scientific | 14-557-409 | Precision up to 0.1 mg |
| Aspirator | Amazon | 6.49986E+11 | Mosquito collection device |
| Bench paper | VWR | 89126-794 | Place under workspace |
| Cotton swabs | Amazon | B092S8JVQN | Use for sorting insects |
| Cotton wool balls | Amazon | B0769MKZWT | Use for sucrose solution |
| Dispenser | Fisher Scientific | 1482225 | Repeater pipettor |
| Drosophila melanogaster (Canton-S strain) | University of Wisconsin-Madison | NA | Insecticide susceptible |
| Fine-tipped paint brushes | Amazon | B07KT2X1BK | Use for sorting insects |
| Fruit fly stock bottles | Fisher Scientific | AS355 | Use for rearing and sorting fruit flies |
| Hand-held CO2 dispenser | Fisher Scientific | NC1710679 | Use for knocking down insects |
| Holding cups | Amazon | B08DXG7V1S | Clear plastic |
| Ice pack | Amazon | B08QDWMMW5 | Use for knocking down fruit flies |
| Ice trays | Amazon | 9301085269 | Use for knocking down insects |
| Insect forceps | Amazon | B07B4767WR | Insect forceps |
| Insecticide | Sigma-Aldrich Inc | 45423-250MG | Deltamethrin |
| Labeling stickers | Amazon | B07Q4X9GWX | 3/4" Color dot stickers |
| Labeling tape | Amazon | B00X6A1GYK | White tape |
| Netting | Amazon | B07F2PHHWV | Use for covering holding cups and insect handling tent |
| Petri dishes | Fisher Scientific | FB0875712H371 | 100 mm x 15 mm |
| PVC Pipe | Lowe’s | 23971 | Insect handling tent materials |
| Rubber bands | Amazon | B00006IBRU | Use for securing mesh/net on cups |
| Sucrose | Amazon | B01J78INO0 | Granulated White Sugar |
| Weighing paper | VWR | 12578-165 | 4" x 4" |
References
- World Health Organization. Vector-borne diseases. World Health Organization. , (2020).
- World Health Organization. Global plan for insecticide resistance management in malaria vectors. World Health Organization. , (2012).
- Liu, N. Insecticide resistance in mosquitoes: impact, mechanisms, and research directions. Annual Review of Entomology. 60 (1), 537-559 (2015).
- Hemingway, J., Ranson, H. Insecticide resistance in insect vectors of human disease. Annual Review of Entomology. 45 (1), 371-391 (2000).
- World Health Organization. Monitoring and managing insecticide resistance in Aedes mosquito populations. World Health Organization. , (2016).
- World Health Organization. Test procedures for insecticide resistance monitoring in malaria vector mosquitoes (Second edition). World Health Organization. , (2016).
- McAllister, J. C., Scott, M. CONUS manual for evaluating insecticide resistance in mosquitoes using the CDC bottle bioassay kit. Centers for Disease Control and Prevention. , (2020).
- Duneau, D., et al. Signatures of insecticide selection in the genome of Drosophila melanogaster. G3: Genes, Genomes, Genetics. 8 (11), 3469-3480 (2018).
- Pittendrigh, B., Reenan, R., ffrench-Constant, R. H., Ganetzky, B. Point mutations in the Drosophila sodium channel gene para associated with resistance to DDT and pyrethroid insecticides. Molecular & General Genetics: MGG. 256 (6), 602-610 (1997).
- Rinkevich, F. D., Du, Y., Dong, K. Diversity and convergence of sodium channel mutations involved in resistance to pyrethroids. Pesticide Biochemistry and Physiology. 106 (3), 93-100 (2013).
- Lissenden, N., et al. Review and meta-analysis of the evidence for choosing between specific pyrethroids for programmatic purposes. Insects. 12 (9), 826 (2021).
- Owusu, H. F., Chitnis, N., Müller, P. Insecticide susceptibility of Anopheles mosquitoes changes in response to variations in the larval environment. Scientific Reports. 7 (1), 3667 (2017).
- Brito-Sierra, C. A., Kaur, J., Hill, C. A. Protocols for testing the toxicity of novel insecticidal chemistries to mosquitoes. Journal of Visualized Experiments: JoVE. (144), e57768 (2019).
- Burgess, E. R., King, B. H., Geden, C. J. Oral and topical insecticide response bioassays and associated statistical analyses used commonly in veterinary and medical entomology. Journal of Insect Science. 20 (6), 1-9 (2020).
- Namias, A., Jobe, N. B., Paaijmans, K. P., Huijben, S. The need for practical insecticide-resistance guidelines to effectively inform mosquito-borne disease control programs. eLife. 10 (1), 65655 (2021).
- Zhu, X., et al. Manipulating solid forms of contact insecticides for infectious disease prevention. Journal of the American Chemical Society. 141 (1), 16858-16864 (2019).
- Dang, K., Singham, G. V., Doggett, S. L., Lilly, D. G., Lee, C. Y. Effects of different surfaces and insecticide carriers on residual insecticide bioassays against bed bugs, Cimex spp. (Hemiptera: Cimicidae). Journal of Economic Entomology. 110 (2), 558-566 (2017).
- Spielmeyer, A., Schetelig, M. F., Etang, J. High-throughput analysis of insecticides on malaria vectors using liquid chromatography tandem mass spectrometry. PLoS ONE. 14 (2), 0211064 (2019).
- Bagi, J., et al. When a discriminating dose assay is not enough: measuring the intensity of insecticide resistance in malaria vectors. Malaria Journal. 14 (1), 210 (2015).
- Pridgeon, J. W., Becnel, J. J., Clark, G. G., Linthicum, K. J. Permethrin induces overexpression of multiple genes in Aedes aegypti. Journal of Medical Entomology. 46 (3), 1-8 (2009).
- World Health Organization. Guidelines for efficacy testing of insecticides for indoor and outdoor ground-applied space spray applications. World Health Organization. , (2009).
- Estep, A. S., et al. Quantification of permethrin resistance and kdr alleles in Florida strains of Aedes aegypti (L.) and Aedes albopictus (Skuse). PLoS Neglected Tropical Diseases. 12 (10), 0006544 (2018).
- Waits, C. M., et al. A comparative analysis of resistance testing methods in Aedes albopictus (Diptera: Culicidae) from St. Johns County, Florida. Florida Entomologist. 100 (3), 571-577 (2017).
- Kostromytska, O. S., Wu, S., Koppenhöfer, A. M. Diagnostic dose assays for the detection and monitoring of resistance in adults from Listronotus maculicollis (Coleoptera: Curculionidae) populations. Journal of Economic Entomology. 111 (5), 2329-2339 (2018).
- Aktar, W., Sengupta, D., Chowdhury, A. Impact of pesticides use in agriculture: their benefits and hazards. Interdisciplinary Toxicology. 2 (1), 1-12 (2009).
- Maïga, H., et al. Guidelines for routine colony maintenance of Aedes mosquito species. IAEA Physical and Chemical Sciences. , (2017).
- Gjullin, C. M., Hegarty, C. P., Bollen, W. B. The necessity of a low oxygen concentration for the hatching of aedes mosquito eggs. Journal of Cellular Physiology. 17 (2), 193-202 (1941).
- Stocker, H., Gallant, P. Getting started: an overview on raising and handling Drosophila. Methods in Molecular Biology. 420 (1), 27-44 (2008).
- Jass, A., Yerushalmi, G. Y., Davis, H. E., Donini, A., MacMillan, H. A. An impressive capacity for cold tolerance plasticity protects against ionoregulatory collapse in the disease vector Aedes aegypti. Journal of Experimental Biology. 222 (1), 214056 (2019).
- Bartholomew, N. R., Burdett, J. M., Vandenbrooks, J. M., Quinlan, M. C., Call, G. B. Impaired climbing and flight behaviour in Drosophila melanogaster following carbon dioxide anaesthesia. Scientific Reports. 5 (1), 15298 (2015).
