A subscription to JoVE is required to view this content. Sign in or start your free trial.
Erythrocyte Sedimentation Rate: A Physics-Driven Characterization in a Medical Context
In This Article
Summary
Erythrocyte sedimentation rate (ESR) is a physical parameter, often used in routine health checks and medical diagnosis. A theoretical model that allows to extract physically-meaningful parameters from the whole sedimentation curve, based on modern colloidal knowledge, has recently been developed. Here, we present a protocol to automatically collect the ESR over time, and extract the parameters of this recent model from this automated data collection. These refined parameters are also likely to improve the medical testimony.
Abstract
Erythrocyte (or red blood cell) sedimentation rate (ESR) is a physical derived parameter of blood which is often used in routine health checks and medical diagnosis. For instance, in the case of inflammation, a higher ESR is observed due to the associated increase in fibrinogen and other plasma proteins. It was believed that this increase was due to the formation of larger aggregates of red blood cells (RBCs) caused by the increase in fibrinogen. Indeed, fibrinogen is an agent-fostering aggregation of RBCs and in the Stokes regime-assumed to be observed in blood-larger aggregates sediment faster. However, all models of ESR measurements based on this hypothesis require further specific physical assumptions, not required in any other system. Besides, modern studies in the field of colloidal suspensions have established that attractive particles form percolating aggregates (i.e. aggregates as wide as the container). The sedimentation of these colloids then follows a so-called "colloidal gel collapse". Recently, it has been shown that RBCs actually follow the same behavior. This hypothesis also allows to efficiently and analytically model the sedimentation curve of RBCs, from which robust and physically-meaningful descriptors can be extracted. This manuscript describes how to perform such an analysis, and discusses the benefits of this approach.
Introduction
The erythrocyte sedimentation rate (ESR) is a medical in vitro clinical tool, formally introduced in evidenced-based medicine during the twentieth century1,2,3,4. It is currently used worldwide as a nonspecific inflammatory test, or to monitor the evolution of some specific conditions5,6,7,8. This is mainly due to an increase in the fibrinogen concentration, but also in other plasma components such as IgM1,9,10,11. According to the current Westergren standard protocol, ESR values are reported as the measurement of the cell-free plasma layer at a given time point (30 min or 1 h) after leaving a vertical tube of a typical size of 20 cm vertically at rest12. However, this measurement method has been criticized since qualitatively different stages in the sedimentation process have been reported, including a delay before reaching the maximum settling velocity13. This delay lasts more than 1 h in approximately half of healthy samples14. The velocity during this phase obeys a different scaling than during the second, faster phase of the sedimentation15. Restricting the readout to the average settling velocity during the first hour then compares a different mix of various blood properties between different individuals.
Moreover, it has recently been demonstrated that the usual theoretical considerations behind this protocol were erroneous16,17,18. At physiological hematocrit (above approximately 25%), red blood cells (RBCs) do not sediment as separate aggregates, but rather as a continuous, so-called percolating, network of RBCs17,18, obeying a different set of physical equations than the usually mentioned Stokes sedimentation16,17. It has been shown that considering a physical description based on the time-resolved measurements of the sedimentation (whole curve) was more robust in some novel medical contexts19,20. Moreover, these measurements could be used to shed light on the physical mechanisms altering the ESR in pathologies in which cell shapes are altered19,20. Additionally, a slow ESR can have a useful medical interpretation, as indicated in the measurements of a cohort of neuroacanthocytosis syndrome patients19,20. This article reviews how to practically implement the measurement of physically-meaningful parameters, based on the whole ESR kinetics. More accurately, the method presented here extracts the maximum sedimentation speed Um, the value of which can be corrected to consider the effect of the hematocrit of the donor16,17. This parameter is more accurate and thus more reliable than the traditional measurement16,17,19,20.
In addition, in some fundamental research, instead of monitoring the inflammation state of a given patient, it is interesting to exclude the effect of hematocrit on the ESR21,22,23, or to investigate the role of RBCs in a modified ESR19,20,24,25 between different donors. It might be useful to compare samples which are not directly full blood samples from patients. Therefore, resuspending RBCs with a controlled hematocrit in the autologous plasma, or in a plasma-substituent, might be used as the first step of ESR measurement. For instance, solutions of Dextran 70 kDa with a concentration of 55 mg/mL in phosphate buffered saline (PBS) produces a sedimentation range within the control range for healthy cells19. This manuscript also shows how such steps should be conducted, and that the presented analysis is also relevant in these cases.
Protocol
Blood sample collection and experiments were approved by the "Ärztekammer des Saarlandes", ethics votum 51/18, and performed after informed consent was obtained according to the Declaration of Helsinki. Standard measurements should be performed with ethylenediaminetetraacetic acid (EDTA)-anticoagulated blood (standard EDTA concentration of 1.6 mg/mL blood, European norm NF EN ISO 6710), in Westergren tubes. The volume required to fill the Westergren tube depends on the manufacturer (as lower parts sometimes contain a wider reservoir); The volume should be about 1 mL of full blood, and 800 µL for the tubes indicated in the Table of Materials. The method described below is however valid no matter the specific suspension and container shape, as long as the hematocrit of the probed samples is higher than 25%16. Volumes, containers, suspending medium, and additives should therefore be selected according to the specific objectives of the performed research.
1. Experiments and measurements
NOTE: Record the sedimentation rate of the sample every minute.
- Sample preparation (if required): If a control hematocrit or suspending liquid is required, start by washing the cells and preparing the samples (as an example, we show how to prepare samples with various fibrinogen levels, by mixing autologous serum and plasma). Serum can indeed be approximated as fibrinogen-free plasma26,27, and can be used in order to decrease the aggregation of RBCs, in otherwise physiological conditions. In order to prepare several samples, collect the blood in standard EDTA tubes of 9 mL and the serum in standard serum tubes (with silica beads as clotting activators) of 9 mL.
- Centrifuge the blood samples (i.e., 9 mL of standard EDTA and serum tubes in the chosen example) at 3,000 x g for at least 7 min, for optimal compaction of the packed erythrocytes. Replace the supernatant with PBS or the desired suspending liquid, if a sufficient quantity is available. If it is simply required to control the hematocrit in autologous plasma, proceed directly to step 1.1.3. Otherwise, gently mix after inclusion of the supernatant for washing.
- Repeat three times. Perform the last wash with the desired suspending liquid in any case.
- In the chosen example, prepare mixtures of autologous plasma and serum with determined proportions (e.g., 25%/75%, 50%/50%, or 75%/25% of plasma-serum volume fraction). For example, when preparing 2.5 mL of the 25%/75% plasma-serum mixture, add 0.625 mL of plasma to 1.875 mL of serum.
- Extract the required volume of packed cells and suspend it in the desired liquid. Process the packed cells as a highly viscous liquid (using standard pre-pipetting and/or reverse-pipetting or a positive displacement pipette28). In the chosen example, for a 4 mL sample at a hematocrit of 45%, suspend 1.8 mL of packed cells within 2.2 mL of the plasma-serum mixture.
- If the hematocrit of the sample is not controlled, determine it by high-speed microcentrifugation (other standard methods are also suitable).
- Extract the required amount of sample for hematocrit determination: fill the micro-hematocrit capillaries by immersing its lower tip in the liquid. Stop it by covering the top opening when the required amount of sample rises in the tube by capillary suction.
- Seal the capillaries with sealing wax. Place them in the micro-hematocrit centrifuge and run it at 15,000 x g(12,000 rpm) for 5 min, or according to the manufacturer's instructions.
- Read the hematocrit level on the capillary and write it down for reference.
- Set up a camera for recording the samples' sedimentation. To avoid overloading the memory or draining the battery, power the device from the mains and save the pictures directly to a computer or external hard drive.
