A subscription to JoVE is required to view this content. Sign in or start your free trial.
Reactivation of Demembranated Cell Models in Chlamydomonas reinhardtii
In This Article
Summary
The in vitro reactivation of motile cells is a crucial experiment in understanding the mechanisms of cell motility. The protocol describes reactivating the demembranated cell models of Chlamydomonas reinhardtii, a model organism to study cilia/flagella.
Abstract
Since the historical experiment on the contraction of glycerinated muscle by adding ATP, which Szent-Györgyi demonstrated in the mid-20th century, in vitro reactivation of demembranated cells has been a traditional and potent way to examine cell motility. The fundamental advantage of this experimental method is that the composition of the reactivation solution may be easily changed. For example, a high-Ca2+ concentration environment that occurs only temporarily due to membrane excitation in vivo can be replicated in the lab. Eukaryotic cilia (a.k.a. flagella) are elaborate motility machinery whose regulatory mechanisms are still to be clarified. The unicellular green alga Chlamydomonas reinhardtii is an excellent model organism in the research field of cilia. The reactivation experiments using demembranated cell models of C. reinhardtii and their derivatives, such as demembranated axonemes of isolated cilia, have significantly contributed to understanding the molecular mechanisms of ciliary motility. Those experiments clarified that ATP energizes ciliary motility and that various cellular signals, including Ca2+, cAMP, and reactive oxygen species, modulate ciliary movements. The precise method for demembranation of C. reinhardtii cells and reactivation of the cell models is described here.
Introduction
The in vitro reactivation of demembranated motile cells is a valuable tool for studying the molecular basis for the regulatory mechanism of cell motility. Szent-Györgyi first demonstrated in vitro contraction of rabbit skeletal muscle fibers extracted with 50% glycerol by adding adenosine triphosphate (ATP)1. This experiment was the first to prove that ATP energizes muscle contraction. The methodology was soon applied to the study of ATP-energized ciliary/flagellar motility, such as sperm flagella2, Paramecium cilia3, and Chlamydomonas reinhardtii cilia (also called flagella)4 using non-ionic detergents for demembranation.
The unicellular green alga C. reinhardtii is a model organism for studying cilia: it swims with two cilia by beating them like a human's breaststroke5. Ciliary motion is driven by dynein, a minus-end directed microtubule-based motor protein6,7. Ciliary dyneins can be classified into outer-arm dyneins and inner-arm dyneins. Mutants lacking each kind of dynein have been isolated as slow-swimming mutants with different motility abnormalities. Detailed in vitro motility analysis of these mutants has significantly advanced dynein research8.
Many important findings have been achieved utilizing this method and its derivatives since the in vitro reactivation experiment of demembranated C. reinhardtii cells (cell models) was established. Reactivation of cell models in a series of Ca2+ buffers, for example, showed9 that two cilia are differently regulated by submicromolar Ca2+, and this asymmetrical cilia control enables the phototactic orientation of C. reinhardtii10. Furthermore, both cilia show waveform conversion from the forward swimming mode (called asymmetric waveform) to the backward swimming mode (called symmetric waveform that appears for a short period when cells are photo- or mechano-shocked)11,12. This waveform conversion is regulated by submillimolar Ca2+, which was shown by the reactivation of the so-called nucleoflagellar apparatus (a complex containing two cilia, the basal bodies, the structures linking the basal bodies with the nucleus, and the remnant of the nucleus)11 or demembranated axonemes of isolated cilia13. Other than Ca2+, redox (reduction-oxidation) poise is a signal that regulates the ciliary beating frequency, which was shown by reactivation of cell models in redox buffers containing different ratios of reduced glutathione vs. oxidized glutathione14. In addition, cyclic adenosine monophosphate (cAMP) asymmetrically regulates two cilia, which was shown by the reactivation of axonemes with photocleavable caged cAMP15. These in vitro findings, combined with genetic findings, have led to a deeper understanding of the molecular mechanisms of cilia regulation in C. reinhardtii.
A protocol for reactivating the cell models is described here. The method is simple, allows for various modifications, and can be applied to multiple organisms that move with cilia. However, because demembranated cells are fragile, it requires some tips to reactivate the motility of cell models with good efficiency while preventing deciliation.
Protocol
A wild-type strain of Chlamydomonas reinhardtii, CC-125, was used for the present study. CC-125 was obtained from Chlamydomonas Resource Center (see Table of Materials) and maintained on a Tris-acetate-phosphate (TAP)16, 1.5% agarose medium at 20-25 °C.
1. Cell culture
- Culture Chlamydomonas reinhardtii (CC-125) in TAP medium16 in a 12 h/12 h light-dark period (light conditions for the light period: ~50 µmol photons m−2 s−1 white light) at 20-25 °C for 2 days (Figure 1).
NOTE: The cells must be in a mid-logarithmic growth phase (Movie 1). Long culture (>4 days, in the late-logarithmic growth or stationary phase) decreases the reactivation efficiency of demembranated cell models.

Figure 1: Liquid culture after 2-day culturing. From a TAP-1.5% agar plate, a chunk of wild-type cells to fill the platinum loop was inoculated to ~150 mL TAP liquid medium in a flask. The cell density after 2-day culture was 2.3 × 106 cells/mL. Please click here to view a larger version of this figure.
Movie 1: Swimming of live cells. Cells were observed under a microscope with a 10x objective lens and an oil-immersion dark-field condenser. Scale bar = 100 µm. Please click here to download this Movie.
2. Preparation of demembranated cell models
NOTE: Before starting the experiment, keep the washing buffer at room temperature and the demembranation, dilution, reactivation buffers, and the ATP solution on ice. The composition of these buffers is provided in Supplementary Table 1.
- Centrifuge ~10 mL of liquid culture at 1000 × g at 20 °C for 3 min.
NOTE: Throughout the protocol from this step, do not use autoclaved plasticware (tubes, pipette tips, etc.), decreasing reactivation efficiency. For conical tubes, reused ones are preferable. The cell density after 2-day culture may be typically 1.0-5.0 × 106 cells/mL. When the cell density is lower than this, take enough culture volume to contain ~5 × 107 cells. - Discard the supernatant, first by decantation and then by a Pasteur pipette, and resuspend the precipitates in ~5 mL of washing buffer.
- Centrifuge cells in the washing buffer at 1000 × g for 3 min at 20 °C.
- Discard the supernatant carefully with a pipette (Figure 2).
NOTE: Pasteur pipettes are preferable. When using micropipettes, avoid using pipette tips that have been autoclaved. - Overlay ~0.5 mL of demembranation buffer on a cell pellet, shake the tube gently by hand to roughly suspend the cells in the buffer, and put the tube on ice (Figure 3A).
NOTE: It is not necessary to entirely suspend the pellet at this moment. Raise the concentration of MgSO4 to 15 mM in the demembranation and reactivation buffers when cell models are reactivated, with a final ATP concentration >1 mM for stable reactivation17. - Suspend the remaining cell pellet gently with a pipette, and place the tube on ice again (Figure 3B).
- Take 5-10 µL of the cell models, dilute 10-fold with the dilution buffer, and observe under the microscope (Movie 2) to confirm all the cell models are demembranated and not swimming (Figure 4).
NOTE: If some cells are still swimming (alive), add the non-ionic detergent used in the demembranation buffer directly to the cell model solution to the final concentration of ~0.15%. Alternatively, repeat steps 2.1-2.5 with the demembranation solution containing 0.15% of detergent.
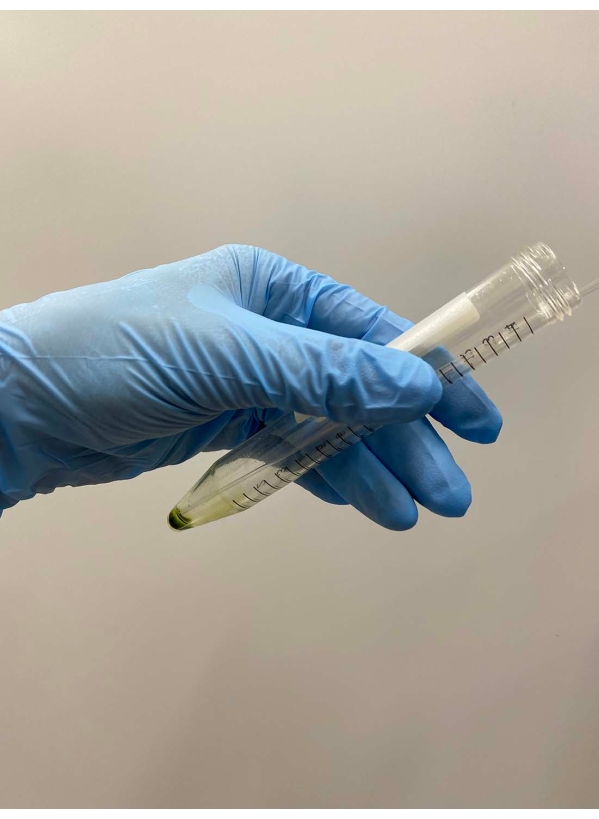
Figure 2: Discarding the supernatant. The rest of the supernatant was carefully removed with a Pasteur pipette after the supernatant was removed by decantation of the tube. Please click here to view a larger version of this figure.
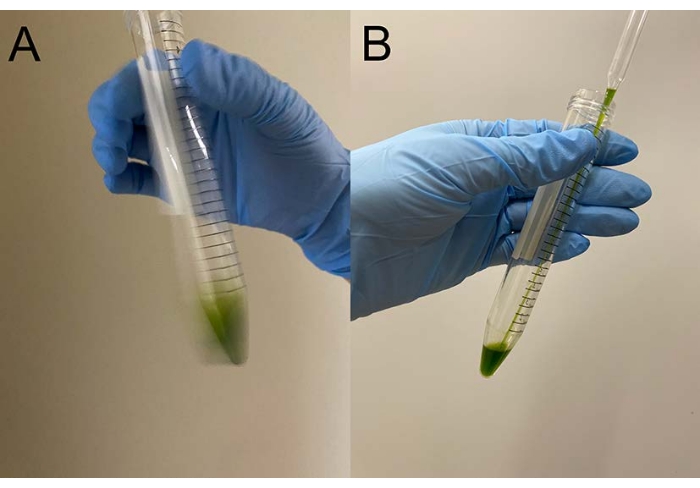
Figure 3: Demembranation. (A) After overlaying 0.5 mL of demembranation solution onto the cell pellet, the solution was mixed by a hand to suspend the cells roughly. (B) After mixing, the rest of the cell pellet was entirely suspended in the solution by a Pasteur pipette. Please click here to view a larger version of this figure.
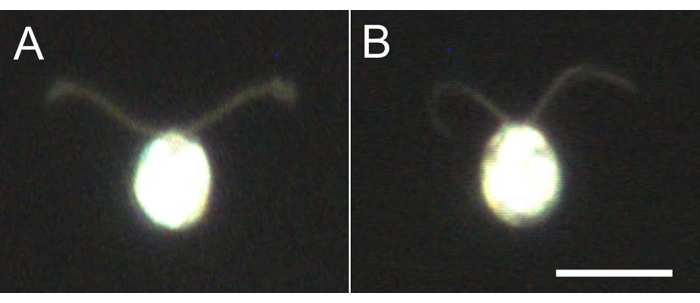
Figure 4: Effects of demembranation. (A) A live cell stuck on a glass slide. (B) A cell model stuck on a glass slide. Note that in the cell model, the cilia became slightly thinner. The images were observed under a microscope with a 20x objective lens and an oil-immersion dark-field condenser. Scale bar = 10 µm. Please click here to view a larger version of this figure.
Movie 2: Confirmation of demembranation. Cell model suspension was observed under a microscope with a 10x objective lens and an oil-immersion dark-field condenser. No cell was swimming. Scale bar = 100 µm. Please click here to download this Movie.
3. Reactivation of demembranated cell models
- Mix 80 µL of reactivation solution, 10 µL of ATP solution, and 10 µL of cell models in a 0.5 mL tube by tapping the tube (Figure 5).
NOTE: The final ATP concentration must be <3 mM because ciliary beating frequency, a parameter for ciliary motion, increases with ATP concentration and saturates at 2-3 mM of ATP17.
CAUTION: Mixing by pipetting or vortexing causes deciliation and decreases reactivation efficiency.- For reactivating with <0.2 mM of ATP, add an ATP-regeneration system, such as 70 U/mL of creatine kinase and 5 mM of creatine phosphate (see Table of Materials).
NOTE: The reactivation solution is prepared at a higher concentration (1.125x, Supplementary Table 1) than the dilution solution so that after mixing the ATP dissolved in water, the contents reach the following final concentrations: 30 mM of Hepes (pH 7.4), 5 mM of MgSO4, 1 mM of dithiothreitol (DTT), 1 mM of EGTA, and 50 mM of potassium acetate (see Table of Materials).
- For reactivating with <0.2 mM of ATP, add an ATP-regeneration system, such as 70 U/mL of creatine kinase and 5 mM of creatine phosphate (see Table of Materials).
- Put ~30 µL of the mixed solution onto a glass slide and gently place a coverslip on it with spacers to avoid mechanical shock to the cell models (Figure 6).
NOTE: White petroleum or double-sided adhesive tapes can be used to make the spacers. - Observe the reactivated cell models under a microscope (Movie 3).

Figure 5: Mixing solution by tapping the tube. (A) To 80 µL of the reactivation solution, 10 µL of ATP solution, and 10 µL of cell models were added in sequential order. (B) The solution was mixed by tapping the tube with a finger. Please click here to view a larger version of this figure.

Figure 6: Making spacers on coverslip edges. (A) A thin layer of white petroleum was applied to the back of a hand. (B) A small amount of white petroleum was scraped off with an edge of a coverslip. (C) A spacer was made on the edge of a coverslip. (D) Another spacer was made on the opposite edge. Please click here to view a larger version of this figure.
Movie 3: Swimming of reactivated cell models. The motility of the cell models was reactivated by adding ATP at a final concentration of 1 mM, and observed under a microscope with a 10x objective lens and an oil-immersion dark-field condenser. Scale bar = 100 µm. Please click here to download this Movie.
Results
The demembranation and reactivation process in C. reinhardtii wild type strain (CC-125) is shown here. The culture 2 days after inoculation became a light green color (step 1.1) (Figure 1). Cells were collected (step 2.1), washed (step 2.2), and demembranated (step 2.5). After demembranation, all cell models became immotile (step 2.7). The demembranated cilia (called axonemes) remain attached to the cell body, which shows that the immotility of the cell models is not caused by ...
Discussion
There are two critical steps in this protocol. The first is a process known as demembranation, which needs to be carried out gently but thoroughly. Deciliation (i.e., detaching of cilia from the cell body) is induced by vigorous pipetting or vortexing, rendering the cell models immobile even after the addition of ATP. Typically, 5 × 107 cells are suspended in ~0.5 mL of demembranation buffer (final cell density: 1 × 108 cells/mL). The reactivation rate of the cell models would decrease if ...
Disclosures
The authors have nothing to disclose.
Acknowledgements
This study was supported by grants from the Japan Society for the Promotion of Science KAKENHI (https://www.jsps.go.jp/english/index.html) to N.U. (19K23758, 21K06295) and K.W. (19H03242, 20K21420, 21H00420), from the Ohsumi Frontier Science Foundation (https://www.ofsf.or.jp/en/) to K.W., and from the Dynamic Alliance for Open Innovation Bridging Human, Environment and Materials (http://alliance.tagen.tohoku.ac.jp/english/) to N.U. and K.W. We thank Ms. Miyuki Shinohara (Hosei Univ.) for her help in preparing the figures.
Materials
| Name | Company | Catalog Number | Comments |
| 0.5 mL plastic tube | QSP | 502-PLN-Q | |
| 15 mL conical tube | SARSTEDT | 62.554.502 | |
| Adenosine 5'-triphosphate disodium salt hydrate (ATP) | Sima-Aldrich | A2383 | |
| Centrifuge | KUBOTA | 2800 | |
| Chlamydomonas strain CC-125 | Chlamydomonas Resource Center | https://www.chlamycollection.org/ | |
| Creatine kinase | Merck | CK-RO | |
| Creatine phosphate | Merck | CRPHO-RO | |
| Dithiothreitol (DTT) | Nakalai tesque | 14128-46 | |
| GEDTA(EGTA) | Dojindo | G002 | |
| Hepes | Dojindo | GB70 | |
| Igepal CA-630 | Sigma-Aldrich | I8896 | IUPAC name is octylphenoxypolyethoxyethanol: IGEPAL CA-630 is a substitute for Nonidet P-40 (NP-40); NP-40 is no longer available in Sigma-Aldrich. |
| MgSO4-7H2O | Nakalai tesque | 21002-85 | |
| Microscope | Olympus | BX-53 | |
| Pasteur pipette | fisher scientific | 13-678-20C | |
| Polyethylene glycol, Mr 20,000 | Merck | 8.18897.1000 | |
| Pottasium acetate | Nakalai tesque | 28434-25 | |
| Sodium Hydroxide | Nacalai | 31511-05 | |
| Sucrose | FUJIFILM Wako Pure Chemical Corporation | 196-00015 |
References
- Szent-Gyorgyi, A. Free-energy relations and contraction of actomyosin. Biological Bulletin. 96 (2), 140-161 (1949).
- Hoffman-Berling, H. Adenosintriphosphat als betriebsstoff von zellbewegungen. Biochimica et Biophysica Acta. 14, 182-194 (1954).
- Naitoh, Y., Kaneko, H. Reactivated Triton-extracted models of Paramecium: modification of ciliary movement by calcium ions. Science. 176 (4034), 523-524 (1972).
- Witman, G. B., Plummer, J., Sander, G. Chlamydomonas flagellar mutants lacking radial spokes and central tubules. Structure, composition, and function of specific axonemal components. Journal of Cell Biology. 76 (3), 729-747 (1978).
- Rüffer, U., Nultsch, W. Flagellar coordination in Chlamydomonas cells held on micropipettes. Cell Motility and the Cytoskeleton. 41 (4), 297-307 (1998).
- Sale, W. S., Satir, P. Direction of active sliding of microtubules in Tetrahymena cilia. Proceedings of the National Academy of Sciences of the United States of America. 74 (5), 2045-2049 (1977).
- Fox, L. A., Sale, W. S. Direction of force generated by the inner row of dynein arms on flagellar microtubules. Journal of Cell Biology. 105 (4), 1781-1787 (1987).
- Kamiya, R., Yagi, T. Functional diversity of axonemal dyneins as assessed by in vitro and in vivo motility assays of Chlamydomonas mutants. Zoolog Science. 31 (10), 633-644 (2014).
- Kamiya, R., Witman, G. B. Submicromolar levels of calcium control the balance of beating between the two flagella in demembranated models of Chlamydomonas. Journal of Cell Biology. 98 (1), 97-107 (1984).
- Okita, N., Isogai, N., Hirono, M., Kamiya, R., Yoshimura, K. Phototactic activity in Chlamydomonas 'non-phototactic' mutants deficient in Ca2+-dependent control of flagellar dominance or in inner-arm dynein. Journal of Cell Science. 118, 529-537 (2005).
- Hyams, J. S., Borisy, G. G. Isolated flagellar apparatus of Chlamydomonas: characterization of forward swimming and alteration of waveform and reversal of motion by calcium ions in vitro. Journal of Cell Science. 33, 235-253 (1978).
- Fujiu, K., Nakayama, Y., Yanagisawa, A., Sokabe, M., Yoshimura, K. Chlamydomonas CAV2 encodes a voltage- dependent calcium channel required for the flagellar waveform conversion. Current Biology. 19 (2), 133-139 (2009).
- Bessen, M., Fay, R. B., Witman, G. B. Calcium control of waveform in isolated flagellar axonemes of Chlamydomonas. Journal of Cell Biology. 86 (2), 446-455 (1980).
- Wakabayashi, K., King, S. M. Modulation of Chlamydomonas reinhardtii flagellar motility by redox poise. Journal of Cell Biology. 173 (5), 743-754 (2006).
- Saegusa, Y., Yoshimura, K. cAMP controls the balance of the propulsive forces generated by the two flagella of Chlamydomonas. Cytoskeleton. 72 (8), 412-421 (2015).
- Gorman, D. S., Levine, R. P. Cytochrome f and plastocyanin: their sequence in the photosynthetic electron transport chain of Chlamydomonas reinhardi. Proceedings of the National Academy of Sciences of the United States of America. 54 (6), 1665-1669 (1965).
- Takano, W., Hisabori, T., Wakabayashi, K. Rapid estimation of cytosolic ATP concentration from the ciliary beating frequency in the green alga Chlamydomonas reinhardtii. Journal of Biological Chemistry. 296, 100156 (2021).
- Wakabayashi, K., Yagi, T., Kamiya, R. Ca2+-dependent waveform conversion in the flagellar axoneme of Chlamydomonas mutants lacking the central-pair/radial spoke system. Cell Motility and the Cytoskeleton. 38 (1), 22-28 (1997).
- Yueh, Y. G., Crain, R. C. Deflagellation of Chlamydomonas reinhardtii follows a rapid transitory accumulation of inositol 1,4,5-trisphosphate and requires Ca2+ entry. Journal of Cell Biology. 123 (4), 869-875 (1993).
- Wakabayashi, K., Ide, T., Kamiya, R. Calcium-dependent flagellar motility activation in Chlamydomonas reinhardtii in response to mechanical agitation. Cell Motility and the Cytoskeleton. 66 (9), 736-742 (2009).
- Ueki, N., Wakabayashi, K. Detergent-extracted Volvox model exhibits an anterior-posterior gradient in flagellar Ca2. Proceedings of the National Academy of Sciences of the United States of America. 115 (5), 1061-1068 (2018).
- Tanno, A., et al. The four-celled Volvocales green alga Tetrabaena socialis exhibits weak photobehavior and high-photoprotection ability. PLoS One. 16 (10), 0259138 (2021).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved