A Flame-Free Method for Sterilizing C. elegans Picks, Spatulas, and Scalpels
In This Article
Summary
This article describes a method for sterilizing worm picks, spatulas, and scalpels using a micro-incinerator in place of an open flame.
Abstract
Caenorhabtidis elegans (C. elegans) is an optimal model organism for research and education at primarily undergraduate institutions. Undergraduates can quickly learn the sterile technique required to maintain C. elegans cultures. Sterilization of platinum picks used to transfer worms from one plate to another is traditionally done by holding the pick in a flame from a Bunsen burner or ethanol lantern. However, Bunsen burners require a gas source, and both pieces of equipment pose the risk of accidental fire associated with an open flame. Demonstrated here is a technique for sterilizing worm picks, spatulas, and scalpels using an infrared bacteriological loop micro-incinerator. This equipment requires only an electrical outlet and minimizes potential fire hazards. By lowering risk and gas requirements, this technique is well suited for research and teaching in an undergraduate setting.
Introduction
The model organism C. elegans is well-suited for both research and education at primarily undergraduate institutions (PUIs) due to the low cost, ease of maintenance, and range of applications1,2,3,4. In order to handle worms - for example, to move a worm from one plate to another, experimenters can use a worm pick. A variety of picks can be made or purchased for use with C. elegans. Picks are most commonly made using a platinum or platinum/iridium tip, mounted in a glass, metal, or wooden handle. Glass handles can be made in-house by melting a Pasteur pipette around a platinum wire until the wire is secure. Additional information on C. elegans husbandry, including how to grow and maintain worms and their food sources, can be found in WormBook5 and other sources6,7,8.
When working with C. elegans, aseptic techniques are typically used to prevent contamination with microbes and fungi. Examples of aseptic techniques include sterilization of instruments, autoclaving of reagents, and conducting work in sterile fields. Worm picks are typically sterilized using an open flame9. Additionally, sterilization of the worm pick incinerates worms, thus preventing accidental mix-ups of strains when working with multiple worm strains. Typical methods for sterilizing worm picks involve an open flame from either a Bunsen burner, ethanol lantern, or standard lighter (Table 1). We were motivated to seek safer alternatives to existing methods in the laboratory when an undergraduate student unknowingly spilled ethanol while filling an ethanol lantern and accidentally started a small fire when igniting the lantern. Unfortunately, many accidents have been reported using ethanol lanterns10,11,12. Fortunately, alternative sterilization methods have been validated for use in microbiology, and the purpose of this article is to demonstrate how to use this equipment to sterilize instruments for use with C. elegans.
In microbiology laboratories, the aseptic technique is also critical. Serological loops and wires made of platinum are sterilized either using an open flame13 or a micro-incinerator14,15,16. Other names for micro-incinerators include micro-sterilizers or bacto-incinerators. Advantages of the micro-incinerator over traditional flame methods include reduced fire hazard, elimination of spattering of incinerated materials, and ability to work in a laminar flow hood/biosafety cabinet16,17,18. In fact, both the American Society of Microbiology and the World Health Organization recommends using micro-incinerators over the use of an open flame17,19,20. In comparison with Bunsen burners, micro-incinerators also do not require a gas line, which some laboratories might not have, or might not have located at each bench for students to use. Inspired by these advantages, a protocol was developed to replace the use of flame with micro-incinerators for sterilization of commonly used instruments such as picks, spatulas, and scalpels in the C. elegans laboratory. This method may be appropriate for instructors and researchers seeking to enhance safety and/or flexibility when working with C. elegans.
Protocol
1. Prepare the micro-incinerator
- Attach a loop holder accessory guide to the micro-incinerator by clipping it onto the outer barrel.
- Plug in micro-incinerator to a standard 120 V or 230 V electrical outlet as appropriate.
NOTE: This can be done on a benchtop or in a laminar flow hood. - Turn the micro-incinerator to the high setting and allow it to warm up for 10-20 min depending on the manufacturer's instructions to reach an optimum temperature of 800-825 oC.
NOTE: If keeping the incinerator on for longer than 3 h, the lower temperature setting (500 oC) can be used as a standby setting. According to manufacturers' user manuals, this extends the usable life of the equipment.
2. Sterilize the pick, spatula, or scalpel
- Insert the instrument into the cylindrical sterilization area without touching the sides by sliding along the guide21.
NOTE: If sterilizing a scalpel, it is critical to avoid touching the ceramic wall with the blade. Scraping the ceramic wall may compromise the integrity of the heating unit. - Hold the instrument in the sterilization area for 5-7 s.
- Remove the instrument without touching the sides by sliding backward along the guide.
- For picks, allow the instrument to cool for 3-5 s before touching a worm to avoid burning it.
NOTE: Scalpels or spatulas that are not allowed to cool will singe the agar. - After picking worms, re-insert the pick in the chamber for 5-7 s to incinerate any worms on the pick.
3. Comparative method - sterilization of instruments using a Bunsen burner
- Connect Bunsen burner to the gas line using rubber tubing. Make sure to secure the tubing tightly and position the burner away from overhead objects.
- Turn on gas by turning the knob on the gas line.
- Ignite the burner using a striker or lighter.
- Adjust flame using the gas knob and the air intake until a blue cone is visible.
- Hold the pick, spatula, or scalpel in the flame until it glows red.
- For picks, allow the instrument to cool for 3-5 seconds before touching a worm to avoid burning it.
NOTE: Scalpels or spatulas that are not allowed to cool will singe the agar. - After picking worms, re-insert the pick in the flame to incinerate any worms on the pick.
4. Experiment
- Culture OP50 Escherichia coli (E. coli) bacteria in Luria Broth22 overnight on a 37 oC shaker.
- After overnight culture, dilute the culture in sterile water at a ratio of 1:100.
NOTE: This dilution factor was chosen to ensure the separation of colonies after plating. - Dip a worm pick sterilized using a Bunsen burner in the bacterial solution, re-sterilize with the Bunsen burner, and swirl it in 100 mL of sterile water.
- Plate the 100 µL of water on a 10 cm LB agar22,23 Petri dish, spread the water using a sterile cell spreader, and incubate at room temperature for 24 h with the lid on.
- Perform steps 4.3-4.4 using a worm pick sterilized using a micro-incinerator.
- As a positive control, perform steps 4.3-4.4 without re-sterilization.
- As a negative control, perform steps 4.3-4.4 using sterile water in place of the bacterial solution.
- Count the colonies manually after the incubation period.
Representative Results
A simple experiment (section 4) was devised to demonstrate the relative contamination rates using a micro-incinerator versus a flame (Figure 1, Table 2). While these results represent contamination rates across methods, it would not be necessary to repeat this method to use the micro-incinerator technique. The experiment was run in triplicate. Negative control was sterile water with no bacteria. The positive control used neither sterilization technique: the pick was dipped in the diluted OP50 culture then transferred directly to the sterile water. All replicates and conditions were run in parallel on the same day. In all three negative control plates, zero colonies were counted. A mean count of 4.7 (± 0.3 SEM) colonies was obtained when the Bunsen burner was used to sterilize picks. A mean count of 3.3 (±1.2 SEM) colonies was observed in the micro-incinerator condition. However, a mean count of 298.3 (±17.9 SEM) colonies was obtained in the positive control condition. A 2-tailed t-test assuming equal variance comparing the Bunsen burner to micro-incinerator yielded no statistically significant difference, p = 0.35. Thus, the micro-incinerator achieved sterility results equally effective to the Bunsen burner.