- Set up a steady camera in front of the holder where the ESR tubes will be left at rest. Use a white, illuminated background (white sheets of paper in the background work perfectly).
- Using empty Westergren tubes, preferably without markings, set the focus and the field of view of the camera to obtain the highest resolution where the samples will be placed. Preferably, ensure that the borders of the pictures are aligned in the vertical and horizontal direction.
- Take a picture of a scaled tube to extract the pixel resolution. The use of RGB pictures is recommended.
- Set the light and the exposure time of the camera to have a white background, but no saturation. The example in Figure 1 has been obtained using a Canon EOS M50, with an exposure time of 1/15s, an aperture of F8.0, Tungsten white balance, in single shot mode with manual focus, and an ISO speed of 1,000.
- Prepare and place the ESR tubes.
- Fill the bottom container of the Westergren tube with the volume corresponding to the manufacturer's instructions. Insert the Westergren tube in the bottom container as indicated by the manufacturer.
- As soon as the first tube is ready, place it in the holder and start the camera recording. Recording one picture every minute usually gives a good resolution of the ESR kinetic curve.
- Prepare and place the next samples. Make sure not to stand in front of any sample when a picture is taken.
- Let the measurements run for at least 2 h, in order to compare with standard measurements at 30 min, 1 h, and 2 h. However, it is also better to see the saturation and arrest of the sedimentation. For healthy samples, or in case of inflammation, recording the samples overnight, between 12 h and 24 h, is more than sufficient since the curvature of the fastest samples is visible within 3 h13,19. However, if a condition decreases the sedimentation velocity, as a decrease in fibrinogen concentration does (i.e., in the chosen example, high serum volume fraction), recordings of 50 h or more might be required to obtain the most accurate information16,19.
2. Image analysis
NOTE: Once the images are recorded, extract the ESR curve. An example of Matlab code is provided as Supplementary File 1 (MatlabCodeImageAnalysisSampled.m).
- Select or identify a region of interest (ROI) where only one tube is visible, the lower border being located under the lowest position of the erythrocyte cell-free plasma interface but within the sample (see Figure 1A, B). If required, rotate the picture so that the vertical direction is aligned along the first component of the picture.
- Convert the ROI of the RGB picture into a gray-scale picture or matrix Gr. Usually, combining the three-color channels (red [R], green [G] and blue [B]) as Gr = 2 * R - B - G is very efficient with a clear background (see Figure 1C and MatlabCodeImageAnalysisSampled.m lines 121-128).
- Binarize the picture. For a healthy sample, using the Otsu threshold29 usually gives a consistent result (see Figure 1D and line 133 of MatlabCodeImageAnalysisSampled.m). For samples with a high hematocrit or with some hemolysis, it might be better to adjust it manually or use another automatic method (as done in lines 129-131 of MatlabCodeImageAnalysisSampled.m), depending on the exact contrast obtained between the various phases inside the tube.
- Obtain the average of the pixel (or elements) values of Gr along the horizontal direction. This step minimizes the noise and efficiently averages the possible interface irregularities (see Figure 1E and MatlabCodeImageAnalysisSampled.m line 137).
- Before computing the variations, smooth the curve with a moving average, especially if the tubes contain some horizontal markings (see Figure 1F). For the provided examples, a moving window of ~2.5 mm (50 pixels) was used for this process (see line 138 of MatlabCodeImageAnalysisSampled.m). Then, identify the interface position as the point with the highest intensity variation (see Figure 1G).
- Repeat for each picture and each sample. For each sample, save the positions of the interface along time in an appropriate format to fit the physical model with any suitably fit software.
3. Fitting of the physical model
- Using any suitably fit software, knowing the hematocrit and the initial height of the blood column h0, find the values of the delay time t0, the dimensionless time γ, and the final packed volume fraction Φm of the erythrocytes that minimizes the sum of squared residual deviations for the physical model17. A Matlab code (ShapeAnalyzerIntegrated.m) is provided as Supplementary File 2 as an example of how to perform such a fit. See Supplementary File 2 for further instructions. As described elsewhere16,17, erythrocytes at physiological hematocrit sediment as a soft particle gel, where the important physical parameters are the difference in density between the plasma and RBCs of the donor Δρ, the RBC characteristic diameter rRBC, the plasma viscosity at room temperature η, and the donor's hematocrit Φ. Using these parameters, and assuming that the gravitational stress is the main driving process for the plasma to flow upward through the porous network formed by the erythrocytes after a delay time t0, one obtains the temporal evolution16,17:
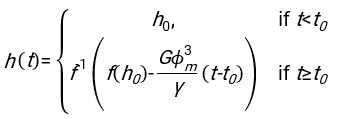
with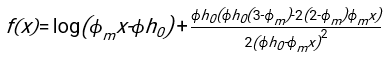 ,
, 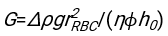 , and
, and 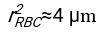 being the average disk radius of an erythrocyte. An example of a Matlab code performing this is provided as an attachment (ShapeAnalyzerIntegrated.m fits the function defined in SedimFit.m [Supplementary File 3]). Alternatively, G/γ can also be used directly as a fit parameter with units of 1/t.
being the average disk radius of an erythrocyte. An example of a Matlab code performing this is provided as an attachment (ShapeAnalyzerIntegrated.m fits the function defined in SedimFit.m [Supplementary File 3]). Alternatively, G/γ can also be used directly as a fit parameter with units of 1/t. - Once the quantitative curve is extracted from the picture, save the physical parameters of the sample. Traditional ESR values at 30 min, 1 h, or 2 h can still be extracted from the curve for reference (see ShapeAnalyzerIntegrated.m lines 123-132).
Results
An example of an image sequence correctly acquired is provided as Supplementary Movie 1 (MovieS1.avi). A series of characteristic fits of the model is shown for various conditions in Figure 2. Fibrinogen concentration was determined from the fibrinogen concentration in the plasma Fib0, assuming that the serum does not have any fibrinogen at all. Hence, Fib = C Fib0, where C is the plasma volume fraction in the plasma-...
Discussion
For the automated protocol to work efficiently, it is important to have a clear background and proper illumination. A dark background might prevent the existence of an efficient binarization threshold. For samples with some hemolysis, which usually occurs (increases) over time, it is important to verify first that the chosen binarization threshold is relevant for both the initial and final pictures.
When it comes to the binarization process of the picture, the choice of the ROI and binarizatio...
Disclosures
The authors have no conflicts of interest to declare relevant to the content of this article.
Acknowledgements
This work was supported by the research unit FOR 2688 - Wa1336/12 of the German Research Foundation and by the Marie Skłodowska-Curie grant agreement No. 860436-EVIDENCE. T. J. and C. W. acknowledge funding from French German University (DFH/UFA). A. D. acknowledges funding by the Young Investigator Grant of Saarland University.
Materials
| Name | Company | Catalog Number | Comments |
| Anticoagulant (EDTA or Heparin) tube (for blood sample) | SARSTEDT | 267001 or 265 | Anticoagulated blood sample to characterize |
| Camera EOS M50 | Canon | Kit EF-M18-150 IS STM | Any camera should work, provided that sector alimentation, connection to computer for automated shooting and adapted objective are available |
| Centrifuge | HERMLE | 302.00 V03 - Z 36 HK | Requirements: at least 3000 x g ofr 7 min. |
| Micro-centrifuge | MLW | TH21 | or any other way to determine the hematocrit |
| Micro-hematocrit capilaries | Fisher scientific | 11884040 | or other capillaries/containers for hematocrit determination |
| Phosphate Buffered Saline (PBS) | ThermoFisher | 10010023 | 1x PBS, pH 7.4, 298 Osm |
| Pipettes (e.g. positive displacement pipette) | Gilson | FD10006 | Pipette required to manipulate blood and/or packed cells.Other models are of course suitable, but be careful to treat blood and pakced cells as highly viscous fluids. |
| Wax sealing plate | Hirschmann | 9120101 | Sealing wax for the micro-hematocrit capillaries |
| Westergren tubes | Praxindo | A9244560 | Any other standard Wetsergren tube should work too |
| White background with illumination | / | / | White sheet(s) of paper behind the samples, with usual room light is perfcetly sufficient. |
References
- Bedell, S. E., Bush, B. T. Erythrocyte sedimentation rate. From folklore to facts. The American Journal of Medicine. 78, 1001-1009 (1985).
- Grzybowski, A., Sak, J. Edmund Biernacki (1866-1911): Discoverer of the erythrocyte sedimentation rate. On the 100th anniversary of his death. Clinics in Dermatology. 29 (6), 697-703 (2011).
- Kushner, I., Mackiewicz, A. . The Acute Phase Response: An Overview. Acute Phase Proteins. , (1993).
- Tishkowski, K., Gupta, V. Erythrocyte Sedimentation Rate. StatPearls Publishing. , (2022).
- Menees, S. B., Powell, C., Kurlander, J., Goel, A., Chey, W. D. A meta-analysis of the utility of C-reactive protein, erythrocyte sedimentation rate, fecal calprotectin, and fecal lactoferrin to exclude inflammatory bowel disease in adults with IBS. The Americal Journal of Gastroenterology. 110 (3), 444-454 (2015).
- Brigden, M. L. Clinical utility of the erythrocyte sedimentation rate. Americal Family Physician. 60 (5), 1443-1450 (1999).
- Liu, S., et al. Preliminary case-control study to evaluate diagnostic values of C-reactive protein and erythrocyte sedimentation rate in differentiating active Crohn's disease from intestinal lymphoma, intestinal tuberculosis and Behcet's syndrome. The American Journal of the Medical Sciences. 346 (6), 467-472 (2013).
- Greidanus, N. V., et al. Use of erythrocyte sedimentation rate and C-reactive protein level to diagnose infection before revision total knee arthroplasty: A prospective evaluation. The Journal of Bone and Joint Surgery. 89 (7), 1409-1416 (2007).
- Flormann, D., Kuder, E., Lipp, P., Wagner, C., Kaestner, L. Is there a role of C-reactive protein in red blood cell aggregation. International Journal of Laboratory Hematology. 37 (4), 474-482 (2015).
- Brust, M., et al. The plasma protein fibrinogen stabilizes clusters of red blood cells in microcapillary flows. Scientific Reports. 4, 4348 (2014).
- Gray, S. J., Mitchell, E. B., Dick, G. F. Effect of purified protein fractions on sedimentation rate of erythrocytes. Proceedings of the Society for Experimental Biology and Medicine. 51 (3), 403-404 (1942).
- Kratz, A., et al. ICSH recommendations for modified and alternate methods measuring the erythrocyte sedimentation rate. International Journal of Laboratory Hematology. 39 (5), 448-457 (2017).
- Hung, W. T., Collings, A. F., Low, J. Erythrocyte sedimentation rate studies in whole human blood. Physics in Medicine and Biology. 39 (11), 1855-1873 (1994).
- Woodland, N. B., Cordatos, K., Hung, W. T., Reuben, A., Holley, L. Erythrocyte sedimentation in columns and the significance of ESR. Biorheology. 33 (6), 477-488 (1996).
- Holley, L., Woodland, N., Hung, W. T., Cordatos, K., Reuben, A. Influence of fibrinogen and haematocrit on erythrocyte sedimentation kinetics. Biorheology. 36 (4), 287-297 (1999).
- Dasanna, A. K., et al. Erythrocyte sedimentation: Effect of aggregation energy on gel structure during collapse. Physical Review. E. 105 (2-1), 024610 (2022).
- Darras, A., et al. Erythrocyte sedimentation: collapse of a high-volume-fraction soft-particle gel. Physical Review Letters. 128 (8), 088101 (2022).
- Darras, A., et al. Imaging erythrocyte sedimentation in whole blood. Frontiers in Physiology. 12, 729191 (2022).
- Darras, A., et al. Acanthocyte sedimentation rate as a diagnostic biomarker for neuroacanthocytosis syndromes: Experimental evidence and physical justification. Cells. 10 (4), 788 (2021).
- Rabe, A., et al. The erythrocyte sedimentation rate and its relation to cell shape and rigidity of red blood cells from chorea-acanthocytosis patients in an off-label treatment with dasatinib. Biomolecules. 11 (5), 727 (2021).
- Giavarina, D., Capuzzo, S., Pizzolato, U., Soffiati, G. Length of erythrocyte sedimentation rate (ESR) adjusted for the hematocrit: reference values for the TEST 1 method. Clinical Laboratory. 52 (5-6), 241-245 (2006).
- Bull, B. S. Is a standard ESR possible. Laboratory Medicine. 6 (11), 31-39 (1975).
- Bull, B. S., Brecher, G. An evaluation of the relative merits of the Wintrobe and Westergren sedimentation methods, including hematocrit correction. American Journal of Clinical Pathology. 62 (4), 502-510 (1974).
- Reinhart, W. H., Singh, A., Straub, P. W. Red blood cell aggregation and sedimentation: the role of the cell shape. British Journal of Haematology. 73 (4), 551-556 (1989).
- Jan, K., Usami, S., Smith, J. A. Influence of oxygen tension and hematocrit reading on ESRs of sickle cells: Role of RBC aggregation. Archives of Internal Medicine. 141 (13), 1815-1818 (1981).
- Issaq, H. J., Xiao, Z., Veenstra, T. D. Serum and plasma proteomics. Chemical Reviews. 107 (8), 3601-3620 (2007).
- Yu, Z., et al. Differences between human plasma and serum metabolite profiles. PLoS One. 6 (7), 21230 (2011).
- . Proper Pipetting Techniques - DE Available from: https://www.thermofisher.com/de/de/home/life-science/lab-plasticware-supplies/lab-plasticware-supplies-learning-center/lab-plasticware-supplies-resource-library/fundamentals-of-pipetting/proper-pipetting-techniques.html (2023)
- Otsu, N. A threshold selection method from gray-level histograms. IEEE Transaction on Systems, Man, and Cybernetics. 9 (1), 62-66 (1979).
- Solomon, C., et al. A comparison of fibrinogen measurement methods with fibrin clot elasticity assessed by thromboelastometry, before and after administration of fibrinogen concentrate in cardiac surgery patients. Transfusion. 51 (8), 1695-1706 (2011).
- Norouzi, N., Bhakta, H. C., Grover, W. H. Sorting cells by their density. PLoS One. 12 (7), 0180520 (2017).
- Trudnowski, R. J., Rico, R. C. Specific gravity of blood and plasma at 4 and 37 degrees C. Clinical Chemistry. 20 (5), 615-616 (1974).
- Késmárky, G., Kenyeres, P., Rábai, M., Tóth, K. Plasma viscosity: A forgotten variable. Clinical Hemorheology and Microcirculation. 39 (1-4), 243-246 (2008).
- Teece, L. J., et al. Gels under stress: The origins of delayed collapse. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 458, 126-133 (2014).
- Lindström, S. B., Kodger, T. E., Sprakel, J., Weitz, D. A. Structures, stresses, and fluctuations in the delayed failure of colloidal gels. Soft Matter. 8 (13), 3657-3664 (2012).
- Bartlett, P., Teece, L. J., Faers, M. A. Sudden collapse of a colloidal gel. Physical Review. E, Statistical, Nonlinear, and Soft Matter Physics. 85, 021404 (2012).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved