Deploying Community Scientists to Conduct Nondestructive Genetic Sampling of Rare Butterfly Populations
In This Article
Summary
Here, we present a straightforward protocol for noninvasive genetic sampling of butterfly populations based on the field collection of residual egg debris. It can be used to confirm species identity and quantify genetic variation. This protocol can be easily adapted to broader groups for community science involvement.
Abstract
Global insect declines continue to accelerate. Effective genetic sampling is critically needed to advance the understanding of many taxa and address existing knowledge gaps. This protocol represents a demonstrated method for nondestructively sampling rare butterflies for population genetic structure or DNA barcoding analyses. It uses the chorion of hatched butterfly ovae to yield sufficiently high quantity and quality DNA for successful gene sequencing to confirm species identity and quantify genetic variation. It may be particularly useful when other tissue sampling techniques are impractical or unavailable. While developed for a lepidopteran, it nonetheless could easily be adapted for use with other insect species. It was specifically designed with ease of use as a goal to help maximize broad implementation by individuals of varying experience and skill levels, such as community scientists, conservation practitioners, and students, and for use over large geographic areas to facilitate broad population sampling. The data generated can help inform taxonomic and listing decisions, conservation and management actions, and enhance basic ecological research.
Introduction
Effective population genetic sampling of rare, declining, and/or listed insect taxa is often critical to help inform conservation and management actions. Lethal or injurious tissue sampling methods are routinely utilized for many genetic analyses. However, these methods are undesirable or not permitted because they may cause harm to vulnerable extant populations or negatively impact behavior and fitness. Various nonlethal or noninvasive sampling techniques involving tissue such as whole legs, antennal or wing clippings, larval exuviae, or hemolymph from defensive secretions and other products, such as frass, have been suitable for insect genetic research1,2,3,4,5,6,7,8,9. The overall feasibility and applicability of these various tissue sampling techniques vary significantly based on the biology, ecology, behavior, size, and rarity of the focal organism, situation, and individuals collecting the material. For example, whole legs or appendage clippings require temporary capture and careful handling of several individuals of an appropriate life stage, typically by experienced personnel. Similarly, tissue sources, such as larval exuviae or frass, are likely only to be practical if the organisms are in captivity or temporarily held for an adequate period.
For research questions being asked at the population level, such as those concerning potential taxonomic differentiation or genetic structure, sampling at a broader temporal or geographic scale (i.e., regional or continental) is often required. As a result, certain noninvasive tissue sources may be completely impractical or at the very least inefficient to collect in quantity. Sample collection at such scales may additionally be logistically or financially impractical or prohibitive for individual researchers or even smaller field teams to undertake. While the direct engagement of community scientists has been increasingly adopted by the research community to help address many of these challenges and accelerate big data-driven collection, adoption has been limited for studies involving nondestructive, noninvasive, or eDNA sampling10,11,12.
To help overcome some of these potential limitations, we demonstrated that the chorion from hatched butterfly ovae could yield sufficiently high quantity and quality DNA for successful gene sequencing to confirm species identity and quantify genetic variation. We subsequently tested various collection protocols using this technique13. This pilot work served as the basis for further methodological refinement. This paper describes in detail the resulting straightforward yet comprehensive revised field collection protocol rolled out for community scientists and other nonexpert personnel. This protocol is part of a range-wide population structure analysis of the frosted elfin butterfly (Callophrys irus) to help inform future conservation actions and a federal determination of possibly listing under the U.S. Endangered Species Act. Although potentially more labor-intensive than some noninvasive methods, the lack of necessary organism contact and ease of use make it a potentially viable model that can be applied to other population genetic research programs targeting a selection of both common insects and those of conservation concern, which leave residual egg debris behind from recently hatched nymphs or larvae. The protocol language presented here was specifically developed for butterflies in the family Lycaenidae and for use by nonexperts defined here as community scientists or other individuals (e.g., agency biologists, interns) with limited entomological experience.
Protocol
1. Dissemination of supplies to community scientists
- Carefully review and gather the full list of collection-related supplies needed by consulting the complete Table of Materials.
- If sample collection will take place at multiple locations and/or by multiple individuals/teams, gather and organize all supplies needed into separate deployable units (Figure 1 and Figure 2).
- Transport supplies (deployable units) to community scientists or other personnel responsible for field collection.
- If personnel are not local, carefully pack supplies (each deployable unit) into a separate cardboard shipping box with a complete shipping label and ship.
NOTE: Expedited shipping is not required at this stage.
2. Community scientist collection preparation
- Upon receipt of collection supplies, including the collection tacklebox, carefully review the laminated supply list and conduct a full inventory. Notify the project leader if any supplies are missing.
- Prefill 1.5 mL microcentrifuge tubes with 180 µL of lysis buffer, apply labels with a preprinted unique ID label to each microcentrifuge tube, and place all tubes in the 64-well microcentrifuge tubes storage box. Firmly secure the lid after loading.
NOTE: If label printers are not available, apply unique ID numbers to the side and lid of each vial using an ethanol-proof marker. - Ensure all digital equipment (e.g., smartphone or camera) is fully charged, and any spare batteries are packed.
- Partially fill a small cooler with ice for field storage and local transportation of the collected samples.
- Pack all collection supplies into the collection tacklebox or similar sturdy, weatherproof container for safe transport and consolidated storage.
- Review the nondestructive genetic sample collection protocol in detail prior to heading into the field.
NOTE: Use the laminated and condensed, illustrated protocol for additional guidance in the field (Supplemental Figure S1 and Supplemental Figure S2).
3. Sample collection in the field
- Upon arriving at the field site, use a pencil or all-weather pen to record in the weatherproof field notebook the time of day, date, overall property name (e.g., Apalachicola National Forest), specific field site location name or description, and GPS reading if available, and the full name and roles of all persons present.
- Locate a suitable habitat with the presence of larval host plant species specific to the target butterfly.
NOTE: Use care to avoid or minimize impact to sensitive habitats, plant populations, and wildlife. In addition, be sure to secure all appropriate landowner permissions and/or permits prior to accessing a site and sample collection events. - Using a smartphone or camera, take detailed photographs of all field sites, sample collection locations, host plants, and any other information that may be relevant to the project. Use smartphones or other cameras that record GPS coordinates.
NOTE: Photo Geotagging needs to be enabled on all smartphones. - Comprehensively search all the larval host plant parts, such as leaves, flower buds, flowers, or developing fruit, known to be selected by ovipositing (egg-laying) adult female butterflies for hatched eggs. For most Lycaenidae, look for white, hatched eggs with a distinct hole in the center resembling a doughnut from which the neonate larva emerged (Figure 3). Look for unhatched eggs that are somewhat darker in color, often green or bluish, and will be completely intact without a hole in the middle (Figure 4). Use a hand lens or other magnifying device to inspect each egg closely.
- Once a hatched egg has been located, put nitrile gloves on both hands.
- Using clean, sterile, pointed forceps, grab and gently pull the hatched egg off the host plant and place it directly into a labeled microcentrifuge tube prefilled with lysis buffer taken from the 64-well storage box.
NOTE: Some host plant carryover material (smaller than 15 mm in diameter) is acceptable and normal during collection. - Collect at a minimum a total of 5 samples per host plant patch/site (each containing 1-20 hatched eggs), aiming for >50 eggs total at a location if available.
NOTE: This will help ensure at least some tissue is collected at every sample plant/patch and increase the chances of detecting DNA (personal observation). - After each egg collection, carefully clean the forceps tips by dipping them in a vial of 95% ethanol or by dousing with 95% ethanol from a squeeze bottle or by using alcohol wipes.
NOTE: This will help minimize tissue carryover between sampling events. - If available, collect several hatched eggs (1-20 eggs) from multiple plants in the same host patch into a single microcentrifuge tube and firmly close the lid. For butterfly species utilizing larger herbaceous or woody host species, collect multiple eggs from different locations on the same plant if host patches are limited.
NOTE: Plants are in the same host patch if their leaves touch one another. If there is a noticeable physical separation between plants or patches, this should be considered a different sample. If collecting another tissue type, such as larval exuviae or a single leg, place only a single leg, leg fragment, or larval exuvia into a microcentrifuge tube (one sample per individual). - Make sure that all collected material is fully submerged in the lysis buffer within each 1.5 mL microcentrifuge tube. Tap the bottom of the tube in question on a hard surface to resubmerge any samples if needed.
- Wipe the forceps to dry with a clean disposable lab wipe.
NOTE: Careful forceps cleaning is essential to avoid cross contamination between samples. - When collecting a new sample, discard used nitrile gloves and replace them with a clean pair.
- In a weatherproof field notebook, use a pencil or all-weather pen to record the unique ID label assigned from the microcentrifuge tube along with the type of sample (e.g., eggs, larval exuviae, or leg), the approximate number of hatched eggs, and collection information such as collector name(s), date, location, host plant species, and any additional notes (Figure 2).
- Place the closed microcentrifuge tube with egg debris sample back in the 64-well microcentrifuge storage box.
- Maintain the 64-well microcentrifuge storage box in a cooler with ice while in the field.
- Maintain all other collection supplies in the collection tacklebox while in the field for safe and consolidated storage and transport between sites.
4. When field collection is complete
- Ensure all microcentrifuge tubes containing samples are in the 64-well microcentrifuge storage box in a cooler with ice for transport.
- Place all field collection supplies back in the collection tacklebox.
- Transport all samples back to the office or laboratory and carefully double-check each microcentrifuge tube to ensure that all sample material is completely submerged in lysis buffer.
- Place the 64-well microcentrifuge storage box with samples in a freezer until shipment or processing.
NOTE: An average commercial freezer of-18 °C is acceptable for short-term storage. - Carefully assess all supplies in the collection toolbox and replace any as needed.
NOTE: This is particularly important if multiple field collection events are scheduled. - Store the collection tacklebox in a secure location until the next field collection event.
- Download all digital images and ensure all are backed up securely on a server or cloud-based storage system.
- Scan or photograph the original weatherproof, field notebook pages, or field data sheets containing collection data until all information can be entered into a project spreadsheet or database.
5. Submitting samples and data
- Remove all 64-well microcentrifuge storage boxes with samples from the freezer.
- Carefully package the 64-well microcentrifuge storage boxes with samples and the field notebook or field data sheets in a standard shipping box, attach the provided shipping label with the original sender's account number, and send via express carrier-guaranteed, overnight, next-day delivery to the project director.
- Email the package tracking number and a copy of the scanned notebook pages or field data sheets to the project director.
6. DNA extraction
- Once the 64-well microcentrifuge storage boxes are received, store them at -20 °C for the preservation of DNA in samples until ready for DNA extraction.
- In the lab, extract DNA by grinding insect tissue stored in lysis buffer after thawing from the -20 °C. Add 20 µL of Proteinase K and let the sample soak overnight at 56 °C in a heated incubator for no more than 24 h.
- Add the appropriate cleaning and washing buffers, per the specific manufacturer's recommendation13.
- Transfer the sample suspended in buffer into a spin column attached to a 2 mL collection vial.
- Spin down at 6,021 × g for 60 s using a centrifuge. Add the appropriate washing buffers and spin down appropriately.
- Release bound DNA using elusion buffer (30 µL) and spin down the sample into a fresh 1.5 mL microcentrifuge tube. Store at -20 °C.
NOTE: Proceed to sequencing if the DNA concentration exceeds 0.010 ng/mL. See Figure 5 for DNA concentration by tissue type.
7. Data analysis of DNA sequences
- Trim or edit low-quality base calls in resulting sequences.
- Query sequences against public databases for confirmation of species identification.
- Align sequences to each other for comparison of differences between individuals.
Representative Results
Materials for collecting up to 563 samples were sent to eight conservation and community scientists from nine states across the species range in eastern North America. Materials were sent out over three months during 2021 prior to local peak flight times. To date, we have received a total of 160 C. irus tissue samples that have been collected (Table 1). Genomic DNA was extracted following a protocol for this sample type detailed by Storer et al.14. Out of these 160 samples, DNA was successfully extracted from 88 with an average concentration of 1.67 ng/μL (SE ± 2.98), and the highest DNA yield was 26.8 ng/μL. The concentrations were quantified using a high-sensitivity assay kit per manufacturer's instructions with 2 µL of extract.
While the total number of samples received is substantially lower than that of the overall total number of materials deployed for collection, this is predominately an artifact of providing an overabundance of collection materials to the primary collector or team leader to enable them to have the maximum flexibility for including multiple collection sites and/or community scientists involved if desired. Moreover, C. iris is a rare and declining taxon represented by limited and often relatively small populations throughout its extant range. Despite this constraint, the total number of tissue samples received is substantial, especially compared to what would be expected with a more traditional sampling of individual adult butterflies.

Figure 1: Individual deployable units of all necessary supplies awaiting shipment to community scientists or other personnel responsible for field collection. Please click here to view a larger version of this figure.
Figure 2: Individual deployable unit including all supplies, clear plastic collection tacklebox, and completed express carrier label awaiting shipment to community scientists or other personnel responsible for field collection. Please click here to view a larger version of this figure.

Figure 3: Photo of hatched frosted elfin butterfly (Callophrys irus) egg on wild lupine (Lupinus perennis) showing a distinct hole in the center from which the neonate larva emerged. Note that the overall color of the hatched egg is white. Please click here to view a larger version of this figure.

Figure 4: Photo of unhatched hatched frosted elfin butterfly (Callophrys irus) eggs on wild lupine (Lupinus perennis). Note that unhatched eggs lack a noticeable central hole and that their overall color is bluish green. Please click here to view a larger version of this figure.
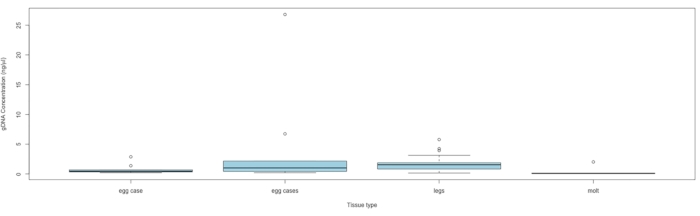
Figure 5: DNA concentration by tissue type. The box midlines represent the median, each box extends the IQR, and the box whiskers are 1.5 × IQR, with any points outside being outliers. Samples containing a single egg case had only one egg case, and samples containing multiple egg cases had at least two cases, with the number of cases ranging from 2 to 20 (average = 5.3) per sample. Abbreviation: IQR = interquartile range. Please click here to view a larger version of this figure.
| State | Egg case | Egg cases | Frass | Leg | Molt | Grand Total |
| Arkansas | 2 | 2 | ||||
| Florida | 3 | 10 | 7 | 22 | 42 | |
| Michigan | ||||||
| New Hampshire | 24 | 6 | 15 | 45 | ||
| New York | 30 | 30 | ||||
| Ohio | ||||||
| Wisconsin | 9 | 5 | 5 | 19 | ||
| Oklahoma | 16 | 6 | 22 | |||
| Grand Total | 36 | 21 | 16 | 65 | 22 | 160 |
Table 1: Number and type of tissue material collected by state.
Supplemental Figure S1: Front page of two-sided, laminated, condensed, and illustrated protocol for use in the field. Please click here to download this File.
Supplemental Figure S2. Back page of two-sided, laminated, condensed, illustrated protocol for use in the field. Please click here to download this File.
Discussion
This protocol describes a field method for nondestructively sampling rare butterflies for population genetic structure or DNA barcoding analyses. The overall benefits of this protocol are specifically designed to help maximize broad implementation by individuals of varying experience and skill levels such as community scientists, conservation practitioners, and students. These include relatively low overall cost, easy-to-acquire supplies and equipment, a straightforward and approachable method without numerous overly complex steps, and easy deployment over a broad geographic area. It additionally targets residual organic material that eliminates the need to temporarily capture or manipulate, and in the process, potentially harm living organisms to acquire genetic samples. Moreover, it extends the potential period available for sample collection beyond the traditional phenology or lifespan of a particular life stage, enhancing flexibility and overall collection opportunity to help maximize sample numbers.
While the focal taxon for this effort was the frosted elfin butterfly, a widespread but declining habitat specialist, this protocol can be applied more broadly to many other insects, including those of conservation concern. Similarly, while the protocol targets the collection of hatched eggs as the source of genetic material, it can easily be adapted to other, potentially more traditional samples (e.g., legs, wing fragments, antennal clips) or even whole organisms. However, this aspect is also a potential limitation of the protocol. Although the vast majority of the steps are designed to be relatively simple and quick to accomplish, comprehensively searching patches of larval host plants for hatch eggs, which individually are typically <1.0 mm in diameter, is time- and labor-intensive. Nonetheless, such extensive sampling activity is particularly ideal for a larger network of participants such as community scientists.
As with other field-based projects, careful preparation is essential. This includes conducting a full inventory of the supplies needed to ensure no items are missing, any required preparation work has been completed, and they are well organized, securely packed, and ready for field deployment. In addition, all field personnel, particularly those conducting the tissue collection, should review the collection protocol in detail prior to any collection event and address any questions to individuals overseeing the project. Once in the field, the sample collection portion of the protocol requires meticulous attention to detail. This includes locating and carefully inspecting any ovae to ensure that they are hatched and thus appropriate for collection, thoroughly cleaning forceps, replacing gloves to help reduce tissue carryover between sampling events, and ensuring tissue samples are completely submerged in lysis buffer within each 1.5 mL microcentrifuge tube. Lastly, recording accurate and detailed data is essential, which includes linking the unique ID label assigned from the microcentrifuge tube along with the specific sample taken and all other pertinent collection information. While this step is routine in scientific research, it often needs to be thoroughly clarified and reinforced for a community science audience.
Here, we demonstrate the potential leveraging of community scientists for nonlethal sample collection from small, rare, and threatened species. However, there are some potential limitations to keep in mind when analyzing any resulting genetic data. For example, while pooling samples from a single patch increases the chances of recovering enough DNA for detection, it also potentially introduces heterozygosity. In addition, as the protocol involves collecting the chorion from hatched ovae, only female butterflies, representing parental DNA, can be sampled within a population. Nonetheless, as no previous population-level genetic data were available for C. irus, the resulting insights gained can only benefit species conservation and management. For example, while a thorough, detailed study design and careful marker selection would be required to adequately assess population structure, the sampling protocol outlined here and the use of cytochrome c oxidase subunit 1 (CO1) DNA barcoding could be used to detect the occurrence of rare or imperiled taxa. Additional discussion of the utility and application of DNA sequencing from nonlethal sampling described here are detailed by Storer et. al.14.
Beyond the primary goal of collecting samples for genetic analysis, the protocol also emphasizes that participants take detailed digital photographs of all field sites, collection locations, larval host plants present, and any other elements of the surrounding habitat that might be relevant. Such documentation helps provide a general habitat assessment that is useful to illustrate existing site conditions such as plant phenology, host resource density, and management history (e.g., recent prescribed fire). Such information is particularly useful for projects where field samples are taken over a broader temporal period to enable more detailed environmental comparisons.
Lastly, as global insect declines continue to accelerate, expanded species assessment and monitoring efforts are critically needed15,16. The use of more widespread participatory engagement from community scientists, students (e.g., U.S. Fish and Wildlife Service interns and fellows), and conservation practitioners offers increasingly viable options for extensive data collection of many types, including DNA samples. Accordingly, the data generated from this protocol have many possible uses. These include helping inform conservation actions (e.g., planning, recovery, and management), listing decisions or species status assessments, and ecological, population, and taxonomic research.
Acknowledgements
The authors wish to thank David Cuthrell, Amanda Dillon, Steve Fuller, Neil Gifford, Heidi Holman, Dean Jue, Sally Jue, Daniel Kennedy, Genevieve Kozak, Rebecca Longenecker, Maureen McClung, Matt Moran, Robin Niver, Brenda Smith, Hunter Trowbridge, and Jessup Weichelt for help with various aspects of the project, including logistics, coordination, permitting, and/or sample collection. This research was funded through a grant from the U.S. Fish and Wildlife Service (Federal Award Identification Number F20AC00356) administered by the Wildlife Management Institute (grant SA 2021-01).
Materials
| Name | Company | Catalog Number | Comments |
| 14 quart Igloo Playmate Cooler | Amazon | NA | portable cooler Amount per deployable unit: 1 cooler |
| 250 DNeasy Mini Spin Columns, Proteinase K, Buffers, Collection Tubes (2 mL) | Qiagen | 69506 | Qiagen DNeasy Blood & Tissue Kit Amount per deployable unit: NA |
| Andwin Scientific Supplier Diversity Partner LAB MARKERS BLACK | FisherSci | NC9280166 | ethanol proof lab marker Amount per deployable unit: 2 markers |
| Cardboard shipping box | shipping box Amount per deployable unit: as needed | ||
| Eisco Polyethylene Wash Bottles, LDPE | FisherSci | S14091 | lab grade spray or squirt bottle (for ethanol or alcohol) Amount per deployable unit: 1 bottle |
| FedEx U.S. express airbill | FedEx | NA | shipping return label Amount per deployable unit: 2 labels |
| Fisherbrand Premium Microcentrifuge Tubes: 1.5 mL | FisherSci | NC9386261 | sterile 1.5 mL microcentrifuge tubes Amount per deployable unit: 128 tubes |
| Forceps, #4a, very fine yet extra strong tips (33 mm), 4-3/8" (111 mm) long | Bioquip | 4523 | straight tip forceps Amount per deployable unit: 2 straight forceps |
| Forceps, #7, curved tips (13 mm), very fine points, 4-1/2" (114 mm) long | Bioquip | 4527 | curved tip forceps Amount per deployable unit: 2 curved forceps |
| Kimberly-Clark Professional Kimtech Science Kimwipes Delicate Task Wipers, 1-Ply | FisherSci | 06-666A | kimwipes (or other sterile wipe) Amount per deployable unit: 1 box |
| Laminated Illustrated field collection protocol | NA | NA | abbreviated protocol Amount per deployable unit: 1 protocol |
| Laminated list of materials | NA | NA | supply list Amount per deployable unit: 1 list |
| Lily Sugar 'N Cream The Original Solid Yarn, 2.5 oz, Medium 4 Gauge, 100% Cotton - Hot Pink - Machine Wash & Dry | Amazon | NA | pink yarn (to secure to forceps for visibility) Amount per deployable unit: 2 yards |
| Loupe by Bausch & Lomb, 10x Coddington Magnifier | Amazon | NA | hand lens Amount per deployable unit: 2 hand lenses |
| MyGift Clear Plastic 2-Tier Trays Craft Supply Storage Box/First Aid Carrying Case w/Top Handle & Latch Lock | Amazon | NA | supply storage box Amount per deployable unit: 1 box |
| Nitrile, Disposable Gloves, L, Powder-Free, 2.8 mil Palm Thickness | Grainger | 60NU14 | nitrile lab gloves (large) Amount per deployable unit: 1 box |
| Nitrile, Disposable Gloves, M, Powder-Free, 2.8 mil Palm Thickness | Grainger | 60NU13 | nitrile lab gloves (medium) Amount per deployable unit: 1 box |
| Qiagen, Inc. BUFFER ATL (200 ML) | Qiagen | 19076 | Qiagen ATL lysis buffer Amount per deployable unit: 180 µl/tube; 128 tubes |
| Rite In The Rain Weatherproof Side Spiral Notebook, Yellow Cover, Universal Page Pattern (No. 373-MX), 11 x 8.75 x 0.5 | Amazon | NA | weatherproof field notebook Amount per deployable unit: 1 notebook |
| Showgard Professional Stamp Tongs 6" 904 Round Tip Tweezers | Amazon | NA | 6" spade tip forceps Amount per deployable unit: 2 long spade forceps |
| Texwipe PolySat Pre-Wetted Wipers | FisherSci | 18-366-231 | alcohol wipes Amount per deployable unit: 100 wipes |
| Thermo Scientific CryoBoxes | FisherSci | 12-565-227 | 64 well microcentrifuge tubes collection/storage boxes Amount per deployable unit: 2 boxes |
| packing material Amount per deployable unit: as needed |
References
- Donald, H. M., Wood, C. W., Benowitz, K. M., Johnson, R. A., Drodie, E. D. Nondestructive sampling of insect DNA from defensive secretion. Molecular Ecology Resources. 12 (5), 856-860 (2012).
- Feinstein, J. DNA sequence from butterfly frass and exuviae. Conservation Genetics. 5 (1), 103-104 (2004).
- Hamm, C. A., Aggarwal, D., Landis, D. A. Evaluating the impact of non-lethal DNA sampling on two butterflies, Vanessa cardui and Satyrodes Eurydice. Journal of Insect Conservation. 14, 11-18 (2010).
- Holehouse, K. A., Hammond, R. L., Bourke, A. F. G. Non-lethal sampling of DNA from bumblebees for conservation genetics. Insectes Sociaux. 50 (3), 277-285 (2003).
- Lushai, G., et al. Application of molecular techniques to non-lethal tissue samples of endangered butterfly population (Parnassius apollo L.) in Norway for conservation management. Biological Conservation. 94 (1), 43-50 (2000).
- Monroe, E. M., Lynch, C., Soluk, D. A., Britten, H. B. Nonlethal tissue sampling techniques and microsatellite markers used for first report of genetic diversity in two populations of the endangered Somatochlora hineana (Odonata: Corduliidae). Annals of the Entomological Society of America. 103 (6), 1012-1017 (2010).
- Oi, C. A., López-Uribe, M. M., Cervini, M., Del Lama, M. A. Non-lethal method of DNA sampling in euglossine bees supported by mark-recapture experiments and microsatellite genotyping. Journal of Insect Conservation. 17 (5), 1071-1079 (2013).
- Scriven, J. J., Woodall, L. C., Goulson, D. Nondestructive DNA sampling from bumblebee feces. Molecular Ecology Resources. 13 (2), 225-229 (2013).
- Watts, P. C., Thompson, D. J., Daguet, C., Kemp, S. J. Exuviae as a reliable source of DNA for population-genetic analysis of odonates. Odonatologica. 34 (2), 183-187 (2005).
- Andrews, K., et al. All hands on deck: local ecological knowledge and expert volunteers contribute to the first delisting of a marine fish species under the Endangered Species Act. Citizen Science: Theory and Practice. 4 (1), 37 (2019).
- Buxton, A., Groombridge, J., Griffiths, G. Comparison of two citizen scientist methods for collecting pond water samples for environmental DNA studies. Citizen Science: Theory and Practice. 3 (2), 2 (2018).
- Granroth-Wilding, H., et al. Non-invasive genetic monitoring involving citizen science enables reconstruction of current pack dynamics in a re-establishing wolf population. BMC Ecology. 17 (1), 44 (2017).
- . DNeasy Blood & Tissue Kit Quick Start Protocol Available from: https://www.qiagen.com/us/resources/download.aspx?id=63e22fd7-6eed-4bcb-8097-7ec77bcd4de6&lang=en (2016)
- Storer, C., Daniels, J., Xiao, L., Rossetti, K. Using noninvasive genetic sampling to survey rare butterfly populations. Insects. 10 (10), 311 (2019).
- Wagner, D. L., et al. Insect decline in the Anthropocene: Death by a thousand cuts. Proceedings of the National Academy of Sciences of the United States of America. 118 (2), 20239891 (2021).
- Montgomery, G. A., et al. Is the insect apocalypse upon us? How to find out. Biological Conservation. 241, 108327 (2020).
Explore More Articles
This article has been published
Video Coming Soon
ABOUT JoVE
Copyright © 2024 MyJoVE Corporation. All rights reserved