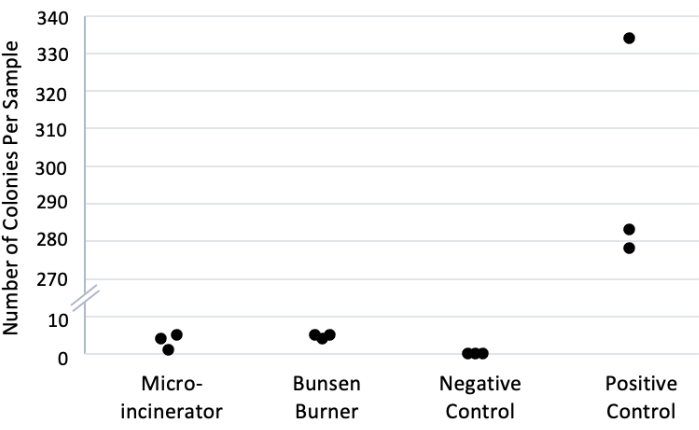
Figure 1: Bacterial counts across sterilization methods. Picks were dipped in 1:100 OP50 culture in sterile water and then sterilized using a Bunsen burner or micro-incinerator. Picks were then dipped in sterile water, plated on LB agar22,23, and incubated for 24 h at room temperature, and colonies were counted manually. Positive control had no sterilization, and negative control used sterile water with no bacteria. n = 3 replicates per condition. Note: y-axis is broken to allow separation of data points in relevant portions of the graph. Please click here to view a larger version of this figure.
| Sterilization Method | Cost | Lab Requirements | Advantages | Disadvantages | |||
| Micro-incinerator | $365–530 | 120 V or 230 V outlet | • Portable • No open flame or exposed heating element • Useable in laminar flow hoods and biological safety cabinets | • Warm up time • Higher cost | |||
| Bunsen Burner | $24–169 | Gas Line | • Fast set up • Low cost | • Open Flame • Not recommended for use in laminar flow hood or biological safety cabinet | |||
| Ethanol Lamp | $11 | None | • Fast set up • Low cost • Refillable | • Open Flame • Safety hazard when refilling • Not recommended for use in laminar flow hood or biological safety cabinet • Not allowed at certain institutions | |||
| Lighter | $5–8 | None | • Low cost • Accessible • Disposable • Refillable | • Open Flame • Hand operated • Not recommended for use in laminar flow hood or biological safety cabinet | |||
Table 1: Comparison of methods for sterilizing instruments. Four methods for sterilizing instruments were compared based on advantages, disadvantages, costs, and laboratory requirements.
| Replicate | Condition | |||
| Micro-incinerator | Bunsen Burner | Negative Control | Positve Control | |
| 1 | 1 | 5 | 0 | 278 |
| 2 | 4 | 4 | 0 | 334 |
| 3 | 5 | 5 | 0 | 283 |
| Mean | 3.3 | 4.7 | 0.0 | 298.3 |
| SEM | 1.2 | 0.3 | 0.0 | 17.9 |
Table 2: Raw data of bacterial counts across sterilization methods. Picks were dipped in 1:100 OP50 culture in sterile water and then sterilized using a Bunsen burner or micro-incinerator. Picks were then dipped in sterile water, plated on LB agar22,23, and incubated for 24 h at room temperature, and colonies were counted manually. Positive control had no sterilization, and negative control used sterile water with no bacteria. n = 3 replicates per condition. Mean and SEM reported below individual counts.
Discussion
C. elegans is a model organism well suited for exercises in undergraduate teaching laboratories. The use of micro-incinerators in place of open flames provides benefits in both research laboratories and classroom laboratories. In fact, undergraduate laboratory courses may pose a higher risk of accidental fires given the number of newly trained scientists in the room. In addition, the risk of fire increases when ethanol is used to sterilize instruments near the source of flame as ethanol vapors are ignitable. The portability also confers an advantage for classrooms where gas lines are not installed at each bench. This method has been employed in teaching and research laboratories at our institution, resulting in no increases in contamination and zero laboratory safety accidents since its incorporation in 2016.
To ensure compatibility with micro-incinerators, a range of picks with different mountings, wire compositions, and wire gauges were tested. Wire composition included 100% platinum as well as 90% platinum/10% iridium with a wire thickness of 30-32 G, and regardless of thickness and composition, the heating method did not compromise wire integrity. Mountings included two different types of commercially available pick handles and in-house-made glass mountings from Pasteur pipettes. Note that picks do not glow red-hot as they do in the flame. However, sufficient sterilization is still achieved as long as the sterilizer has reached the proper temperature. Thus, it is critical to allow the micro-incinerator to warm up for 10 or 20 min, as indicated in the manufacturer's instructions. Keeping the instrument in the chamber for at least 5 s to achieve sterilization is also critical. Leaving the instrument in the chamber longer than 7 s will not cause harm to the instrument but is unnecessary. While this is a relatively straightforward procedure with minimal steps and is unlikely to need troubleshooting, it may take some practice to learn to steady the instrument in the barrel without touching the sides.
In order to replace an open flame, a sterilization method must cover all of the applications used in a laboratory. In addition to sterilizing instruments, C. elegans laboratories may also use Bunsen burners to create a sterile field to do other tasks such as pouring plates or inoculating cultures13. However, whether this creates a sterile field or draws in still-viable contaminants remains controversial14. Although not an option at all institutions, a biosafety cabinet or laminar flow hood can be used for these purposes, allowing a laboratory to function without the use of open flames. Instruction manuals for most micro-incinerators recommend against use for sterilization of scalpel blades because scraping of the inner walls damages the sterilizer. However, if carefully using a guide, one can sterilize a scalpel blade or spatula without touching the inner walls. This extends the use of the technique to allow for chunking without using a flame and allows a C. elegans laboratory to function without switching techniques between sterilizing different objects.
As outlined in Table 1, micro-incinerators offer increased safety, increased compatibility with laminar flow hoods, and increased portability over flame-based methods but have limitations. They are more costly than the other methods and require a warm-up time before sterilization temperatures are achieved. In conclusion, this method which is routinely used in many microbiology laboratories, may be applicable in some C. elegans research and teaching laboratories seeking to improve laboratory safety without compromising sterility.
Acknowledgements
The authors would like to acknowledge Suzanne Howard and Justin Finne. This work was funded by the Wellesley College Neuroscience Department. N2 worms were provided by the CGC which is funded by the NIH Office of Research Infrastructure Programs (P40 OD010440). The publication fees for this article were supported by the Wellesley College Library and Technology Services Open Access Fund.
Materials
| Name | Company | Catalog Number | Comments |
| 90% platinum 10% iridium wire | Tritech | PT-9010 | Other sources and wire compositions may be used. |
| Agarose | Sigma Aldrich | A6013 | LB agar ingredient |
| Bunsen burner | Fisher Scientific | 50-110-1225 | Other Bunsen burners may be used |
| Ethanol Lamp | Carolina | 706604 | Included here as a reference to Table 1 |
| Lighter | Carolina | 706636 | Included here as a reference to Table 1 |
| Loop holder accessory | Fisher | 22-630-002 | Referred to in the manuscript as "guide" |
| Micro-incinerator | Thomas Scientific | 1154J15 | There are many companies that sell similar equipment. Similar models also sold by Benchmark Scientific (B1001), Fisher Scientific (22-630-001), Carolina (703400), and BT Lab Systems (BT1702). |
| N2 worms | CGC | N2 | |
| NaCl | Sigma Aldrich | S5886 | LB ingredient |
| OP50 E. coli | CGC | OP50 | |
| Petri dish | Fisher | 08-772B | |
| Pick handle | Tritech | TWPH1 | |
| Scalpel blade | Fisher | 12-000-161 | |
| Scalpel handle | Fisher | 12-000-164 | |
| Spatula | Fisher | 14-374 | Other spatulas will work |
| Sterile cell spreaders | VWR | 76206-438 | Other cell spreaders may be used as long as they are sterile |
| Tryptone | Sigma Aldrich | T7293 | LB ingredient |
| Yeast Extract | Sigma Aldrich | Y1625 | LB ingredient |
References
- Attix, H., et al. Wild caught nematode identification and early embryo development: An accessible undergraduate research experience. microPublication Biology. 2021, (2021).
- Rose, J. K. Demonstrating connections between neuron signaling and behavior using C. elegans learning assays and optogenetics in a laboratory class. Journal of Undergraduate Neuroscience Education. 16 (3), 223-231 (2018).
- Lemons, M. L. An inquiry-based approach to study the synapse: Student-driven experiments using C. elegans. Journal of Undergraduate Neuroscience Education: JUNE: A Publication of FUN, Faculty for Undergraduate Neuroscience. 15 (1), 44-55 (2016).
- Raley-Susman, K. M., Gray, J. M. Exploration of gerontogenes in the nervous system: a multi-level neurogenomics laboratory module for an intermediate neuroscience and behavior course. Journal of Undergraduate Neuroscience Education. 8 (2), 108-115 (2010).
- Stiernagle, T. Maintenance of C. elegans. WormBook: The Online Review of C. Elegans Biology. , 1-11 (2006).
- Porta-de-la-Riva, M., Fontrodona, L., Villanueva, A., Cerón, J. Basic Caenorhabditis elegans methods: synchronization and observation. Journal of Visualized Experiments: JoVE. (64), e4019 (2012).
- JoVE. Biology I: yeast, Drosophilia, and C. Elegant. C. Elegans Maintenace. JoVE Science Education Database. , (2022).
- Hope, I. A. . C. elegans: A Practical Approach. , (1999).
- Meneely, P. M., Dahlberg, C. L., Rose, J. K. Working with Worms: Caenorhabditis elegans as a Model Organism. Current Protocols Essential Laboratory Techniques. 19 (1), 35 (2019).
- Waechter-Brulla, D. Improving safety in the microbiology laboratory through active learning and investigation. American Society for Microbiology. , (2000).
- Young, J. A. It says in the books that ethanol burns with a cool flame. Journal of Chemical Education. 77 (11), 1488 (2000).
- Mojtabai, F., Kaufman, J. A. . Learning by accident. V. 2. , (2005).
- JoVE. General Laboratory Techniques. Introduction to the Bunsen Burner. JoVE Science Education Database. , (2022).
- Bykowski, T., Stevenson, B. Aseptic Technique. Current Protocols in Microbiology. 56 (1), 98 (2020).
- Katz, D. S. The streak plate protocol. American Society for Microbiology Laboratory Protocols. , (2008).
- Public Health Agency of Canada. Agency of Canada Canadian Biosafety Handbook. Government of Canada. , (2016).
- Emmert, E. A. B. ASM Task Committee on Laboratory Biosafety Biosafety guidelines for handling microorganisms in the teaching laboratory: development and rationale. Journal of Microbiology & Biology Education. 14 (1), 78-83 (2013).
- Collins, C. H., Pal, S. B. Health Hazards in Microbiology. Handbook of Laboratory Health and Safety Measures. , (1990).
- Laboratory Biosafety Manual. World Health Organization Available from: https://www.who.int/publications/I/item/9789240011311 (2020)
- Fleming, D. O. Laboratory Safety Principles and Practices. American Society for Microbiology. , (1995).
- Gordon, R. C., Davenport, C. V. Simple modification to improve usefulness of the Bacti-Cinerator. Applied Microbiology. 26 (3), 423 (1973).
- Cold Spring Harbor. LB (Luria-Bertani) liquid medium. Cold Spring Harbor Protocols. 2006 (1), (2006).
- Cold Spring Harbor. Media containing agar or agarose. Cold Spring Harbor Protocols. 2006 (1), (2006).
This article has been published
Video Coming Soon
ABOUT JoVE
Copyright © 2024 MyJoVE Corporation. All rights reserved