Intracranial Cannula Implantation for Serial Locoregional Chimeric Antigen Receptor (CAR) T Cell Infusions in Mice
In This Article
Summary
Central nervous system (CNS) tumors are the leading cause of cancer-related death in children, and locoregional immune-based therapies are increasingly being tested for patients in clinical trials. This protocol describes methods for locoregional cannula implantation in mice for the preclinical evaluation of immunotherapeutic infusions targeting CNS tumors.
Abstract
Pediatric CNS tumors are responsible for the majority of cancer-related deaths in children and have poor prognoses, despite advancements in chemotherapy and radiotherapy. As many tumors lack efficacious treatments, there is a crucial need to develop more promising therapeutic options, such as immunotherapies; the use of chimeric antigen receptor (CAR) T cell therapy directed against CNS tumors is of particular interest. Cell surface targets such as B7-H3, IL13RA2, and the disialoganglioside GD2 are highly expressed on the surface of several pediatric and adult CNS tumors, raising the opportunity to use CAR T cell therapy against these and other surface targets. To evaluate the repeated locoregional delivery of CAR T cells in preclinical murine models, an indwelling catheter system that recapitulates indwelling catheters currently being used in human clinical trials was established. Unlike stereotactic delivery, the indwelling catheter system allows for repeated dosing without the use of multiple surgeries. This protocol describes the intratumoral placement of a fixed guide cannula that has been used to successfully test serial CAR T cell infusions in orthotopic murine models of pediatric brain tumors. Following orthotopic injection and engraftment of the tumor cells in mice, intratumoral placement of a fixed guide cannula is completed on a stereotactic apparatus and secured with screws and acrylic resin. Treatment cannulas are then inserted through the fixed guide cannula for repeated CAR T cell delivery. Stereotactic placement of the guide cannula can be adjusted to deliver CAR T cells directly into the lateral ventricle or other locations in the brain. This platform offers a reliable mechanism for the preclinical testing of repeated intracranial infusions of CAR T cells and other novel therapeutics for these devastating pediatric tumors.
Introduction
Despite improvements in chemotherapy, radiotherapy, and surgery, central nervous system (CNS) tumors are the deadliest malignancy in pediatrics1, underscoring an important need for new approaches with more successful outcomes. With significant advances in the field of immunotherapy, adoptive cellular therapy (ACT) approaches have shown promising results in various cancers, especially hematological malignancies2. Chimeric antigen receptor (CAR) T cell therapy, a specific type of ACT, takes advantage of the immune system's natural ability to recognize and kill harmful cells by redirecting the specificity of T cells to generate tumor-targeting T cells3. CAR T cell therapy has demonstrated substantial success in the treatment of leukemias and lymphomas4, making it a promising immunotherapeutic approach and encouraging its investigation in solid tumors. However, thus far, CAR T cell therapy in solid tumors has achieved little clinical success and faces many challenges, such as inefficient tumor penetration, limited targetable antigens, and the suppressive tumor microenvironment5.
Recent clinical trials have begun evaluating CAR T cell therapy for pediatric CNS tumors, providing proof of concept and early evidence of T cell activity in preliminary reports6,7,8. While most initial preclinical data focused on intravenous delivery of the CAR T cells, recent preclinical evidence has suggested the superiority of locoregional delivery in the CNS9,10, which has also been utilized successfully in several clinical trials6,7,8,11. Preclinical studies to date that have incorporated the locoregional delivery of CAR T cells in the CNS have relied on a single intracranial dose of CAR T cells delivered stereotactically9,10. However, clinical trials in humans have required repeated infusions of CAR T cells in the CNS6,7,8,11, underscoring a need to evaluate multiple repeated infusions in preclinical development. The goal of this procedure is to successfully test serial CAR T cell infusions using a catheter in orthotopic murine models of pediatric brain tumors. The advantage of this technique is the avoidance of multiple surgical procedures to provide repeated intra-CNS treatments. Cannulas have primarily been used for microdialysis sampling of neurotransmitters and the delivery of neuroactive substances in neuroscience and behavioral research in rodents12, with limited reports of their use for the delivery of anti-cancer therapeutics. Building on the prior reports, this protocol uses a stereotactically placed indwelling cannula system to deliver CAR T cells in xenograft murine models of CNS tumors. The protocol can be utilized to test additional therapeutics in murine models of neurological or neuro-oncologic disorders, and may be helpful to test novel therapeutics where bypassing the blood-brain barrier is critical for efficacy.
