Using a Combination of Indirect Calorimetry, Infrared Thermography, and Blood Glucose Levels to Measure Brown Adipose Tissue Thermogenesis in Humans
In This Article
Summary
Here, we present a protocol to quantify the physiological significance of the impact of brown adipose tissue (BAT) activity on human metabolism. This is achieved by combining carbohydrate loading and indirect calorimetry with measurements of supraclavicular changes in temperature. This novel approach can help develop a pharmacological target for BAT thermogenesis in humans.
Abstract
In mammals, brown adipose tissue (BAT) is activated rapidly in response to cold in order to maintain body temperature. Although BAT has been studied greatly in small animals, it is difficult to measure the activity of BAT in humans. Therefore, little is known about the heat-generating capacity and physiological significance of BAT in humans, including the degree to which components of the diet can activate BAT. This is due to the limitations in the currently most used method to assess the activation of BAT-radiolabeled glucose (fluorodeoxyglucose or 18FDG) measured by positron emission tomography-computerized tomography (PET-CT).
This method is usually performed in fasted subjects, as feeding induces glucose uptake by the muscles, which can mask the glucose uptake into the BAT. This paper describes a detailed protocol for quantifying total-body human energy expenditure and substrate utilization from BAT thermogenesis by combining indirect calorimetry, infrared thermography, and blood glucose monitoring in carbohydrate-loaded adult males. To characterize the physiological significance of BAT, measures of the impact of BAT activity on human health are critical. We demonstrate a protocol to achieve this by combining carbohydrate loading and indirect calorimetry with measurements of supraclavicular changes in temperature. This novel approach will help to understand the physiology and pharmacology of BAT thermogenesis in humans.
Introduction
Brown adipose tissue (BAT) most notably differs from white adipose tissue (WAT) in its mitochondrial content, sympathetic innervation, multilocular lipid droplets, heat-generating ability, and anatomical distribution. BAT was considered to exist only in infants and small mammals until the confirmation of its presence in human adults in 20091,2,3. Thus, until relatively recently, the role of BAT in human physiology and metabolic homeostasis has been poorly understood. Extensive studies in small animals have shown that during cold exposure, more than half of metabolism is due to the non-shivering thermogenic ability of BAT4. Several studies have demonstrated that upon mild cold exposure (17-18 °C), increases in energy expenditure and glucose uptake into the BAT correlate strongly with BAT thermogenesis in humans5,6,7. Furthermore, BAT thermogenesis can contribute up to 10% of resting energy expenditure in humans during cold exposure (for a review, see Van Schaik et al.8). Studying the physiology and impact of BAT on human health and disease is currently restricted by protocol limitations. It is, therefore, essential to have an accurate method for measuring the true metabolic impact of BAT to better understand the impact of BAT thermogenesis on obesity and its metabolic complications in humans.
The anatomical distribution of human BAT makes obtaining accurate measurements of the BAT challenging. Within humans, the BAT is distributed inside the depots of WAT in the abdomen, thorax, and, most notably, the neck9. Autopsy and cadaveric studies have been used to characterize the BAT anatomically10,11, but these methods cannot provide functional information. It is challenging to distinguish the BAT using conventional imaging techniques because of the similar densities of WAT and BAT8. An additional confounding issue is that beige fat depots are also located within the same narrow layers of fascia or in certain depots with the WAT8, which makes it challenging to distinguish using conventional imaging techniques.
To overcome this issue, the BAT volume is typically measured through combining positron emission tomography (PET) and computed tomography (CT). The radiolabeled glucose analog 18F-fluourodeoxyglucose (18F-FDG) is the most common tracer used for studying BAT12. However, it suffers several limitations, such as exposing subjects to ionizing radiation and being invasive and expensive. Additionally, the greatest limitation of the 18F-FDG tracer is that it measures the uptake of a glucose analog, which is not ideal given that free fatty acids are the preferred substrates for BAT thermogenesis13. The 18F-FDG PET/CT technique does not measure the uptake of free fatty acids as a substrate for thermogenesis and, therefore, does not measure the physiological importance of BAT thermogenesis. There are alternative techniques used to assess human BAT, which include the measurement of the uptake of oxygen-15 labeled water (15O-O2) 14, 11C-acetate15, a long chain fatty acid (18F-fluoro-6-thia-heptadecanoic acid)16, or adenosine17, as well as magnetic resonance spectroscopy18 and magnetic resonance imaging 19, but these are still extremely expensive and expose subjects to ionizing radiation. Therefore, a reliable, inexpensive, and importantly, safe gold standard for the quantification of human BAT is lacking.
Infrared thermography (IRT) is an alternative non-invasive imaging technique20,21 that measures the skin temperature overlaying a known BAT depot. While this infers increased energy expenditure, if the measured temperature does not exceed the core temperature, then it cannot be determined whether the measured change in temperature is simply a consequence of altered blood flow. Further, a measured increase in local temperature does not provide values of altered energy expenditure, which is frequently the desired endpoint. A number of research groups have used IRT to measure an increase in temperature in depots of human BAT following a caffeine intervention or cold stimulus; this depot is the supraclavicular fossa22,23,24,25,26,27.
However, it is not clear whether the action of caffeine on BAT is direct or mediated via neural circuitry. There is evidence that caffeine induces browning features in adipocytes in vitro22, and previous work has demonstrated that caffeine (100 mg) increases heart rate variability, which may be an indicator of an increase in sympathetic nerve drive systemically in the body27. This is in line with evidence in rodents, in which caffeine via the central nervous system increases thermogenesis without an adverse cardio-dynamic impact28.
As the preferred substrate for BAT thermogenesis is free fatty acids derived from triglycerides13, and active BAT sequesters circulating lipids to sustain thermogenesis29, measures of substrate utilization are important in assessing the physiological activation of BAT. The respiratory exchange ratio (RER) is the ratio of the volume of oxygen consumed (V̇O2) and carbon dioxide produced (V̇CO2)30. An RER of 0.7 is indicative of fatty acid metabolism, and an RER of 1.0 is indicative of carbohydrate metabolism31. Therefore, evidence of a preference for fatty acid utilization over and above an increase in energy expenditure is a key correlate of BAT thermogenesis.
Additionally, given that the uptake of glucose is a known correlate of BAT activity (see above), a fall in blood glucose in parallel with the change in substrate utilization are key correlates of BAT thermogenesis. Previous studies utilizing indirect calorimetry alone, or together with temperature recording in fasted individuals, have reported little to no acute change in substrate utilization32,33. As this is likely masked by the fasted state (where preabsorptive metabolism favors fat utilization), we propose combining IRT and indirect calorimetry with carbohydrate loading.
This article aims to provide a step-by-step approach that clinical researchers can use to reliably and, importantly, safely quantify the physiological importance of BAT in humans by combining IRT, indirect calorimetry, and blood glucose levels. This technique is best used after subjects have been carbohydrate-loaded and exposed to either pharmacological BAT agents or environmental stimuli. The results of this approach can be used to study BAT activity, substrate utilization, and energy expenditure following activation of the BAT in individual study subjects27.
Protocol
All participants (n = 8) provided written informed consent, and all experiments were approved by the University Human Ethics Committee; data were derived from Van Schaik et al.27.
1. Equipment and software installation
- Measure the fat mass via dual-energy X-ray absorptiometry (DXA) as per Van Schaik et al.27.
- Estimate the substrate utilization and energy expenditure from expired gas; measure this using a respiratory gas analyzer as per the manufacturer's guidelines.
- Collect blood samples via finger (capillary) puncture, and determine the blood glucose levels using a glucometer as per the manufacturer's guidelines.
- Use a non-contact infrared thermometer to determine core body temperature measurements as per the manufacturer's guidelines (the error of this device is ±0.2 °C).
2. Procedures prior to the participant visits
- Screen all the participants for their health status.
- Set the following exclusion criteria: a body mass index of >30 kg/m2 (due to BAT activity being inversely correlated with adiposity34,35, participants using prescribed medications, and diabetes mellitus.
- Before or after the testing session, ensure that participants undergo a DXA scan to measure their fat mass, as BAT activity is inversely correlated with adiposity34,35.
- For 24 h prior to arriving for the study, ensure that the participants abstain from any strenuous exercise or activity and are water-fasted for 10 h before arriving in the lab.
3. Procedures on the study day
- Ensure that the room temperature at which the data are collected is set to a constant temperature to minimize external confounds due to differences in room temperature.
NOTE: This can result in incorrect thermal or metabolic measurements. For the purposes of this experiment, a temperature-controlled room maintained at 22 °C under thermal neutral conditions was used. - Ask the participants to arrive at the lab at 08:00 a.m. to account for daily hormone rhythms.
- Measure the participants' height and weight.
- Ask the participants to lie on a plinth for a minimum of 30 min before the baseline measurements are taken.
- Over a 120 min period, measure the participants' IRT, indirect calorimetry, blood glucose, and core temperature every 15 min following the expired O2and CO2sampling (Figure 1).
- Following the baseline measurements, ensure that the participants are carbohydrate-loaded through the consumption of three carbohydrate gels (90 g glucose each) between the time points of 0 min and 15 min.
- Ensure that the participants ingest the treatment 45 min following the carbohydrate load. To follow this protocol, use 100 mg of caffeine capsules as the intervention27.
NOTE: A washout period of 7 days between intervention and placebo is required, meaning a period of 7 days is required between caffeine and placebo treatment.
4. Indirect calorimetry
- Estimate the energy expenditure and substrate utilization values from the expired gas, as measured using a respiratory gas analyzer. Complete the calibration of the respiratory gas analyzer following the manufacturer's instructions.
- Fit the cold-sterilized silicone mask to the participant to allow delivery of room air and the acquisition of metabolic data. Ensure that the mask is equipped with a presterilized non-rebreathing valve (two-way non-rebreathing valve) and fix it on the participant's face with a mesh attachment and check for leaks.
- Ensure that the inspiratory and expiratory tubes are connected.
- Export the digital data file in a spreadsheet format.
- Sample the expired O2 and CO2with 5 s averaging. This measures the energy expenditure and respiratory exchange ratio (Figure 1). Remove the face mask to complete the additional measures.