- Jung, Y., Kennedy, A., Chiu, H., Mohammad, F., Claridge-Chang, A., Anderson, D. J. Neurons that function within an integrator to promote a persistent behavioral state in Drosophila. Neuron. 105 (2), 322-333 (2020).
- Aldridge, R. L., Kaufman, P. E., Bloomquist, J. R., Gezan, S. A., Linthicum, K. J. Impact of topical application site on the efficacy of permethrin and malathion to Culex quinquefasciatus. Journal of the American Mosquito Control Association. 32 (4), 300-307 (2016).
- Rinkevich, F. D., et al. Distinct roles of the DmNav and DSC1 channels in the action of DDT and pyrethroids. Neuro Toxicology. 47 (1), 99-106 (2015).
- Balmert, N. J., Rund, S. S. C., Ghazi, J. P., Zhou, P., Duffield, G. E. Time-of-day specific changes in metabolic detoxification and insecticide resistance in the malaria mosquito Anopheles gambiae. Journal of Insect Physiology. 64 (1), 30-39 (2014).
- R Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing. R Core Team. , (2021).
- Ritz, C., Baty, F., Streibig, J. C., Gerhard, D. Dose-response analysis using R. PLoS ONE. 10 (12), 0146021 (2015).
- Abbott, W. S. A method of computing the effectiveness of an insecticide. Journal of the American Mosquito Control Association. 3 (2), 302-303 (1987).
- Ravichandran, S. Data analysis through SAS with special emphasis on Probit analysis. National Academy of Agricultural Research Management (NAARM). , (2021).
- Smith, L. B., et al. CYP-mediated resistance and cross-resistance to pyrethroids and organophosphates in Aedes aegypti in the presence and absence of kdr. Pesticide Biochemistry and Physiology. 160 (1), 119-126 (2019).
- Finney, D. J. . Probit Analysis. , (1971).
- Silva, J. J., Kouam, C. N., Scott, J. G. Levels of cross-resistance to pyrethroids conferred by the Vssc knockdown resistance allele 410L+1016I+1534C in Aedes aegypti. PLOS Neglected Tropical Diseases. 15 (7), 0009549 (2021).
- Fan, Y., Scott, J. G. The F1534C voltage-sensitive sodium channel mutation confers 7- to 16-fold resistance to pyrethroid insecticides in Aedes aegypti. Pest Management Science. 76 (1), 2251-2259 (2020).
- Miller, A. L. E., Tindall, K., Leonard, B. R. Bioassays for monitoring insecticide resistance. Journal of Visualized Experiments: JoVE. (46), e2129 (2010).
- Glunt, K. D., et al. Long-lasting insecticidal nets no longer effectively kill the highly resistant Anopheles funestus of southern Mozambique. Malaria Journal. 14 (1), 298 (2015).
- ffrench-Constant, R. H., Roush, R. T., Roush, R. T., Tabashnik, B. E. Resistance detection and documentation: the relative roles of pesticidal and biochemical assays. Pesticide Resistance in Arthropods. , (1990).
- Akdag, K., et al. Synthesis and larvicidal and adult topical activity of some hydrazide-hydrazone derivatives against Aedes aegypti. Marmara Pharmaceutical Journal. 18 (1), 120-125 (2014).
- Richards, S. L., Byrd, B. D., Reiskind, M. H., White, A. V. Assessing insecticide resistance in adult mosquitoes: perspectives on current methods. Environmental Health Insights. 14 (1), (2020).
- Cooperband, M., Golden, F., Clark, G., Jany, W., Allan, S. Prallethrin-induced excitation increases contact between sprayed ultra-low volume droplets and flying mosquitoes (Diptera: Culicidae) in a wind tunnel. Journal of Medical Entomology. 47 (1), 1099-1106 (2010).
- Barbosa, D. S., Rodrigues, M. M. S., Silva, A. A. E. Evaluation of attractive toxic sugar baits (ATSB) against Aedes aegypti (Diptera: Culicidae) in laboratory. Tropical Biomedicine. 36 (2), 578-586 (2019).
This article has been published
Video Coming Soon
ABOUT JoVE
Copyright © 2025 MyJoVE Corporation. All rights reserved