Protocol
All protocol procedures were approved by the Institutional Animal Care and Use Committee of the Children's Hospital of Philadelphia (IAC 19-000907), which is accredited by the AAALAC. This study used 6-12-week-old NOD scid gamma (NSG) mice with orthotopic xenograft tumors; however, the protocol can be utilized on any mouse strain. NSG mice were housed in sterile barrier conditions and surgery performed under sterile biosafety cabinets. When human material such as tumor cells or T cells are being used, procedures and handling must be completed in ABSL-2 biosafety cabinets.
1. Preparing the mouse for surgery
- Anesthetize the mouse in an induction chamber with isoflurane (2-4%) at an oxygen flow rate of 1 L/min until an adequate plane of anesthesia is reached (approximately 5 min).
- Weigh the mouse using a scale to the nearest 0.1 g and administer subcutaneous slow-release (SR) buprenorphine (1 mg/kg) or other analgesic.
NOTE: SR buprenorphine provides analgesia for 72 h. - Shave the fur on the top of the mouse's head using electric clippers or depilatory agents.
- Use a spatula to gently open the bottom of the stereotactic arm and insert the guide cannula using forceps. Tighten the screw on the arm to secure the cannula so that approximately 1/2 to 2/3 of the white plastic portion of the cannula is protruding from the bottom of the opening, along with the entire 5 mm metal length of the cannula.
- Insert and secure the mouse's top teeth in the bite bar of the stereotaxic apparatus. Pull the nose cone forward and tighten it, ensuring the mouse is inhaling isoflurane.
- Mount the mouse on the warmed stereotaxic apparatus using ear cuffs or ear bars, avoiding excessive pressure.
NOTE: Warmed tray should have a rectal thermometer inserted, and the warming tray should adjust to maintain a normal body temperature of the mouse during the procedure. - Apply sterile ophthalmic ointment to both eyes using a cotton-tipped applicator.
- Wipe the surgical site with povidone-iodine on a pad or applicator, followed by an alcohol pad. Perform this step three times in total.
- Before beginning the procedure, perform a toe pinch with forceps to assess for adequate sedation.
2. Surgical procedure
NOTE: All aspects of the surgical procedure utilize sterilized instruments and aseptic techniques. The mice continue under anesthesia with isoflurane (2-4%) throughout the duration of the procedure, approximately 10-20 min.
- Gently pick up the scalp between the ears with forceps. Using sterile scissors, cut the lifted scalp parallel to the skull and remove an oval flap of skin (0.75-1 cm in length) to expose the skull.
NOTE: Scissors are preferred over a scalpel to provide a clean, oval-shaped opening and to prevent unnecessary damage to surrounding skin and tissue. - Push away fascia using a scalpel or cotton-tipped swabs and a hemostatic cotton pellet to help slow excessive bleeding as needed.
NOTE: Using the wooden side of a sterile cotton tip can also push away fascia and help avoid excessive bleeding. - Identify the landmarks bregma and lambda, the respective anterior and posterior marks on the skull where the cranial plates meet13.
NOTE: Identification can be augmented by wiping the top of the exposed skull with hydrogen peroxide. - Gently score the skull using a scalpel to create a surface for the acrylic to attach. Scoring should include multiple linear lines approximately 0.5-1 cm in length at 90° angles to each other.
- Using the stereotaxic arm, localize the cannula to the landmark of interest (bregma or lambda). Once localized, raise the cannula tip 1-2 mm above the skull surface and move to the desired coordinates. For intratumoral injections, this uses the same A/P and M/L coordinates as the tumor placement.
- On the exposed skull, away from the area where the cannula will enter, make two screw holes with an 18 G needle or a surgical drill. Ensure that the holes are spaced out to include enough room for the cannula. Using a drill bit, twist through the screw holes until they catch on the skull. Insert and fasten two screws into the holes using a flat-tipped screwdriver. Then, gently pull the screws up to ensure they are secured.
NOTE: Do not insert the screws until they are flush with the skull, or they may damage the mouse brain underneath. Leave at least a 1-2 mm gap between the screw and the skull. - Using an 18 G needle or surgical drill, drill through the skull at the identified coordinates to create a hole for the cannula to be inserted.
- Using the stereotactic arm, lower the cannula to the desired D/V coordinate.
NOTE: The D/V coordinate of cannula implantation needs to account for the projection length of the dummy and treatment cannulas, and may be more superficial than the orthotopic injection of tumor cells (Figure 1). - In a porcelain 12-well plate, fill one well with acrylic resin powder (approximately 0.3 g) and 10-15 drops (approximately 0.5-0.75 mL) of acrylic resin liquid. This produces a viscous white-colored material. Draw up the mixture into a 1 mL syringe and use it to coat and cover the skull, filling in the spaces around the cannula and screws.
NOTE: The viscous material hardens into cement over time, so this step should be completed promptly after mixing. - While the cement is still pliable, loosen the screw on the stereotactic arm and use a spatula in the opening at the bottom to gently release the cannula from the holder and slowly retract the stereotactic arm upward away from the mouse.
- Once the cement is completely dry, insert the dummy cannula into the guide cannula and tightly secure it by turning clockwise.
- Once the procedure is complete, place the mouse back in its warmed home cage to carefully monitor, ensuring adequate recovery and recording any post-procedure observations, including that the mouse has fully regained consciousness, before returning to the colony.
NOTE: It is generally recommended to place only half of the cage on a heating pad to allow for the animal to move to the cooler side to avoid overheating. - Administer additional analgesics as needed if the mice display behaviors indicative of pain post-operatively, such as meloxicam 5 mg/kg subcutaneously delivered once daily for up to 3 days.
3. Preparing CAR T cells
- Measure the pre-transfected CAR T cell concentration using a cell counter.
- Centrifuge pre-transfected T cells at 200 x g for 5 min at room temperature (RT).
- Aspirate the supernatant using a sterile Pasteur pipet on a vacuum aspiration system and resuspend the pellet in phosphatae buffered saline (PBS) to a desired concentration. Typical delivery volumes are 2-5 µL. Typical cell doses are 0.5-5 x 106 cells.
4. CAR T cell infusions
- Prepare the treatment cannula by feeding the top through a small piece of PKG tubing.
- Fill the treatment syringe with the CAR T cell suspension and insert it through the other end of the PKG tubing, enough to cover the top of the treatment cannula.
- Anesthetize the mouse with isoflurane (2-4%) at an oxygen flow rate of 1 L/min.
- Stabilize the guide cannula using forceps at the base, and then carefully unscrew and remove the dummy cannula, allowing for access into the guide cannula.
NOTE: A stereotaxic setup is not necessary, but can be used to stabilize the head for treatment. - Infuse the CAR T cells for 1 min and hold the treatment cannula in place for an additional 1 min following the cessation of infusion.
- Remove the treatment cannula and screw the dummy cannula tightly back in place.
- Administer subcutaneous meloxicam (5 mg/kg) for optional pain control.
Representative Results
Successful cannula implantation into the mouse CNS
Mice with successful cannulation have a secure guide cannula in place, that does not interfere with activities of daily living (Figure 2A) and that can easily pass a treatment cannula and deliver fluid without resistance (Figure 2B). In our experience, the majority of cannulas remain in place for over 4 weeks, although 0-25% can be dislodged over time. Verification of correct placement can be confirmed with Evans blue dye injected into the cannula. For example, a cannula inserted into the lateral ventricle shows Evans blue dye throughout the ventricular system as it travels through the cerebral spinal fluid, confirming correct placement (Figure 2C). Cannulas inserted into the tumor show Evans blue dye at the site of tumor implantation.
Effective delivery of CAR T cells into the CNS for treatment of xenograft tumors.
The efficacy of the intracranial cannula system and the therapeutic efficacy of CAR T cells in murine models can be measured through a variety of mechanisms, including bioluminescent imaging and overall survival. GPC2-directed CAR T cells were tested against murine medulloblastoma and diffuse midline glioma models, 7316-4509 and 7316-3058, respectively, using repeated CAR T cell dosing via the stated intracranial cannula system14. Orthotopic tumor placement and engraftment were confirmed by bioluminescent imaging, and cannulas were placed into the tumor bed using the same coordinates as the orthotopic tumor placement. The treatments consisted of GPC2 CAR T cell infusions once to twice a week for 2-4 weeks, totaling four to six doses. Following treatment, GPC2-directed CAR T cell therapy induced significant tumor regression in the medulloblastoma model 7316-4509 (p < 0.01) (Figure 3A) and significantly prolonged survival in thalamic diffuse midline glioma 7316-3058 (p < 0.05) (Figure 3B)14.
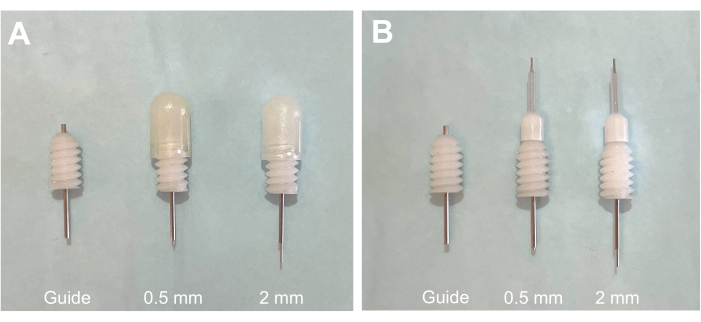
Figure 1: Guide cannula with projection dummy and treatment cannulas. (A) Guide cannula with 0.5 mm projection and 2 mm projection dummy cannulas in place. (B) Guide cannula with 0.5 mm projection and 2 mm projection treatment cannulas in place. Please click here to view a larger version of this figure.
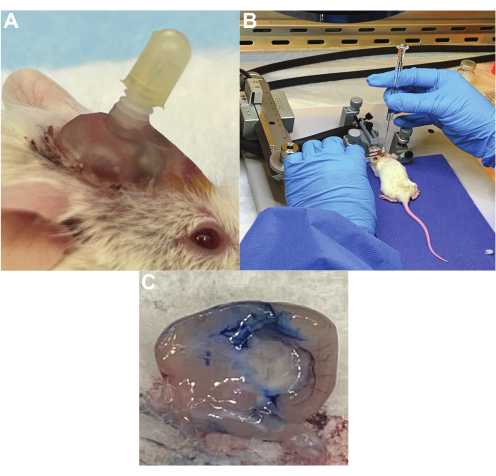
Figure 2: Treatment cannula delivery system for repeated doses of CAR T cells. (A) Cannula implanted in a mouse skull with a cap in place when not in use. (B) Infusion of CAR T cells into a pontine tumor via the treatment cannula while the mouse is under anesthesia. (C) Verification of cannula placement in the lateral ventricle using Evans blue dye. Dye was inserted through the cannula, followed by euthanasia and brain excision, with dye present throughout the ventricles. Please click here to view a larger version of this figure.
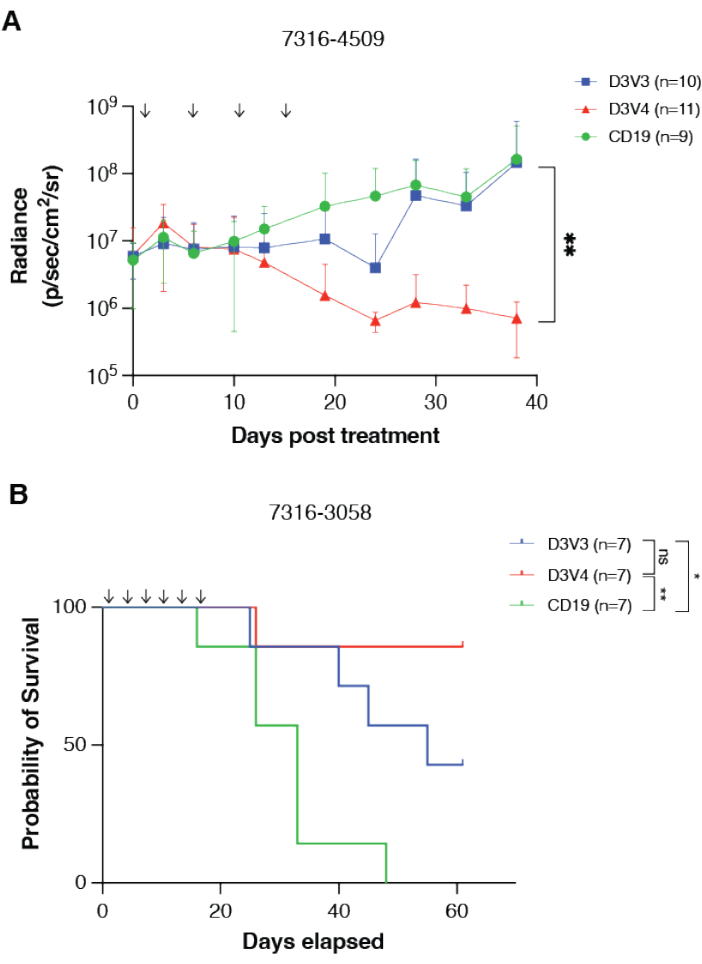
Figure 3: GPC2-directed CAR T cells mediate antitumor responses and prolong survival in pediatric brain tumors in vivo. (A) Quantification of bioluminescence of the orthotopic group 4 medulloblastoma xenograft 7316-4509 treated with either GPC2- or CD19-directed mRNA CAR T cells. Doses are indicated by arrows on the graph. Data displayed as mean with SD, n = 9-11 mice per arm. (B) Overall survival of mice implanted with thalamic DMG xenograft 7316-3058 treated with six repeated doses of 2 x 106 CAR T cells. Doses are indicated by arrows on the graph. n = 7 mice per arm. **p < 0.01; *p < 0.05; ns = not significant. This figure has been reproduced with permission from Foster et al.14. Please click here to view a larger version of this figure.
Discussion
CAR T cell therapy has revolutionized the treatment of hematological cancers and shows promising value in treating solid brain tumors6,7,8. This protocol was designed to enable the preclinical evaluation of locoregional CAR T cell delivery for the treatment of pediatric CNS tumors. The cannula system replicates an Ommaya or Rickham reservoir, an intraventricular catheter system currently being used in ongoing clinical trials of CAR T cell therapy in pediatric CNS tumors6,7,8, underscoring the relevance and translational potential of these methods. This system allows for the repeated delivery of CAR T cells that bypass the blood-brain barrier, again similar to methods being employed in ongoing clinical trials. Locoregional delivery can provide maximal efficacy in the CNS9 and may also reduce the risk of systemic toxicities associated with trafficking from circulation15. While stereotactic delivery can provide a single dose into the CNS, the advantage of this system is the opportunity to provide multiple repeated doses into a specified location in the CNS without the need for multiple surgeries. The limitations of this procedure include a fixed delivery site without the ability to change location or make adjustments once the cannula is in place, and the potential for dislodgement of the cannula.
A critical step in this protocol is the implantation of the fixed guide cannula at a D/V coordinate that takes into consideration the projection of the treatment cannulas. The treatment cannula will protrude beyond the tip of the guide cannula, and so care must be taken to ensure the placement will result in the delivery of CAR T cells to the region of interest. Projection lengths of the treatment cannula can be customized, and in the author's experience, 0.5 mm is a useful projection length. This length ensures that the therapeutic does not remain in the guide cannula upon dispensing, but also does not require significant adjustment of the D/V coordinates for the guide cannula to the region of interest. An additional important step in this protocol is the time the treatment cannula is left in place following CAR T cell infusion. The treatment cannula should be held in place for at least 1 min following the end of infusion, to prevent leaks and the loss of locoregional delivery of CAR T cell therapy.
Troubleshooting this method is straightforward, with most complications involving difficulty removing the dummy cannula or inserting the treatment cannula into the fixed guide cannula, likely due to dried blood on the interior of the guide cannula. This can be easily resolved by gently passing the dummy cannula through the guide cannula until there is less resistance and the debris has been cleared. The acrylic resin can occasionally dislodge from the skull, resulting in the loss of the cannula system. In our experience, this is generally limited by scoring the skull with a scalpel and the placement of two screws. In addition, any items from the cage that may accidentally apply force to the cannula while the mouse is moving around are removed, such as particular mouse enrichment huts with small openings.
In conclusion, described here is a protocol for the insertion of a cannula system in murine models of CNS tumors for the repeated delivery of CAR T cells. Cannula placement can be adjusted to multiple locoregional delivery locations, testing the efficacy of different delivery sites. In addition, this system can be used for additional therapeutics beyond CAR T cells to evaluate efficacy when bypassing the blood-brain barrier, and may also be useful for the evaluation of therapeutics in murine models of non-oncologic disorders.
Acknowledgements
Funding for this work was provided by the Matthew Larson Foundation, Grayson Saves Foundation, Hyundai Hope on Wheels Young Investigator Award, Kortney Rose Foundation, National Institutes of Health NCI K12 CA076931-19 and 1K08CA263179-01, and the Department of Defense W81XWH-21-1-0221.
Materials
| Name | Company | Catalog Number | Comments |
| 18 G needles | BD | 511097 | 1 1/2 inch metal hub |
| Acrylic resin liquid | Lang Dental | B1323 | |
| Acrylic resin powder | Lang Dental | B1323 | |
| Alcohol wipes | BD | 326895 | |
| Centrifuge 5240 | Eppendorf | 5420000040 | Centrifuge |
| Cotton tipped swabs | Puritan | 826-WC | Handle Width = 2.11 mm (0.083), Head Width = 1.27 mm (0.050), Handle Length = 147.62 mm (5.812), Overall Length = 152.4 mm (6), Head Length = 12.7 mm (0.500) |
| Drill bit holder | P1 Technologies | DH-1 | Drill bit holder for D56-D70 |
| Drill bit | P1 Technologies | D58 | 1.07 mm |
| Dummy cannula | P1 Technologies | C315DCS-5/SPC | Configuration: Small cap; Length: Cut 5.00 mm below pedestal; Projection: 0.50 mm |
| Flat tip screwdriver | P1 Technologies | SD-80 | Screwdriver |
| Graefe forceps | Fine Science Tools | 11051-10 | Forceps |
| Guide cannula | P1 Technologies | C315GS-5/SPC | Configuration: 5.00 mm pedestal height; Length: Cut 5.00 mm below pedestal |
| Hemostatic cotton pellets with racemic epinephrine | Pascal | 1151602 | |
| MOXI Z Mini automated cell counter Kit | Moxi | MXZ001 | Cell counter |
| NOD scid gamma (NSG) mice | Jackson Laboratory | 5557 | 6 to 12-week-old males and females |
| Pasteur pipet | VWR | 14673-043 | |
| PKG tubing | P1 Technologies | C313CT | Diameter: 0.58 mm x 1.27 mm |
| Porcelain 12 well plate | Flinn Scientific | AP6064 | |
| Povidone iodine | Medline | MDS093943 | |
| Scalpel | World Precision Instrument | 50-822-457 | Disposable Scalpel, no.10, sterile, 10/box, Plastic Handle with 6" Ruler |
| Screws | P1 Technologies | 0-80 X 3/32 | 2.4 mm |
| Stereotaxic Frame | David Kopf Instruments | 940 | Model 940 Small Animal Stereotaxic Instrument with Digital Display Console |
| Student fine scissors | Fine Science Tools | 91460-12 | Scissors |
| Treatment cannula | P1 Technologies | C315IS-5/SPC | 33GA; Configuration: Standard internal; Length: Cut 5.00 mm below pedestal; Projection: 0.50 mm |
| Treatment syringes | Hamilton | 87908 | 5 µL, Model 75 Cemented Needle Special (SN) Syringe, 75SN/22/0.5"/PT3 |
| Vactrap XL | Foxx Life Sciences | 305-4401-FLS | Vacuum System |
References
- Curtin, S. C., Minino, A. M., Anderson, R. N. Declines in cancer death rates among children and adolescents in the United States, 1999-2014. NCHS Data Brief. 257, 1-8 (2016).
- Rohaan, M. W., Wilgenhof, S., Haanen, J. B. A. G. Adoptive cellular therapies: the current landscape. Virchows Archiv. 474 (4), 449-461 (2019).
- Sadelain, M., Brentjens, R., Riviere, I. The basic principles of chimeric antigen receptor design. Cancer Discovery. 3 (4), 388-398 (2013).
- Maude, S. L., et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. The New England Journal of Medicine. 378 (5), 439-448 (2018).
- Wagner, J., Wickman, E., DeRenzo, C., Gottschalk, S. CAR T cell therapy for solid tumors: Bright future or dark reality. Molecular Therapy. 28 (11), 2320-2339 (2020).
- Vitanza, N. A., et al. Locoregional infusion of HER2-specific CAR T cells in children and young adults with recurrent or refractory CNS tumors: an interim analysis. Nature Medicine. 27 (9), 1544-1552 (2021).
- Majzner, R. G., et al. GD2-CAR T cell therapy for H3K27M-mutated diffuse midline gliomas. Nature. 603 (7903), 934-941 (2022).
- Vitanza, N. A., et al. Intraventricular B7-H3 CAR T cells for diffuse intrinsic pontine glioma: preliminary first-in-human bioactivity and safety. Cancer Discovery. 13 (1), 114-131 (2023).
- Theruvath, J., et al. Locoregionally administered B7-H3-targeted CAR T cells for treatment of atypical teratoid/rhabdoid tumors. Nature Medicine. 26 (5), 712-719 (2020).
- Donovan, L. K., et al. Locoregional delivery of CAR T cells to the cerebrospinal fluid for treatment of metastatic medulloblastoma and ependymoma. Nature Medicine. 26 (5), 720-731 (2020).
- Brown, C. E., et al. Regression of glioblastoma after chimeric antigen receptor T-Cell therapy. The New England Journal of Medicine. 375 (26), 2561-2569 (2016).
- Bourne, J. A. Intracerebral microdialysis: 30 years as a tool for the neuroscientist. Clinical and Experimental Pharmacology and Physiology. 30 (1-2), 16-24 (2003).
- Zhou, P., et al. Automatically detecting bregma and lambda points in rodent skull anatomy images. PLoS One. 15 (12), 0244378 (2020).
- Foster, J. B., et al. Development of GPC2-directed chimeric antigen receptors using mRNA for pediatric brain tumors. Journal for Immunotherapy of Cancer. 10 (9), 004450 (2022).
- Akhavan, D., et al. T cells for brain tumors: Lessons learned and road ahead. Immunological Reviews. 290 (1), 60-84 (2019).
This article has been published
Video Coming Soon
ABOUT JoVE
Copyright © 2024 MyJoVE Corporation. All rights reserved