- Calculate the substrate oxidation rates (carbohydrate and lipid oxidation) and total energy expenditure using the non-protein Weir equations 1-331,36:
Fat oxidation rate (g/min−1) = (1.695 VO2)-(1.701 VCO2) (1)
Carbohydrate oxidation rate (g/min−1) = (4.585 VCO2) -(3.226 VO2) (2)
Energy Expenditure (kcal/min) = (3.94 × VO2)+ (1.1 × VCO2) (3)
5. Plasma blood glucose measurements
- Conduct blood glucose readings via finger prick and a glucometer following each round of expired gas measurements (Figure 2).
6. Core temperature
- Record the core temperature (Tcore) following each round of expired gas measurements. Ideally, measure the core temperature either rectally or intra-aurally (Figure 2).
NOTE: Owing to COVID-19 safe practices, minimize person-to-person contact. - Ensure that the participants are supine and their head is in a neutral position. Consistently direct the non-contact thermometer toward the center of the participant's forehead.
7. Infrared thermography
- Conduct the IRT following each round of expired gas measurements (Figure 2).
- Ask the participants to sit up in an upright posture looking straight ahead, with the chest area to neck region exposed (Figure 3).
- Use a thermal imaging camera to acquire infrared images of the anterior neck and upper chest region.
- Position the camera on a tripod at the level of the neck 1 m from the subject's face (Figure 4D). Use the following settings: detector type = uncooled microbolometer; detector pitch = 17 µm; camera spectral range = 7.5-14.0 µm; thermal sensitivity = 20 mK at 30 °C; lenses = 36 mm; resolution = 1,024 pixels x 768 pixels.
- Turn the camera on.
- Adjust the focus of the camera by rotating the focus ring.
NOTE: It is very important to adjust the focus correctly. Incorrect focus adjustment affects the temperature measurement. - Point the laser pointer to the midline of the neck of the participant.
- Take the image.
NOTE: The image will save automatically if a memory card is used.
8. Image analysis
- Choose three regions of the anterior thorax and neck for the analysis of the surface temperature: bilaterally the skin overlying BAT in the supraclavicular fossa (SCF) and the lateral region of the neck, with the sternal area considered as a control reference point (Tref), as this area does not contain BAT (Figure 4A-C).
- Place triangular regions of interest (ROIs) in the left and right SCF areas and a circular ROI over the sternal region.
- When the required regions have been cross-located, confirm that the software displays the average and standard deviation of the temperature for each selected region.
9. Data analysis
- Use a double-blind approach for the analysis of the interventions using the techniques described. Have a researcher not involved in the data collection or analysis code the interventions generically.
- Perform the statistical analysis.
- Calculate averages for the IRT, core temperature, and blood glucose data from the measured single time point.
- Calculate averages for the RER, fat oxidation, carbohydrate oxidation, and energy expenditure in 10 min epochs.
- For energy expenditure, sum the rate of energy expenditure for each group, and separate it into pre- and post intervention.
NOTE: Refer to Van Shaik et al. for statistical tests to analyze the data27.
Results
Figure 1 and Figure 2 present a flowchart of the study design. Images of the protocol setup are represented in Figure 3. The participant characteristics can be found in Table 1. Representative examples of IRT of the images of a participant, including baseline (Figure 4A), post carbohydrate load (Figure 4B), and 60 min following caffeine supplementation (Figure 4C), with a representative image of the camera setup, are presented in Figure 4D. Notably, Figure 4A-C provides a visual representation of the changes in supraclavicular fossa temperature (Tscf) following the intervention; the differences in temperature are particularly marked between Figure 4B and Figure 4C.
In Figure 5A-C, results from Van Schaik et al. show the Tscf (Figure 5A), the temperature of a reference point (Tref; Figure 5C), and the core temperature (Tcore; Figure 5B) from baseline (0 min) to completion of the data collection (120 min). The data show a caffeine intervention compared to placebo27. The results described in this manuscript are purely representative of this published paper. Additionally, the data on Tscf do not show a group effect. The statistics can be found in the supplemental data of Van Schaik et al.27.
The marked increase in supraclavicular temperature coincides with changes in substrate utilization and rapid lowering of the blood glucose levels following the intervention, as shown in Figure 6. These results, combined with the lack of change in temperature for the Tref and Tcore temperatures (Figure 5B,C) are indicative of BAT thermogenesis. Additionally, as the energy expenditure increases (Figure 6E), RER decreases (Figure 6A), which coincides with fat oxidation increasing (Figure 6B) following the intervention.
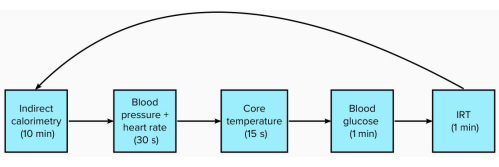
Figure 1: Schematic of measures with time to complete in each 15 min period. Please click here to view a larger version of this figure.
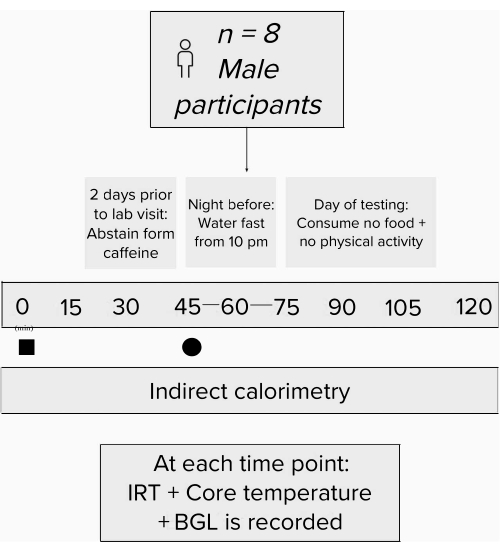
Figure 2: Flow chart schematic of the study design. Experimental process. Black square = time of carbohydrate load; black circle = time of intervention. Abbreviations: IRT = infrared thermography; BGL = blood glucose levels. Please click here to view a larger version of this figure.
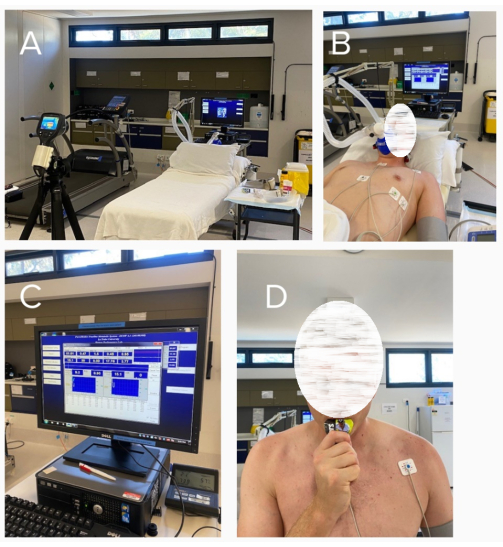
Figure 3: Representative images of the protocol. (A) Setup without the participant present; (B) data collection of the participants at baseline; (C) indirect calorimetry computer; (D) participant consuming the carbohydrate load post baseline measures. Please click here to view a larger version of this figure.
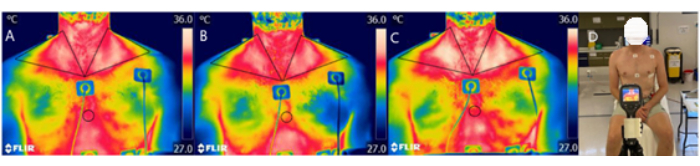
Figure 4: Representative examples of the IRT and camera setup. Thermal images from a participant, at (A) baseline, (B) post carbohydrate load, and (C) 60 min following the intervention of caffeine, with (D) a representative image of the camera setup. Abbreviation: IRT = infrared thermography. Please click here to view a larger version of this figure.
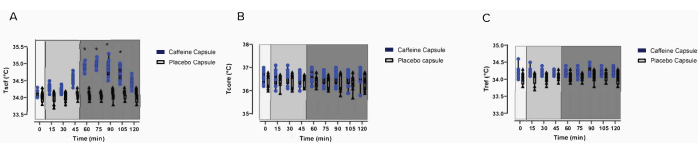
Figure 5: Effects of the intervention on the temperature measures. Baseline raw temperature changes of (A) Tscf, (B) Tcore, and (C) Tref in participants following a carbohydrate load (timepoint = 0) and the administration of a caffeine intervention or a placebo capsule (time = 45 min to 120 min)27. This figure is modified from Van Schaik et al.27. (A-C) Light grey box 1 = time of carbohydrate load; box 2 = pre-intervention; dark grey box 3 = post-intervention; blue circles = caffeine intervention; black triangles = placebo intervention. The data are expressed as the minimum to maximum, with all points shown in the box and whisker plots. The variance is expressed as mean ± SD, n = 8 per intervention; * represents the caffeine interaction effect (*p < 0.05). The data values were analyzed using a repeated-measures three-way analysis of variance. Abbreviations: Tscf = temperature in the supraclavicular fossa; Tcore = core temperature; Tref = control reference point. Please click here to view a larger version of this figure.
