Magnetic Resonance-Guided Stereotaxy for Infusions to the Pig Brain
In This Article
Summary
The protocol presented here demonstrates stereotaxy to the pig brain using convection-enhanced infusions, with real-time magnetic resonance imaging (MRI) visualization guidance and real-time infusion distribution visualization.
Abstract
The overall goal of this procedure is to perform stereotaxy in the pig brain with real-time magnetic resonance (MR) visualization guidance to provide precise infusions. The subject was positioned prone in the MR bore for optimal access to the top of the skull with the torso raised, the neck flexed, and the head inclined downward. Two anchor pins anchored on the bilateral zygoma held the head steady using the head holder. A magnetic resonance imaging (MRI) flex-coil was placed rostrally across the head holder so that the skull was accessible for the intervention procedure. A planning grid placed on the scalp was used to determine the appropriate entry point of the cannula. The stereotactic frame was secured and aligned iteratively through software projection until the projected radial error was less than 0.5 mm. A hand drill was used to create a burr hole for insertion of the cannula. A gadolinium-enhanced co-infusion was used to visualize the infusion of a cell suspension. Repeated T1-weighted MRI scans were registered in real time during the agent delivery process to visualize the volume of gadolinium distribution. MRI-guided stereotaxy allows for precise and controlled infusion into the pig brain, with concurrent monitoring of cannula insertion accuracy and determination of the agent volume of distribution.
Introduction
In this protocol, we describe the application of an interventional magnetic resonance imaging (iMRI) stereotactic system for cannula placement and real-time visualization of infusions into the pig brain. The development of iMRI systems allows for accurate catheter placement1. iMRI allows for visualization of the distribution of the infusion agent in the brain of patients under general anesthesia1,2 to evaluate the accuracy of the procedure in real time.
The MR-guided stereotactic system is a targeted platform that allows for sub-millimeter targeting accuracy1. It uses a skull-mounted aiming device in conjunction with dedicated software that provides anatomical imaging of the brain with projected lead insertion trajectories and adjustment parameters. iMRI guidance for stereotactic surgical intervention to the brain has proven effective in clinical applications, such as deep brain stimulation in the treatment of Parkinson's disease2,3,4,5, focal ablation for the treatment of epilepsy6,7, and convection-enhanced delivery (CED) of drugs to the central nervous system8,9.
The CED method is used to directly deliver therapeutic agents to the central nervous system using fluid convection. This is based on a small hydrostatic pressure gradient that enables the flow of an infusate from the tip of the infusion cannula into the surrounding extracellular space10. Stereotactic methods are used to deliver high concentrations of macromolecules, small molecules11,12, cell transplantation13,14,15, or therapeutic agents into the chosen brain tissue target, circumventing the blood-brain barrier. Factors such as permeability, diffusion coefficients, back pressure, uptake, and clearance mechanisms affect the diffusion of the therapeutic agents16. This technique utilizes a gadolinium-based co-infusate1 for clinical CED, to monitor the infusion agent in real time into the parenchymal target. Parameters such as the volume of distribution in the tissue and related kinetics following targeted accuracy are monitored with iMRI.
CED studies of infusion agents via an MR-guided stereotaxy system have been studied in non-human primates, resulting in accurate, predictable, and safe procedures. Infusion cannula placement accuracy has been shown to reach sub-millimeter placement error17. The system provides a predictable infusion distribution, with an observed linear increase in distribution volume with infusion volume, leading to a subsequently introduced reflux-resistant cannula for CED infusions18. This iMRI infusion procedure was reported to incur no untoward effects in non-human primates19.
Here, we expand the application of MR-guided sterotaxy to the pig brain, to deliver and monitor the distribution of an infusion agent consisting of a 300 µL cell suspension. The size of the pig brain allows for imaging and neurosurgical interventions that can be applied clinically to humans, which is not possible in smaller animal models of disease20. Furthermore, the immune system of the pig produces similar responses to that of humans in terms of responses to biological or other therapeutic agents21. Therefore, working with this animal species for stereotactic drug delivery procedures has direct translational clinical implications and may be logistically easier than with non-human primate research.
We used a pig model (domestic swine, female, 25 kg, 14 weeks of age) for MR-guided stereotaxy. The visual implementation of the stereotactic procedure in pigs is reported in this study. We describe the adaptations of the space to accommodate a pig head, visualization of the procedure both in video and images, and concurrent MR imaging to evaluate infusate distribution in the pig brain. MR-guided stereotaxy was performed in a 3T MRI space.
With this experiment, our group demonstrates the performance of MR-guided stereotaxy in the pig brain, and a basic imaging timeline to track infusions within the brain. The general technique for clinical stereotaxy performed in humans can be applied to the swine skull and brain.
The overall goal of this procedure is to perform MR-guided stereotaxy in the pig brain with real-time MRI visualization guidance. This is achieved by first positioning the subject prone in the MRI bore for optimal access to the top of the skull. The second step is to plan the surgical insertion with MRI-assisted visualization guidance, which involves the placement and scan of a fiducial grid to determine the appropriate entry point for a pre-planned trajectory. This is achieved with a high-resolution (1 mm isotropic) T1-weighted 3D magnetization prepared rapid gradient echo (MPRAGE) scan, in a duration of 7 min and 44 s. Next, we secure the stereotactic frame on the head, and adjust the alignment iteratively through software projection until the projected radial error is less than 0.5 mm. Fast 2D turbo spin echo scans (duration of 13 s) in oblique orientations provide image guidance. Then, an incision is made on the skin, and a hand drill is used to create a burr hole for insertion of the infusion cannula at the predefined coordinates. The final step is to monitor the infusion with repeated T1-weighted MRI scans (3D MPRAGE; 1 min 45 s) in real time with gladolinium co-infusion. The results show that MR-guided stereotaxy allows for precise and controlled infusion into the pig brain, based on real-time MR guidance and subsequent T1-weighted 3D MPRAGE MRI scans (1 mm isotropic resolution) used to visualize the volume of distribution.
Protocol
The study was approved by the Institutional Animal Care and Use Committee at Houston Methodist Research Institute, IACUC approval number IS00006378. All experimental methods were performed in accordance with the relevant national and institutional guidelines and regulations.
1. Animal positioning
- Position the subject for optimal access to the top of the skull: place the subject on the MRI table in preparation for the MRI scan.
NOTE: Subject information: domestic swine, female, 25 kg, 14 weeks of age.- Sedate the subject with ketamine (600 mg intramuscularly [IM]) and midazolam (5 mg IM). Administer the analgesics hydromorphone (4 mg IM), carprofen (100 mg per os), and fentanyl (25 µg topical), the antibiotic ceftriaxone (550 mg intravenously [IV]), and NaCl (0.9% IV).
- Intubate the subject. Maintain anesthesia with 2%-3% isoflurane.
- Monitor the subject's vital signs throughout the procedure.
- Mechanically ventilate at 16-19 respirations/min with a ventilator.
- Place the subject on the MRI table in preparation for the MRI scans.
- Place the subject in a prone position with the head facing the MRI bore.
- Place a standard MRI four channel flex coil on the head holder.
- Stabilize the subject's head with the head holder.
- Raise the torso with towels and foam pads. The goal is for the head to fall slightly downward, with the neck flexed and the snout nearly touching the table. This will help to ensure that the stereotactic frame and infusion cannula fit within the bore of the MRI scanner. Anchor the MRI head holder pins on the bilateral zygoma to keep the head affixed to the MRI table.
- Check that the top of the skull is inclined toward the back of the scanner with the neck flexed. This position enables the surgeon to have access to the top of the scalp when the subject enters the MRI.
- Once set, the MRI table is moved into the bore of the scanner until the subject's head reaches the end of the bore.
2. Planning surgical insertion with MRI-assisted visualization guidance
- Prepare the area in a sterile fashion, taking care to avoid the prepared material from getting into the subject's eyes. Place sterile towels around the surgical area. Place a sterile drape with an opening toward the top of the skull that the surgeon can access.
- Place the fiducial planning grid on the subject's scalp by affixing the adhesive side of the grid over the patient's head, centered around the location of where the burr hole will be.
- Peel off the top fluid-filled layer of the grid while firmly holding the lower layer in position.
- Perform the MRI scout scan with the grid set in place. The scan often requires intravenous MR contrast agent administration to visualize the vasculature: use a 1 mmol/mL concentration of the contrast agent gadolinium contrast agent for an infusion volume of 2.5 mL.
NOTE: The scout scan is a preliminary image taken before the definitive imaging study. The purpose is for the surgeon to ensure that imaging is carried out close to the region of interest, and to define imaging boundaries. The recommended dose at the 1 mmol/mL concentration, as per the manufacturer, for the contrast agent is 0.1 mL per kilogram that the animal weighs. - Select the precise brain location for cannula insertion in the MR-guidance software.
- Ensure the software allows the visualization of the surgeon's planned trajectory for cannula placement, based on the target selected. Ensure the software outputs the trajectory visualization and the corresponding entry point.
NOTE: For this study, a site in the frontal cortex was selected to target white matter. This is a location where many human gliomas arise and grow22. It is also a preferential site for dissemination along white matter tracts23.
NOTE: Consider the surgeon's decision for an entry point, target, and desired trajectory to minimize pial and sulcal transgressions and avoid blood vessels. - Adjust the suggested trajectory, including the desired entry and target points, by manually dragging the projected entry and target points in the software to avoid blood vessels and minimize pial and sulcal transgressions. The trajectory can be changed and viewed in three dimensions.
- Once the desired trajectory is identified based on the surgeon's preference, run the MR-guidance software to find the entry point on the grid.
- Scroll through the planned trajectory on the scan to find the entry point on the scalp. The software specifies the grid coordinates based on the projection of the planned trajectory on the grid.
3. Securing the stereotactic frame and adjusting the alignment iteratively through software projection
- Assemble the stereotactic frame around the desired entry point coordinates on the grid by first securing the base with six bone-anchored screws and four offset screws.
- Secure the six bone-anchored screws to the skull over the grid, through the scalp. The six anchor screws are used to stabilize the stereotactic frame and avoid any movement during drilling.
- Secure the four offset screws located at the base of the tower through the skin, anchored on the skull. They act as a counter force to tighten the center bone screws, by lifting the frame base to the center screws, and to stabilize the base.
- Once the stereotactic frame base is secure, continue with frame assembly.
- Perform the high-resolution T1-weighted MPRAGE MRI scan, an option in the MRI software, with the frame set in place to capture the frame fiducials and confirm the trajectory.
- Confirm the desired projected cannula insertion trajectory with the software, visualizing the MRI scan and planned trajectory.
- Subsequent 2D turbo spin echo MRI scans are taken to confirm alignment of the frame with the subject once the frame is in place. If there is a misalignment between the current frame position and the desired trajectory, the software outputs adjustment parameters.
NOTE: The software calculates the radial difference between the projection of the current position of the stereotactic frame and the defined target point. This error is used to calculate the projected error, which is in turn used to calculate the required adjustments to the frame to minimize it.
- Subsequent 2D turbo spin echo MRI scans are taken to confirm alignment of the frame with the subject once the frame is in place. If there is a misalignment between the current frame position and the desired trajectory, the software outputs adjustment parameters.
- Perform the pitch-roll and X-Y adjustments by turning the thumb wheels, as indicated by the output adjustment parameters in the software.
- Repeat the software-enabled MRI visualization of the trajectory and perform rotational and translational adjustments (using the thumb wheels) of the targeting cannula as necessary.
- Using the MR-guidance software, measure the thickness of the skull at the desired trajectory and the total distance to the brain.
NOTE: The software calculates the distance from the top of the frame (screwed to the skull) to the target point to estimate the total length.
4. Drilling and inserting the cannula for infusion
- Use an iodine scrub before performing the incision to prevent infection.
- Make a 3 cm incision on the scalp, using a scalpel under the stereotactic frame.
- Set up the frame for drill insertion by performing the adjustments prior to creating the access hole.
- Remove and replace the center guide tube with one that fits a 3.4 mm drill bit for drilling.
- Ensure that an assistant is present to hold the frame in place while the surgeon drills with a manual drill to add additional stability to the frame.
- Let the surgeon drill with a manual twist drill to create a 3.4 mm diameter burr hole.
- Set up the frame for the second drill insertion to widen the burr hole and avoid bony collisions that may alter the trajectory.
- Set up the drill with the 4.5 mm drill bit; replace the center guide tube with one that fits this bigger drill bit.
- Create a 4.5 mm burr hole.
- Perform an MRI scan to ensure the targeting cannula has returned to the planned trajectory, as drilling through the frame can sometimes shift the cannula.
- Pierce the dura with a sharp stylet.
- Insert the pre-primed frame-compatible infusion cannula. Ensure the cannula has a consistent neutral or positive back pressure to limit the introduction of air bubbles.
NOTE: The software provides a specified depth to the planned target. - Measure the depth on the stereotactic frame-compatible infusion cannula and use the cannula-associated depth stop. This depth stop ensures that the cannula reaches the desired location and does not go beyond it. There is also a lock and dock assembly with an additional screw to ensure the cannula stays at the desired depth.
5. Monitoring the infusion with repeated MRI scans
- Perform an MRI scan to assess the insertion of the cannula to the correct target location in the brain.
- Start the infusion of the desired agent as a co-infusion with a gadolinium-based contrast agent.
NOTE: In this experiment, a 1 mM concentration of gadolinium-based contrast agent was used, but this may need to be adjusted based on the application. A total of 300 µL of infusion volume was administered at a rate of 10 µL/ min, although this may be varied as well. - Perform an MRI scan at regular time intervals to monitor the infusion and volume of distribution of the cannula-inserted agent in the brain, which can be inferred due to the co-infusion of gadolinium.
NOTE: A hyperintense area around the cannula tip indicates the presence of the gadolinium-based contrast agent. - Once infusion ends, stop the pump.
NOTE: The infusion rate used in this study was 30 µL/ min, until the 300 µL volume of the cell suspension was completely infused. - Let the cannula stay in the brain for 5 min following termination of the infusion prior to removing the cannula.
NOTE: The infusion cannula is typically left in place for 5 min following the termination of the infusion to reduce backflow21,24. - Remove the cannula manually through the frame.
- Remove the frame from the head by disassembling it in reverse order from how it was built.
- Close the incision with a running 3-0 or 4-0 monocryl suture.
- Turn off the isoflurane to prepare for recovery.
- Extubate the subject and allow the subject to recover under observation by the veterinary team.
Representative Results
The pig position in the MRI scanner provides optimal access for the surgeon to operate and clearance for the stereotactic frame and infusion cannula (Figure 1). The subject's torso was raised with towels and foam pads. This enabled the head to fall slightly downward at the end of the MR bore, and therefore ensured that the stereotactic frame and infusion cannula insertion location were optimally accessible for the surgeon.
The MRI-guided visualization allows for precise planning and insertion of a cannula to the brain (Figure 2). The MR-guidance software provides the insertion point to achieve the desired trajectory.
The stereotactic frame was scanned in the software, and it was adjusted to effectively reach the desired location (Figure 3). In this demonstration, a location in the frontal cortex was chosen. Once the frame was set, the software was used to estimate the thickness of the pig skull, the distance to the desired location from the frame base, and the frame parameter adjustments to reach the desired location. In this case, for the location and insertion angle selected, the thickness of the skull that the cannula would traverse was 4.7 mm, and 4.4 mm from the interior surface of the skull to the surface of the brain (Figure 3A).
Finally, iterative interoperative MRI scans after the cannula infusion showed how the infusion was delivered to the brain tissue (Figure 4). These scans also provided a comparison of the cannula projection (blue rectangle) and projected cannula trajectory (yellow rectangle), which show the effectiveness of this technique in reaching the desired location. MR scans were taken at regular intervals of 4-6 min and finalized with 10 and 30 min scans. The gadolinium-enhanced infusion was tractable in these scans, which provided a real-time visualization of the volume of distribution of the agent.
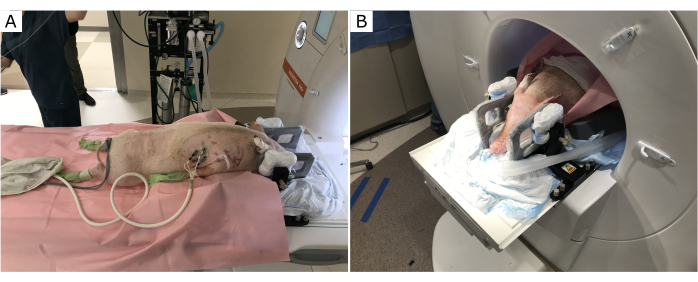
Figure 1: Subject position on the MRI table. The torso is raised, the neck flexed, and the head inclined downward. (A) Before entering the MR bore. (B) Subject positioned through the MR bore for optimal access to the top of the skull. Please click here to view a larger version of this figure.
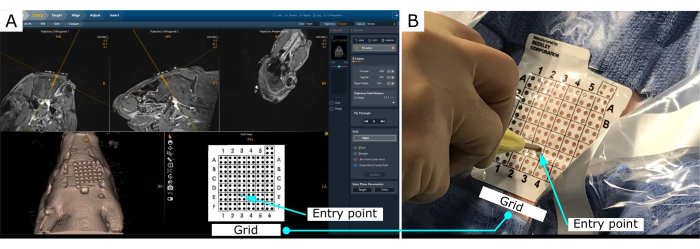
Figure 2: MR-guided stereotaxy visualization. (A) Visualization of the planned trajectory. The software outputs the entry point location in the grid, placed on the scalp. (B) Entry point location on the scalp. Please click here to view a larger version of this figure.
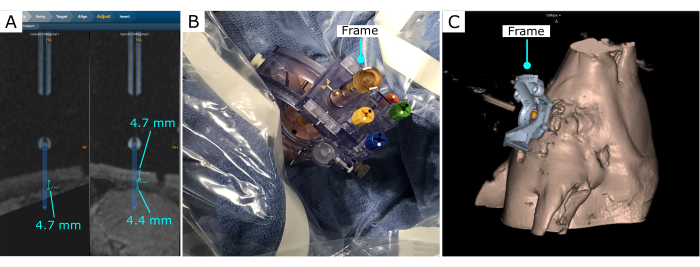
Figure 3: Intervention trajectory after the frame is secured on the skull. (A) Measurements of bone depth and distance to the brain. (B) Stereotactic frame on the skull, with a burr hole created with a hand drill. (C) Stereotactic frame and 3D reconstruction projection on the software. Please click here to view a larger version of this figure.
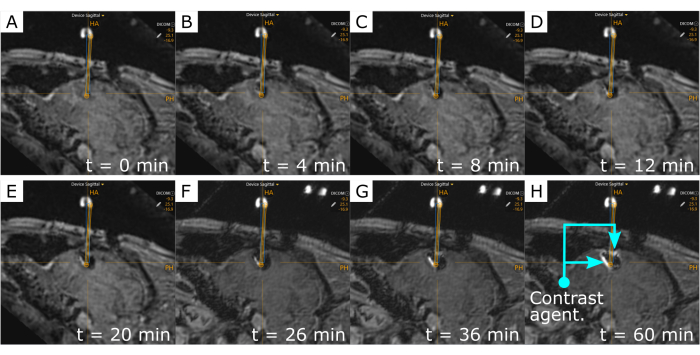
Figure 4: Time-lapse of the gadolinium-enhanced infusion agent. The hyperintense area around the cannula tip indicates the presence of gadolinium. Repetitive MR scans were acquired across time to track the volume of distribution of the agent during infusion: (A) t = 0, (B) t = 4 min, (C) t = 8 min, (D) t = 12 min, (E) t = 20 min, (F) t = 26 min; and after the infusion ended: (G) t = 36 min, and (H) t = 60 min. Visualization of the co-infused agent occurs after 4 mins. The blue rectangle is the measured-cannula placement, while the yellow rectangle shows the projected cannula trajectory. Please click here to view a larger version of this figure.
Discussion
This protocol presents the performance of MR-guided stereotaxy to the pig brain inside a 3T MR machine with the possibility of sub-millimeter targeting accuracy, as achieved in previous studies1,4,17,18,25. Previous cadaver experiments with MR-guided stereotaxy showed a radial error of 0.2 ± 0.1 mm1. In this report, the final depth error with respect to the planned trajectory was 1.4 mm due to online evaluation and adjustment of the trajectory by the surgeons. The final depth error was comparable to radial error findings (under 2 mm) for clinical implementations of iMRI stereotactic procedures in humans26.
Here, we demonstrate the placement of the subject on the MRI table, with its trunk lifted such that the head can fall slightly downward and point outward toward the end of the MR bore. This head placement is critical to provide the surgeon with space to perform the procedure. The stereotactic frame allows for precise and controlled infusion into pig brain models. Additionally, the real-time MR imaging allows for accurate determination of the volume of distribution. Pigs, as large animal models for infusions tracked in real time in MRI, present the possibility of the study of drug delivery to the brain, cell delivery, and other agents of translational value.
The pig has distinct anatomical differences to consider, compared to humans or non-human primates. As pigs grow, the size of the body in the MR bore becomes a challenge. The shape of the head and torso are different from humans, which proves challenging to accommodate for optimal access to the brain for the surgeon, both for the surgical procedure and cannula insertion in the space outside the MR bore. Therefore, it is critical to position the subject in a way that the surgeon has access to the head from the end of the MR bore.
The difference in skull thickness between pigs and humans is a factor to consider. In this protocol, the iMRI visualization allowed for precise estimation of the skull thickness for an efficient burr hole procedure. Given the use of these minimally invasive neurosurgical tools, animal recovery was uneventful.
The MR-guided visualization provides real-time guidance for access to the pig brain, cannula insertion, and monitoring of the infusion agent. The drilling process, tissue deformation, and/or disruption of white matter tracts have been reported to contribute to difficulties in agent delivery to the brain25. Iterative MR scans during the planning and cannula insertion provide the capability for small adjustments. Additionally, infusion parameters such as the rate of infusion or accuracy of the cannula insertion could be changed in real-time or paused, as dictated by the intra-procedural imaging. Finally, an appropriate balance of the gadolinium-based co-infusate must be selected, to obtain a clear evaluation of the volume of distribution of the agent.
The over-concentration of the gadolinium-based contrast agent may have obscured its distribution in the MRI scans27, showing a black spot around the cannula tip, surrounded by a hyperintense area that showed the outer limits of the infusion volume. Available footage of the procedure is limited due to the constraints associated with filming in the limited MRI space around the surgeon's work area. The intraoperative video footage was used to guide the protocol description.
Infusion agents via MR-guided stereotaxy in pigs and other large animal models has resulted in accurate, predictable, and safe procedures. Demonstrating iMRI stereotaxy in pigs provides the basis for the scalability of research treatments that high hold translational value to humans. Pig models have been widely used to study immunological responses due to their similarity to the human response compared to other species28. Therapeutic agents delivered to the brain can be studied in the context of precise target infusion, with the added benefit of real-time MRI visualization of the infusion location, necessary adjustments, and intra-operative evaluation of its distribution in the tissue.
Acknowledgements
The authors declare that this study received philanthropic funding from the John S. "Steve" Dunn, Jr. & Dagmar Dunn Pickens Gipe Chair in Brain Tumor Research at Houston Methodist. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
This work was funded in part by grant number RP190587 from the Cancer Prevention and Research Initiative (CPRIT) and the Houston Methodist Foundation.
The authors thank Vi Phan and Lien My Phan, from the Translational Imaging Center at Houston Methodist Research Institute, for their assistance with MR imaging.
The authors declare that this study received philanthropic funding from Paula and Rusty Walter and Walter Oil & Gas Corp Endowment at Houston Methodist. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Materials
| Name | Company | Catalog Number | Comments |
| 3 Tesla Siemens MAGNETOM Vida | Siemens Healthineers | 70 cm wide-bore 3 Tesla whole body MRI scanner | |
| Four channel flex coil | Siemens Healthineers | Placed ventrally to allow access to the skull | |
| MR Neuro Patient Drape | ClearPoint Neuro, Inc | NGS-PD-05 | MR Neuro Patient Drape, Marker Pen, Track Ball Cover, Cable Cover |
| MR Neuro Procedure Drape Tapered - Long | ClearPoint Neuro, Inc | NGS-PD-02-L | MR Neuro Procedure Drape Tapered, Marker Pen, Track Ball Cover |
| MR Neuro Procedure Drape Tapered w/Extension - Long | ClearPoint Neuro, Inc | NGS-PD-03-L | MR Neuro Procedure Drape Tapered w/Extension, Marker Pen, Track Ball Cover |
| MR Neuro Scanner Bore Drape w/Extension | ClearPoint Neuro, Inc | NGS-PD-04 | MR Neuro Scanner Bore Drape w/Extension |
| Scalp Mount Base | ClearPoint Neuro, Inc | NGS-SM-01 | Scalp Mount Base and centering too |
| Skull Mount Base | ClearPoint Neuro, Inc | NGS-SK-01 | Skull Mount Base |
| SMARTFrame Accessory Kit | ClearPoint Neuro, Inc | NGS -AK-01-11 | Stylet, Lancet, Peel-Away Sheath (2), Ruler, Depth Stop (2) |
| SMARTFrame Guide Tubes | ClearPoint Neuro, Inc | NGS-GT-01 | 15 GA Guide Tube, 18 GA Guide Tube and 16GA Guide Tube |
| SMARTFrame Guide Tubes .052” / 18 ga | ClearPoint Neuro, Inc | NGS-GT-02 | .052” Guide Tubes that fit 18 ga devices (5) |
| SMARTFrame Guide Tubes .060” / 17 ga | ClearPoint Neuro, Inc | NGS-GT-03 | .060” Guide Tubes that fit 17 ga devices (5) |
| SMARTFrame Guide Tubes .064” / CP Stylet | ClearPoint Neuro, Inc | NGS-GT-04 | .064” Guide Tubes that fit ClearPoint Stylets (5) |
| SMARTFrame Guide Tubes .068” / 16 ga | ClearPoint Neuro, Inc | NGS-GT-05 | .068” Guide Tubes that fit 16 ga devices (5) |
| SMARTFrame Guide Tubes .074” / 15 ga | ClearPoint Neuro, Inc | NGS-GT-06 | .074” Guide Tubes that fit 15 ga devices (5) |
| SMARTFrame MR Fiducial | ClearPoint Neuro, Inc | NGS-BM-05 | MR Fiducials (5) |
| SMARTFrame Scalp Mount Rescue Screw – Long | ClearPoint Neuro, Inc | NGS-RS-02 | Short Scalp Mount Rescue Bone Screws (3) |
| SMARTFrame Scalp Mount Rescue Screw – Short | ClearPoint Neuro, Inc | NGS-RS-03 | Long Scalp Mount Rescue Bone Screws (3) |
| SMARTFrame Skull Mount Rescue Screw | ClearPoint Neuro, Inc | NGS-RS-01 | Skull Mount Rescue Bone Screws (3) |
| SMARTFrame Thumb Wheel Extension Set. | ClearPoint Neuro, Inc | NGS -TE-01 | Light Hand Controller |
| SmartFrame XG Device Guide, 2.5 mm | ClearPoint Neuro, Inc | NGS-XG-03 | 2.5-mm Device Guide |
| SmartFrame XG Device Guide, 3.2 mm | ClearPoint Neuro, Inc | NGS-XG-04 | 3.2-mm Device Guide |
| SMARTFrame XG Drill Guide, 4.5 mm | ClearPoint Neuro, Inc | NGS-XG-02 | 4.5-mm Drill Guide |
| SMARTFrame XG Drill Guide, 6.0 mm | ClearPoint Neuro, Inc | NGS-XG-05 | 6.0-mm Drill Guide |
| SMARTFrame XG Exchangeable Device Guides | ClearPoint Neuro, Inc | NGS-XG-01 | Device Guide, 3.4-mm, Device Guide, 14 GA |
| SMARTFrame XG MRI-Guided Trajectory Frame | ClearPoint Neuro, Inc | NGS-SF-02-11 | Stereotactic Frame, Skull Mount Base, Centering Ring, Dock, Standard Device Lock, Large Device Lock, Screwdriver, Roll Lock Screw w/washer |
| SMARTFrame XG MRI-Guided Trajectory Frame, 5 Fr | ClearPoint Neuro, Inc | NGS-SF-02-11-5 | Stereotactic Frame, Centering Ring, Dock, 5 Fr Device Lock, Large Device Lock, Screwdriver, Roll Lock Screw w/washer |
| SMARTFrame XG MRI-Guided Trajectory Frame, 7 Fr | ClearPoint Neuro, Inc | NGS-SF-02-11-7 | Stereotactic Frame, Centering Ring, Dock, 7 Fr Device Lock, Large Device Lock, Screwdriver, Roll Lock Screw w/washer |
| SMARTGrid MR Planning Grid | ClearPoint Neuro, Inc | NGS -SG-01-11 | Marking Grid and Marking Tool |
| SMARTTip MR Drill Kit, 4.5-mm | ClearPoint Neuro, Inc | NGS-DB-45 | 4.5-mm Drill Bit, 3.2-mm Drill Bit, Lancet, Depth Stop, Ruler |
| SMARTTwist MR Hand Drill | ClearPoint Neuro, Inc | NGS-HD-01 | Hand Drill |
| VentiPAC | SurgiVet | V727000 | Mechanical ventilator |
| Wharen Centering Guide | ClearPoint Neuro, Inc | NGS-CG-01 | Wharen Centering Guide |
References
- Larson, P. S., et al. An optimized system for interventional magnetic resonance imaging-guided stereotactic surgery: preliminary evaluation of targeting accuracy. Neurosurgery. 70, 95-103 (2012).
- Foltynie, T., et al. MRI-guided STN DBS in Parkinson's disease without microelectrode recording: efficacy and safety. Journal of Neurology, Neurosurgery and Psychiatry. 82 (4), 358-363 (2011).
- Sidiropoulos, C., et al. Intraoperative MRI for deep brain stimulation lead placement in Parkinson's disease: 1 year motor and neuropsychological outcomes. Journal of Neurology. 263 (6), 1226-1231 (2016).
- Ostrem, J. L., et al. Clinical outcomes using ClearPoint interventional MRI for deep brain stimulation lead placement in Parkinson's disease. Journal of Neurosurgery. 124 (4), 908-916 (2016).
- Lee, P. S., et al. Outcomes of interventional-MRI versus microelectrode recording-guided subthalamic deep brain stimulation. Frontiers in Neurology. 9, 241 (2018).
- Patel, N. K., Plaha, P., Gill, S. S. Magnetic resonance imaging-directed method for functional neurosurgery using implantable guide tubes. Operative Neurosurgery. 61 (5), 358-366 (2007).
- Drane, D. L., et al. Better object recognition and naming outcome with MRI-guided stereotactic laser amygdalohippocampotomy for temporal lobe epilepsy. Epilepsia. 56 (1), 101-113 (2015).
- Chittiboina, P., Heiss, J. D., Lonser, R. R. Accuracy of direct magnetic resonance imaging-guided placement of drug infusion cannulae. Journal of Neurosurgery. 122 (5), 1173-1179 (2015).
- Han, S. J., Bankiewicz, K., Butowski, N. A., Larson, P. S., Aghi, M. K. Interventional MRI-guided catheter placement and real time drug delivery to the central nervous system. Expert Review of Neurotherapeutics. 16 (6), 635-639 (2016).
- Bobo, R. H., et al. Convection-enhanced delivery of macromolecules in the brain. Proceedings of the National Academy of Sciences. 91 (6), 2076-2080 (1994).
- Mittermeyer, G., et al. Long-term evaluation of a phase 1 study of AADC gene therapy for Parkinson's disease. Human Gene Therapy. 23 (4), 377-381 (2012).
- Lonser, R. R., Sarntinoranont, M., Morrison, P. F., Oldfield, E. H. Convection-enhanced delivery to the central nervous system. Journal of Neurosurgery. 122 (3), 697-706 (2015).
- Subramanian, T., Deogaonkar, M., Brummer, M., Bakay, R. MRI guidance improves accuracy of stereotaxic targeting for cell transplantation in parkinsonian monkeys. Experimental Neurology. 193 (1), 172-180 (2005).
- Emborg, M. E., et al. Intraoperative intracerebral MRI-guided navigation for accurate targeting in nonhuman primates. Cell Transplantation. 19 (12), 1587-1597 (2010).
- Silvestrini, M. T., et al. Interventional magnetic resonance imaging-guided cell transplantation into the brain with radially branched deployment. Molecular Therapy. 23 (1), 119-129 (2015).
- Faraji, A. H., Rajendran, S., Jaquins-Gerstl, A. S., Hayes, H. J., Richardson, R. M. Convection-enhanced delivery and principles of extracellular transport in the brain. World Neurosurgery. 151, 163-171 (2021).
- Richardson, R. M., et al. T2 imaging in monitoring of intraparenchymal real-time convection-enhanced delivery. Neurosurgery. 69 (1), 154-163 (2011).
- Richardson, R. M., et al. Novel platform for MRI-guided convection-enhanced delivery of therapeutics: preclinical validation in nonhuman primate brain. Stereotactic and Functional Neurosurgery. 89 (3), 141-151 (2011).
- San Sebastian, W., et al. Safety and tolerability of magnetic resonance imaging-guided convection-enhanced delivery of AAV2-hAADC with a novel delivery platform in nonhuman primate striatum. Human Gene Therapy. 23 (2), 210-217 (2012).
- Sauleau, P., Lapouble, E., Val-Laillet, D., Malbert, C. -. H. The pig model in brain imaging and neurosurgery. Animal. 3 (8), 1138-1151 (2009).
- Yin, D., Forsayeth, J., Bankiewicz, K. S. Optimized cannula design and placement for convection-enhanced delivery in rat striatum. Journal of Neuroscience Methods. 187 (1), 46-51 (2010).
- Larjavaara, S., et al. Incidence of gliomas by anatomic location. Neuro-Oncology. 9 (3), 319-325 (2007).
- Pallud, J., Devaux, B., Daumas-Duport, C., Oppenheim, C., Roux, F. X. Glioma dissemination along the corticospinal tract. Journal of Neuro-Oncology. 73 (3), 239-240 (2005).
- White, E., Bienemann, A., Megraw, L., Bunnun, C., Gill, S. Evaluation and optimization of the administration of a selectively replicating herpes simplex viral vector to the brain by convection-enhanced delivery. Cancer Gene Therapy. 18 (5), 358-369 (2011).
- Chen, M. Y., Lonser, R. R., Morrison, P. F., Governale, L. S., Oldfield, E. H. Variables affecting convection-enhanced delivery to the striatum: a systematic examination of rate of infusion, cannula size, infusate concentration, and tissue-cannula sealing time. Journal of Neurosurgery. 90 (2), 315-320 (1999).
- Sterk, B., et al. Initial clinical experience with ClearPoint smartframe array-aided stereotactic procedures. World Neurosurgery. 162, 120-130 (2022).
- Rohrer, M., Bauer, H., Mintorovitch, J., Requardt, M., Weinmann, H. -. J. Comparison of magnetic properties of MRI contrast media solutions at different magnetic field strengths. Investigative Radiology. 40 (11), 715-724 (2005).
- Dawson, H. D. A comparative assessment of the pig, mouse and human genomes. The Minipig in Biomedical Research. 1, 323-342 (2011).
This article has been published
Video Coming Soon
ABOUT JoVE
Copyright © 2024 MyJoVE Corporation. All rights reserved