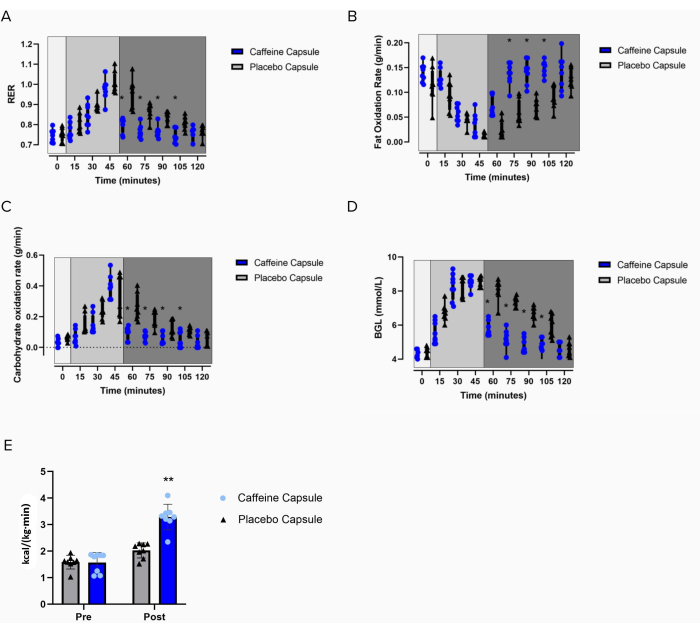
Figure 6: Effects of the intervention on metabolic measures. Changes in (A) RER, (B) the fat oxidation rate, (C) the carbohydrate oxidation rate, (D) blood glucose levels, and (E) energy expenditure in participants following a carbohydrate load (time = 0) and the administration of a caffeine capsule or a placebo capsule (time = 45 min to 120 min). Light grey box 1 = time of carbohydrate load; box 2= pre-intervention; dark grey box 3 = post-intervention; blue circles = caffeine intervention; black triangles = placebo intervention. The data are expressed as the minimum to maximum, with all points shown in the box and whisker plots. (E) Pre- and post-administration of the interventions; grey bar = placebo intervention; blue bar= caffeine intervention. The variance is expressed as mean ± SD, n = 8 per intervention; * represents the caffeine interaction effect (*p < 0.05). The data values were analyzed using a repeated-measures three-way analysis of variance. Please click here to view a larger version of this figure.
| All participants | |
| n | 8 |
| Age, years | 22 ± 2 |
| Height, cm | 176 ± 5 |
| Weight, kg | 74 ± 8 |
| BMI, kg/m2 | 23 ± 2 |
| Body fat, % | 20 ± 8 |
Table 1: Participant demographics. The values are means ± SD unless otherwise indicated. This table is from Van Schaik et al.27.
Discussion
The method we have shown here is a technically simple, safe, and cost-effective protocol for measuring BAT thermogenesis in humans. The protocol addresses concerns related to the reliability of using IRT on its own to distinguish between local warming due to altered skin blood flow and deeper warming due to thermogenesis by correlating IRT with both measures of energy expenditure (EE) and substrate utilization. Since this technique does not use ionizing radiation, it permits repeated-measures analysis, which is not possible with PET imaging techniques. Finally, while PET imaging techniques can identify BAT activation, they do not report on the physiological outcomes (increased temperature and EE) that this protocol measures.
The strength of the protocol described here is that there are four lines of evidence that support the conclusion of evoked BAT thermogenesis: (1) increased measured Tscf, in parallel with unchanged core temperature and stable skin temperature over the adjacent reference region; (2) increased energy expenditure; (3) a change in substrate utilization; and (4) a fall in blood glucose levels. The converging observations are all consistent with the predicted outcomes for BAT thermogenesis. The essential part of the protocol is the carbohydrate loading of the participants to ensure carbohydrate metabolism before intervention. BAT thermogenesis switches substrate metabolism from carbohydrates to free fatty acids, as shown by the fall in RER. While the preferred substrate for BAT thermogenesis is free fatty acids, a significant uptake of glucose into active BAT is well established5,6,7. Therefore, we observe a fall in blood glucose levels concurrent with BAT thermogenesis. It would not be possible to observe the mutual shift in substrate utilization (RER) and the fall in blood glucose levels in a fasted state.
Previous studies have concluded that increased Tscf (measured by IRT) is sufficient to conclude BAT thermogenesis. However, this conclusion is only certain if the Tscf exceeds the core temperature. If the Tscf is less than or equal to the core temperature, then a local change in temperature due to increased skin blood flow cannot be excluded. A systematic review concluded that IRT alone is unable to determine whether increases in supraclavicular skin temperature are due to BAT thermogenesis37. The review noted that the most common method (18F-FDG PET/CT) measures the uptake of glucose into BAT37. However, the preferred substrate for BAT thermogenesis is fatty acids13. This methodological issue prevents any meaningful comparison between PET/CT data in validating IRT data, as either of these measures alone are not a suitable measure of the true metabolic activity of the BAT as it cannot indicate the change in energy expenditure and substrate utilization due to BAT thermogenesis. Nevertheless, with the protocol described here, not only can we quantify the change in temperature, but we can also confirm an increase in energy expenditure-a key physiological outcome of BAT thermogenesis. IRT is a non-contact, non-invasive, and relatively inexpensive method for measuring temperature and temperature changes associated with BAT thermogenesis. In contrast, PET-CT is expensive and exposes individuals to ionizing radiation, thus restricting the applicability of this method to small retrospective analyses of clinical imaging studies. The application of the current protocol to large-scale, randomized clinical trials would be relatively simple and cost-effective.
It is important to note that the decrease in carbohydrate oxidation following caffeine intervention can be explained by the switch in substrate utilization as a result of increased BAT thermogenesis due to the intervention. Measures of insulin signaling would make the results of this study more robust. However, it is not clear based on the results of this study as to whether caffeine would affect insulin signaling via action on the BAT or whether the fall in blood glucose is a result of the BAT taking up more energy substrates.
The 18F-FDG PET/CT method has several inherent limitations when it is used to quantify and measure the physiological activity of BAT, particularly when investigating the influence of nutrients or dietary ingredients on BAT activity. The 18F-FDG PET/CT method requires subjects to be fasted to avoid feeding-induced increases in glucose uptake by the muscular tissue, which can significantly reduce the detection of both the BAT and BAT function38. Furthermore, this technique alone cannot measure the physiological impact or extent of BAT activation. Additionally, the use of ionizing radiation in PET imaging studies is an ethical and health and safety hurdle for designing repeated-measures cross-over studies. In addition, 18F-FDG represents glucose uptake only, which is not the same as measuring glucose metabolism. This method of carbohydrate loading subjects prior to measuring the BAT temperature and combining blood glucose levels with indirect calorimetry allows us to rigorously measure the physiological impact of thermogenesis and changed substrate utilization, which would otherwise not be available in a fasted state.
Strengths and limitations
This protocol has wider implications than purely studying BAT. By carbohydrate-loading participants prior to intervention, the oscillation of blood glucose levels in response to both carbohydrate loading and the caffeine intervention, as well as changes in substrate utilization, can be observed. Therefore, this technique can be used to improve human indirect calorimetry studies and metabolic measures. It is not yet known whether the results from this study can be replicated following other interventions, such as cold exposure or adrenergic stimulation. However, the results of this study have been replicated following intervention with a different dietary ingredient, namely Capsicum annuum27. Additional rigor and confidence in the results could be obtained using a double-blind approach for the analysis of interventions using the techniques described, and this could be easily implemented27.
The potential confound of varied room temperature is not relevant in this protocol, as the room temperature was kept stable from participant to participant. Additionally, the humidity was taken into account during the calibration of the respiratory gas analyzer. This is inferred in the setup of this piece of equipment, as calibration is completed as per the manufacturer's instruction.
The time intervals for the measurement and treatment were determined following a small pilot study in which troubleshooting of the protocol was conducted. Essentially, the time intervals for measurement were determined based on the time needed for the researcher to perform the measurements and for the participant's comfort. The time for the intervention was determined based on the time taken for carbohydrate metabolism to occur following the carbohydrate load to investigate whether the intervention increased free fatty acid oxidation (i.e., BAT thermogenesis) and lowered carbohydrate oxidation.
Notably, there are differences between capillary and venous glucose levels39. However, in the context of out-of-hospital care, the most common way in which blood glucose levels are measured is via a blood sample of capillary origin analyzed by a hand-held, point-of-care glucometer40. Additionally, in healthy individuals (similar to those included in this protocol) in a non-clinical setting, there is a statistically significant, but not clinically significant, difference between capillary and venous blood glucose levels when measured using a point-of-care, capillary-based glucometer41. In this context, capillary sampling would remain the optimal approach due to the fact that most point-of-care glucometers available on the market are engineered to analyze capillary blood samples41. From a clinical perspective, it might be argued that venous blood glucose is the superior method of analysis. However, venous blood sampling is not only expensive and requires specialized equipment (ibid), but it is also invasive. The ethical considerations of increasing the risk of adverse events during the protocol need to be balanced against the reported literature showing the high correlation and reliability of capillary blood glucose as a proxy measure of venous blood glucose42. The key here, of course, is that we have not set out to diagnose diabetes but to measure changes in blood glucose levels, for which capillary blood glucose monitoring is a more than suitable protocol.
Glucose can induce thermogenesis, and single meals can activate the BAT43. However, and rather importantly, the data included in this manuscript show no significant effect of glucose loading in the intervention group or the placebo group. Furthermore, the data included in the manuscript were derived from the results of Van Schaik et al., which included a third intervention (Capsicum annuum), and the glucose load did not produce a significant effect on the measures27.
It should be noted that this protocol has only been used in male participants with low body fat and active BAT (to reduce the number of controllable variables, females were excluded from the study). There is a known inverse correlation between adiposity and BAT mass in humans44. In addition, it is known that previously obese people who have lost weight through diet and exercise have a lower basal metabolic rate and must consume lower-calorie diets to maintain a normal weight45,46. Furthermore, BAT activity can stimulate BAT growth8. The method described here will allow for long-term studies to investigate changes in BAT activity associated with metabolic diseases in a way not afforded by other techniques.
Conclusion
In conclusion, we demonstrate a measurement approach to quantify human brown adipose tissue activity using IRT and indirect calorimetry following a carbohydrate load. The critical steps include 1) carbohydrate loading the participants that are in a fasted state prior to measuring the BAT temperature whilst combining indirect calorimetry and blood glucose levels to allow the quantification of the physiological extent of BAT thermogenesis and altered substrate utilization; 2) assessing relevant IRT BAT depots and temperatures from a reference point and core temperature to demonstrate any increase in Tscf that would be indicative of BAT activation based on the anatomical location. We believe that these quantitative measurements allow for a more accurate evaluation of the contribution of BAT to adult human energy metabolism and thermoregulation. This thorough approach should be used by researchers to study BAT physiology and serve as a new standard for developing human BAT activation approaches in the future.
Disclosures
The authors have no conflicts of interest to disclose.
Acknowledgements
We would like to thank all the study volunteers for their participation in our study. This work was supported by the Holsworth Research Initiative, La Trobe University, and the Defence Science Institute (DSI, Australia).
Materials
| Name | Company | Catalog Number | Comments |
| Automated Sphygmomanometer | Omron SEM-2 advanced, Omron, Kyoto, Japan | ||
| Dual-energy X-ray absorptiometry scanner | Hologic Horizon, Hologic Inc., Bedford, MA, USA | ||
| ECG electrodes | Ambu Blue Sensor R, Malaysia | ||
| Five lead ECG | Medilog AR12 plus; Schiller, Germany | ||
| FLIR E60 camera | FLIR Systems Australia, Melbourne , Australia | ||
| FLIR Research Studio Professional Edition | FLIR Systems Australia, Melbourne , Australia | ||
| Freestyle Optium Xceed | Abbott Diabetes Care, Alameda, Canada | ||
| Glucose Gel | Winners Sports Nutrition, Mt Martha, Victoria, Australia | ||
| MaskA cold-sterilized silicone mask | 7400 series Oro-Nasal Mask, Hans Rudolph | ||
| Medilog Darwin2 software | Professional; Schiller, Germany | ||
| Non-contact Infrared Thermometer | Berrcom, JXB-178, Guangdong, China | ||
| Optium Glucose Strip Xceed | Abbott Diabetes Care, Alameda, Canada | ||
| ParvoMedics TrueOne 2400 respiratory gas analyser | ParvoMedics Inc, East Sandy, UT, USA | ||
| Pre-sterilized Non-rebreathing Valve | Two-way non-rebreathing valve T-Shape configuration, 2600 Medium or 2700 Large, Hans Rudolph |
References
- Cypess, A. M., et al. Identification and importance of brown adipose tissue in adult humans. The New England. Journal of Medicine. 360 (15), 1509-1517 (2009).
- van Marken Lichtenbelt, W. D., et al. Cold-activated brown adipose tissue in healthy men. The New England Journal of Medicine. 360 (15), 1500-1508 (2009).
- Virtanen, K. A., et al. Functional brown adipose tissue in healthy adults. New England Journal of Medicine. 360 (15), 1518-1525 (2009).
- Abreu-Vieira, G., Xiao, C., Gavrilova, O., Reitman, M. L. Integration of body temperature into the analysis of energy expenditure in the mouse. Molecular Metabolism. 4 (6), 461-470 (2015).
- Orava, J., et al. Different metabolic responses of human brown adipose tissue to activation by cold and insulin. Cell Metabolism. 14 (2), 272-279 (2011).
- Chen, K. Y., et al. Brown fat activation mediates cold-induced thermogenesis in adult humans in response to a mild decrease in ambient temperature. Journal of Clinical Endocrinology Metabolism. 98 (7), 1218-1223 (2013).
- Ouellet, V., et al. Brown adipose tissue oxidative metabolism contributes to energy expenditure during acute cold exposure in humans. The Journal of Clinical Investigation. 122 (2), 545-552 (2012).
- Van Schaik, L., Kettle, C., Green, R., Irving, H., Rathner, J. Effects of caffeine on brown adipose tissue thermogenesis and metabolic homeostasis: A review. Frontiers in Neuroscience. 15, 54 (2021).
- Lee, P., et al. Temperature-acclimated brown adipose tissue modulates insulin sensitivity in humans. Diabetes. 63 (11), 3686 (2014).
- Heaton, J. M. The distribution of brown adipose tissue in the human. Journal of Anatomy. 112 (1), 35-39 (1972).
- Sievers, W., et al. Innervation of supraclavicular adipose tissue: A human cadaveric study. PLoS One. 15 (7), 0236286 (2020).
- Chondronikola, M., Beeman, S. C., Wahl, R. L. Non-invasive methods for the assessment of brown adipose tissue in humans. The Journal of Physiology. 596 (3), 363-378 (2018).
- Carpentier, A. C., et al. Brown adipose tissue energy metabolism in humans. Frontiers in Endocrinology. 9, 447 (2018).
- Raiko, J., et al. Human brown adipose tissue [15O] O2 PET imaging in the presence and absence of cold stimulus. European Journal of Nuclear Medicine and Molecular Imaging. 43 (10), 1878-1886 (2016).
- Blondin, D. P., et al. Selective impairment of glucose but not fatty acid or oxidative metabolism in brown adipose tissue of subjects with type 2 diabetes. Diabetes. 64 (7), 2388-2397 (2015).
- Blondin, D. P., et al. Dietary fatty acid metabolism of brown adipose tissue in cold-acclimated men. Nature Communications. 8, 14146 (2017).
- Lahesmaa, M., et al. Regulation of human brown adipose tissue by adenosine and A2A receptors-studies with [15O] H2O and [11C] TMSX PET/CT. European Journal of Nuclear Medicine and Molecular Imaging. 46 (3), 743-750 (2019).
- Koskensalo, K., et al. Human brown adipose tissue temperature and fat fraction are related to its metabolic activity. The Journal of Clinical Endocrinology & Metabolism. 102 (4), 1200-1207 (2017).
- Gifford, A., Towse, T. F., Walker, R. C., Avison, M. J., Welch, E. B. Characterizing active and inactive brown adipose tissue in adult humans using PET-CT and MR imaging. American Journal of Physiology-Endocrinology and Metabolism. 311 (1), 95-104 (2016).
- Law, J., et al. Thermal imaging is a noninvasive alternative to PET/CT for measurement of brown adipose tissue activity in humans. Journal of Nuclear Medicine. 59 (3), 516-522 (2018).
- Brasil, S., et al. A systematic review on the role of infrared thermography in the brown adipose tissue assessment. Reviews in Endocrine and Metabolic Disorders. 21 (1), 37-44 (2020).
- Velickovic, K., et al. Caffeine exposure induces browning features in adipose tissue in vitro and in vivo. Scientific Reports. 9 (1), 9104 (2019).
- Pérez, D. I. V., et al. Physically active men with high brown adipose tissue activity showed increased energy expenditure after caffeine supplementation. Journal of Thermal Biology. 99, 103000 (2021).
- Symonds, M. E., et al. Thermal imaging to assess age-related changes of skin temperature within the supraclavicular region co-locating with brown adipose tissue in healthy children. The Journal of Pediatrics. 161 (5), 892-898 (2012).
- Salem, V., et al. Glucagon increases energy expenditure independently of brown adipose tissue activation in humans. Diabetes, Obesity and Metabolism. 18 (1), 72-81 (2016).
- Lee, P., et al. Hot fat in a cool man: Infrared thermography and brown adipose tissue. Diabetes, Obesity and Metabolism. 13 (1), 92-93 (2011).
- Van Schaik, L., et al. Both caffeine and Capsicum annuum fruit powder lower blood glucose levels and increase brown adipose tissue temperature in healthy adult males. Frontiers in Physiology. 13, 870154 (2022).
- Van Schaik, L., et al. but not anxiogenic, doses of caffeine act centrally to activate interscapular brown adipose tissue thermogenesis in anesthetized male rats. Scientific Reports. 11 (1), 113 (2021).
- McNeill, B. T., Morton, N. M., Stimson, R. H. Substrate utilization by brown adipose tissue: What's hot and what's not. Frontiers in Endocrinology. 11, 571659 (2020).
- Schmidt-Nielsen, K. . Animal Physiology: Adaptation and Environment. , (1997).
- Peronnet, F., Massicotte, D. Table of nonprotein respiratory quotient: An update. Canadian Journal of Sport Sciences. 16 (1), 23-29 (1991).
- Galgani, J. E., Ryan, D. H., Ravussin, E. Effect of capsinoids on energy metabolism in human subjects. British Journal of Nutrition. 103 (1), 38-42 (2010).
- Ohnuki, K., et al. CH-19 sweet, a non-pungent cultivar of red pepper, increased body temperature and oxygen consumption in humans. Bioscience, Biotechnology, and Biochemistry. 65 (9), 2033-2036 (2001).
- Wang, Q., et al. Brown adipose tissue activation is inversely related to central obesity and metabolic parameters in adult human. PLoS One. 10 (4), 0123795 (2015).
- Vijgen, G. H., et al. Brown adipose tissue in morbidly obese subjects. PLoS One. 6 (2), 17247 (2011).
- Cunningham, J. Calculation of energy expenditure from indirect calorimetry: Assessment of the Weir equation. Nutrition. 6 (3), 222-223 (1990).
- Jimenez-Pavon, D., et al. Infrared thermography for estimating supraclavicular skin temperature and BAT activity in humans: A systematic review. Obesity. 27 (12), 1932-1949 (2019).
- Roman, S., et al. Brown adipose tissue and novel therapeutic approaches to treat metabolic disorders. Translational Research. 165 (4), 464-479 (2015).
- Sirohi, R., Singh, R. P., Chauhan, K. A comparative study of venous and capillary blood glucose in a tertiary care hospital. Indian Journal of Public Health Research and Development. 11 (7), 740 (2020).
- Funk, D. L., Chan, L., Lutz, N., Verdile, V. P. Comparison of capillary and venous glucose measurements in healthy volunteers. Prehospital Emergency Care. 5 (3), 275-277 (2001).
- Topping, J., et al. A comparison of venous versus capillary blood samples when measuring blood glucose using a point-of-care, capillary-based glucometer. Prehospital and Disaster Medicine. 34 (5), 506-509 (2019).
- Akinbami, F., et al. Tale of two sites: capillary versus arterial blood glucose testing in the operating room. The American Journal of Surgery. 203 (4), 423-427 (2012).
- Saito, M., Matsushita, M., Yoneshiro, T., Okamatsu-Ogura, Y. Brown adipose tissue, diet-induced thermogenesis, and thermogenic food ingredients: from mice to men. Frontiers in Endocrinology. 11, 222 (2020).
- Yoneshiro, T., et al. Age-related decrease in cold-activated brown adipose tissue and accumulation of body fat in healthy humans. Obesity. 19 (9), 1755-1760 (2011).
- Fothergill, E., et al. Persistent metabolic adaptation 6 years after "The Biggest Loser" competition. Obesity. 24 (8), 1612-1619 (2016).
- Hall, K. D. Energy compensation and metabolic adaptation: "The Biggest Loser" study reinterpreted. Obesity. 30 (1), 11-13 (2021).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved