A subscription to JoVE is required to view this content. Sign in or start your free trial.
Generation of 3D Tumor Spheroids for Drug Evaluation Studies
* These authors contributed equally
In This Article
Summary
This article demonstrates a standardized method for constructing three-dimensional tumor spheroids. A strategy for spheroid observation and image-based deep-learning analysis using an automated imaging system is also described.
Abstract
In recent decades, in addition to monolayer-cultured cells, three-dimensional tumor spheroids have been developed as a potentially powerful tool for the evaluation of anticancer drugs. However, the conventional culture methods lack the ability to manipulate the tumor spheroids in a homogeneous manner at the three-dimensional level. To address this limitation, in this paper, we present a convenient and effective method of constructing average-sized tumor spheroids. Additionally, we describe a method of image-based analysis using artificial intelligence-based analysis software that can scan the whole plate and obtain data on three-dimensional spheroids. Several parameters were studied. By using a standard method of tumor spheroid construction and a high-throughput imaging and analysis system, the effectiveness and accuracy of drug tests performed on three-dimensional spheroids can be dramatically increased.
Introduction
Cancer is one of the diseases most feared by human beings, not least because of its high mortality rate1. In recent years, the possibility of treating cancer has increased as new therapies have been introduced2,3,4,5. Two-dimensional (2D) and three-dimensional (3D) in vitro models are used to study cancer in a laboratory setting. However, 2D models cannot immediately and accurately assess all of the important parameters that indicate antitumor sensitivity; therefore, they fail to fully represent in vivo interactions in drug therapy testing6.
Since 2020, the global three-dimensional (3D) culture market has been greatly boosted. According to one report from NASDAQ OMX, the global value of the 3D cell culture market will exceed USD 2.7 billion by the end of 2025. Compared with 2D culture methods, 3D cell culturing exhibits advantageous properties, which can be optimized not only for proliferation and differentiation but also for long-term survival7,8. By such means, in vivo cellular microenvironments can be simulated to obtain more accurate tumor characterization, as well as metabolic profiling, so that genomic and protein alterations can be better understood. Due to this, 3D test systems should now be included in mainstream drug development operations, especially those with a focus on screening and evaluating novel antitumor drugs. Three-dimensional growths of immortalized established cell lines or primary cell cultures in spheroid structures possess in vivo features of tumors such as hypoxia and drug penetration, as well as cell interaction, response, and resistance, and can be regarded as a stringent and representative model for performing in vitro drug screening9,10,11.
However, these 3D culture models also suffer from several problems that may take some time to solve. Cell spheroids can be formed using these protocols, but they differ in certain details, such as culture time or embedding gels12, so these constructed cell spheroids cannot be well controlled under a restricted size range. The size of the spheroids may influence the consistency of the viability test and imaging analysis. The growth microenvironments and growth factors also vary, which may lead to different morphologies due to differences in the differentiation among cells13. There is now an obvious need for a standard, simple, and cost-effective method for constructing all types of tumors with controlled sizes.
From another perspective, although homogeneous assays and high-content imaging approaches have been developed to evaluate morphology, viability, and growth rate, the high-throughput screening of 3D models remains a challenge for various reasons reported in the literature, such as the lack of uniformity in the position, size, and morphology of tumor spheroids14,15,16.
In the protocol presented here, we list each step in the construction of 3D tumor spheroids and describe a method for spheroid observation and analysis using a high-throughput, high-content imaging system that involves auto-focus, auto-imaging, and analysis, among other advantageous characteristics. We show how this method can produce 3D tumor spheroids of uniform size that are suitable for high-throughput imaging. These spheroids also demonstrate a high sensitivity to cancer drug treatment, and morphological changes in the spheroids can be monitored using high-content imaging. In summary, we demonstrate the robustness of this methodology as a means to generate 3D tumor constructs for drug evaluation purposes.
Protocol
1. Spheroid construction
- Anti-adhesion treatment of the culture plate
- Pipette 100 µL of anti-adhesion reagent into each well of a 48-well plate with a U-shape well bottom, and keep for 10 min. After 10 min, aspirate the coating reagent, and wash twice with sterilized PBS.
- Put the culture plate in an incubator (37 °C in humidified air with 5% CO2) until use.
- Cell preparation, collection, and counting
- Use the culture medium specific to the cells to culture the cells in cell-culture flasks (Supplementary Table 2). For example, NCI-H23, CT-26 cells are cultured in RPMI 1640, and HT-29 cells are cultured in McCoy's 5A medium. These two media are supplemented with 10% heat-inactivated FBS and 1% P/S, respectively.
- Maintain all the cells in standard culture conditions (37 °C in humidified air with 5% CO2) during proliferation. Here, the NCI-H23 cell line is used as an example in the following steps.
- Wash cells cultured in a T25 flask twice with 1x PBS to remove the culture medium (it is better to choose cells in the logarithmic phase and passage the cells at a confluency of 80%-90%).
- Treat the expanded cells with 1 mL of 0.25% trypsin/EDTA for 1- 2 min in an incubator at 37 °C, 5% CO2. Confirm the cell shape (normally circular in this case) under the microscope, and then stop the trypsin treatment. To do this, aspirate the used trypsin/EDTA suspension in the T25 flask, and wash the cells with 4 mL of fresh medium.
- Transfer all of the suspension (5 mL) to a 15 mL tube. Use 1 mL of fresh medium to wash down the residual cells and add it to the tube. Centrifuge the cells at 186.48 x g for 5 min at room temperature.
- Remove the supernatant and add 10 mL fresh medium to the cell pellet, followed by gentle pipetting until the cells are in a homogenous suspension.
- Aspirate 0.1 mL of cell suspension into a new centrifuge tube, add 0.9 mL of fresh medium, and then pipette the suspension well.
- Extract 10 µL of the cell suspension for cell counting. Carry out this process two or three times and take an average value.
- Dilute the suspension to reach a final seeding density of 50,000 cells/mL, according to the concentration obtained from the cell counting process in step 1.2.7.
- Cell culture and spheroid formation
- Add 200 µL of the cell suspension to each well of a 48-well U-bottom plate.
- Wrap the sealing film around the plate and centrifuge it at 119.35 x g for 5 min at room temperature.
- Carefully take the plate out of the centrifuge and pull off the protective film. Then, add 5-8 mL of sterilized water into the water channel surrounding the wells (to prevent evaporation) and incubate at 37 °C for 5 days. Do not change/supplement any water to the water channel during the period.
- Observe the cell aggregation during the following 5 days.
NOTE: In general, the cells start to clump as colonies within 5 days. However, the process of spheroid construction may be quicker or slower with different cell types and cell densities. Due to this, the cells must be observed every day using one of three possible methods. First, the cells can be observed through the bottom of the well plate. When the cells have yet not formed a spheroid, a single layer of cells can be seen at the bottom. When the cells form a spheroid, a dense 3D construct can be observed at the U-bottom of each well. Another method involves checking the cells under a microscope. When the cells become a tumor spheroid, the construction involves three layers (a proliferating layer, an inactive layer, and a necrotic core, from the outside to the inside of the spheroid), which have a transparency gradient. Finally, the color of the culture medium can also be used for observational purposes. This can be helpful when the three-layer structure cannot be clearly seen, even using a digital microscope. When the medium turns from purple-red to yellow, the process of embedding the spheroids in the gel can begin. The medium should not be replaced during the cell aggregation period.
- Gel embedding
NOTE: The gels need to be stored at a temperature below −20 °C. In particular, gels should be placed far away from the fridge door to avoid temperature fluctuations. Note that the gels are in a frozen state at this stage of the process.- Take the frozen gel from the −20 °C fridge and place it on an ice box for the whole time during the experiment.
- Observe the cell spheroids under the microscope. Before the gel embedding begins, the status of the spheroids should again be checked with a digital microscope.
- Carefully remove 150 µL of the medium. The plate should also be placed on the ice box.
- Embed each spheroid in the gel by adding the liquid gel slowly from the wall side of the well while moving the pre-cooled pipette tip around and inside the well. Wait for 5 min and if the gel does not spread evenly, gently pipette the gel with a 10 µL pipette tip. Each well contains a tumor spheroid, 25 µL of 3.5 mg/mL gel, and 50 µL of complete culture medium. Add 75 µL of medium to the controls as well.
NOTE: Each well contains one spheroid. - Incubate the plate at 37 °C for 30 min until hydrogelation is fully completed. Confirm the gelation status under the microscope.
- Overlay 125 µL of the fresh medium on each sample.
- Culture the spheroids for another 7-10 days. Prepare groups of spheroids with four to six wells each and choose at least three of them for analysis.
NOTE: If performing a drug test, prepare two groups for one sample. One group is used for viability testing, while the other is used for image capturing and analysis.
2. Drug treatment
- Dissolve the drug according to the manufacturer's instructions. Prepare 100x working solutions with DMSO. Prepare at least five doses of the drug in serial dilution. Here, the lung cancer therapeutical drug, AMG 510, is used as an example. The composition is shown in Supplementary Table 1.
- Use 0.1% DMSO as a positive control.
- Add 125 µL of drug-treated medium to each well and place the plate back into the incubator (37 °C in humidified air with 5% CO2). At this stage, each well contains a tumor spheroid, 25 µL of 3.5 mg/mL gel, and 175 µL of the medium. The controls contain 200 µL of the medium.
3. Spheroid viability
- Measure the spheroid viability using an Alamar Blue assay kit according to the manufacturer's guidelines. Measure the viability using a microplate photometer (absorbance at 570 nm and 600 nm) after Alamar Blue treatment is carried out.
- Measure the viability at day 1, day 4, day 7, and day 10 after embedding the spheroids in the gel, respectively, or as indicated.
NOTE: When using an Alamar Blue assay, at least 16 h of reacting time is required. Therefore, add 20 µL of Alamar Blue in the afternoons of day 0, day 3, day 6, and day 9. - Aspirate 100 µL of the supernatant medium from each well to a new test plate and add 80 µL of fresh medium to each well of the culture plate. Then, replace another 100 µL of the drug-treated medium. Ensure there are no remains of Alamar Blue in the well.
NOTE: Medium replacement is carried out on each occasion that the viability is tested, and the drug-treated medium of the imaging-analysis groups needs to be replaced on the same day.
4. Spheroid observation and deep learning analysis through images in the drug test
- Imaging
- Aspirate 100 µL of the medium out before imaging.
- Place the plate on the stage. Obtain digital images of the spheroids using an automated microscope with a 10x objective (2x objective first). The microscope can focus and centralize these spheroids automatically.
NOTE: The autofocus function and the algorithm used have been previously reported by Yazdanfar et al.17. - Wait for the automatic imaging. Four images are acquired for each spheroid. An integrated image is formed and processed with the software connected to the high-content imaging system.
- Click on the "Image patch process" button and choose the integrated images in the software.
- Choose "U-NET model", and type in the conversion rate (10x objective images have a conversion rate of 3.966). Click at the bottom of the screen below, to start the image processing. Then, save the diameter, perimeter, and roughness data in spreadsheet software.
- Calculate the excess perimeter index (EPI). The EPI is the ratio between the spheroid perimeter (P0) and the equivalent perimeter (Pe), as calculated by Eq. 1. Measure the area of the spheroid at the focal plane (S) using Image J. Then, calculate the equivalent perimeter of the spheroid using Eq. 2.
EPI = (Po− Pe)/Pe (Eq. 1)
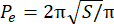 (Eq. 2)
(Eq. 2)
NOTE: The spheroid perimeter is generated by the software with deep-learning algorithms based on the developed U-NET model. - Add 100 µL of fresh medium with the drug and place the plate back into the incubator (37 °C in humidified air with 5% CO2).
- Spheroid inhibition
- Calculate the tumor growth inhibition (TGI) using Eq. 3. The relative spheroid volume (RTV) is the terminal volume over the original volume (Eq. 4). The spheroid volume (V) is calculated automatically by the software using Eq. 5 according to the spheroid diameter output.
TGI = (RTVcontrol− RTVtreatment)/RTVcontrol × 100% (Eq. 3)
RTV = Vterminal/Voriginal (Eq. 4)
V = 4/3π(d/2)3 (Eq. 5)
NOTE: The TGI is obtained with reference to in vivo growth inhibition, and the tumor weights are replaced with RTV. RTVcontrol is the relative spheroid volume of the control group, RTVtreatment is the relative spheroid volume of the drug tested group, Vterminal is the volume of the spheroid on the last day, Voriginal is the volume of the spheroid on the first culture day, and d represents the spheroid diameter.
- Calculate the tumor growth inhibition (TGI) using Eq. 3. The relative spheroid volume (RTV) is the terminal volume over the original volume (Eq. 4). The spheroid volume (V) is calculated automatically by the software using Eq. 5 according to the spheroid diameter output.
Results
Figure 1A,B shows the process used for constructing tumor spheroids in this study. We first seeded the cells in a 48-well U-bottom plate. This step is almost the same as that used in 2D cell culture. We kept the plate in a common incubator with water surrounding the wells so that the deposited cells started to form spheroids in a self-assembly process. Under normal operational conditions, most types of tumor spheroids were completely formed after 5 days when a targeted mediu...
Discussion
The microenvironment plays an important role in tumor growth. It may affect the provision of extracellular matrices, oxygen gradients, nutrition, and mechanical interaction and, thus, affect gene expression, signal pathways, and many functions of tumor cells19,20,21. In many cases, 2D cells do not produce such effects or even produce opposite effects, thus affecting the evaluation of drug treatments. However, the emergence of 3D...
Disclosures
The authors have nothing to disclose.
Acknowledgements
We thank all the members of our laboratories for their critical input and suggestions. This research was supported by the Key Project of Jiangsu Commission of Health (K2019030). Conceptualization was conducted by C.W. and Z.C., the methodology was performed by W.H. and M.L., the investigation was performed by W.H. and M.L., the data curation was performed by W.H., Z.Z., S.X., and M.L., the original draft preparation was performed by Z.Z., J.Z., S.X., W.H., and X.L., the review and editing was performed by Z.C., project administration was performed by C.W. and Z.C., and funding acquisition was conducted by C.W. All the authors have read and agreed to the published version of the manuscript.
Materials
| Name | Company | Catalog Number | Comments |
| 0.5-10 μL Pipette tips | AXYGEN | T-300 | |
| 1.5 mL Boil proof microtubes | Axygen | MCT-150-C | |
| 100-1000μL Pipette tips | KIRGEN | KG1313 | |
| 15 mL Centrifuge Tube | Nest | 601052 | |
| 200 μL Pipette tips | AXYGEN | T-200-Y | |
| 3D gel | Avatarget | MA02 | |
| 48-well U bottom Plate | Avatarget | P02-48UWP | |
| 50 mL Centrifuge Tube | Nest | 602052 | |
| Alamar Blue | Thermo | DAL1100 | |
| Anti-Adherence Rinsing Solution | STEMCELL | #07010 | |
| Certified FBS | BI | 04-001-1ACS | |
| Deionized water | aladdin | W433884-500ml | |
| DMEM (Dulbecco's Modified Eagle Medium) | Gibco | 11965-092 | |
| DMSO | sigma | D2650-100ML | |
| Excel sofware | Microsoft office | ||
| Graphpad prism sofware | GraphPad software | ||
| High Content Microscope and SMART system | Avatarget | 1-I01 | |
| Image J software | National Institutes of Health | ||
| Insulin-Transferrin-Selenium-A Supplement (100X) | Gibco | 51300-044 | |
| Parafilm | Bemis | PM-996 | |
| PBS | Solarbio | P1020 | |
| Penicillin/streptomycin Sol | Gibco | 15140-122 | |
| RPMI 1640 | Gibco | 11875-093 | |
| Scientific Fluoroskan Ascent | Thermo | Fluoroskan Ascent | |
| T25 Flask | JET Biofil | TCF012050 | |
| Trypsin, 0.25% (1X) | Hyclone | SH30042.01 |
References
- Carioli, G., et al. European cancer mortality predictions for the year 2021 with focus on pancreatic and female lung cancer. Annals of Oncology. 32 (4), 478-487 (2021).
- Katti, A., Diaz, B. J., Caragine, C. M., Sanjana, N. E., Dow, L. E. CRISPR in cancer biology and therapy. Nature Reviews Cancer. 22 (5), 259-279 (2022).
- Abrantes, R., Duarte, H. O., Gomes, C., Walchili, S., Reis, C. A. CAR-Ts: New perspectives in cancer therapy. FEBS Letter. 596 (4), 403-416 (2022).
- Shokooohi, A., et al. Effect of targeted therapy and immunotherapy on advanced nonsmall-cell lung cancer outcomes in the real world. Cancer Medicine. 11 (1), 86-93 (2022).
- Chen, K., Zhang, Y., Qian, L., Wang, P. Emerging strategies to target RAS signaling in human cancer therapy. Journal of Hematology & Oncology. 14 (1), 116 (2021).
- Pinto, B., Henriques, A. C., Silva, P. M. A., Bousbaa, H. Three-dimensional spheroids as in vitro preclinical models for cancer research. Pharmaceutics. 12 (12), 1186 (2020).
- Jensen, C., Teng, Y. Is it time to start transitioning from 2D to 3D cell culture. Frontiers in Molecular Biosciences. 7, 33 (2020).
- Qin, Y., Hu, X., Fan, W., Yan, J. A stretchable scaffold with electrochemical sensing for 3D culture, mechanical loading, and real-time monitoring of cells. Advanced Science. 8 (13), 2003738 (2021).
- Wartenberg, M., et al. Regulation of the multidrug resistance transporter P-glycoprotein in multicellular tumor spheroids by hypoxia-inducible factor (HIF-1) ad reactive oxygen species. FASEB Journal. 17 (3), 503-505 (2003).
- Minchinton, A. I., Tannock, I. F. Drug penetration in solid tumours. Nature Reviews Cancer. 6 (8), 583-592 (2006).
- Baker, B. M., Chen, C. S. Deconstructing the third dimension: How 3D culture microenvironments alter cellular cues. Journal of Cell Science. 125 (13), 3015-3024 (2012).
- Brüningk, S. C., Rivens, I., Box, C., Oelfke, U., Ter Haar, G. 3D tumour spheroids for the prediction of the effects of radiation and hyperthermia treatments. Scientific Reports. 10, 1653 (2020).
- Graves, E. E., Maity, A., Thu Le, Q. The tumor microenvironment in non-small-cell lung cancer. Seminars in Radiation Oncology. 20 (3), 156-163 (2010).
- Kunz-Schughart, L. A., Frreyer, J. P., Ebner, R. The use of 3-D cultures for high-throughput screening: The multicellular spheroid model. Journal of Biomolecular Screening. 9 (4), 273-285 (2004).
- Carragher, N., et al. Concerns, challenges and promises of high-content analysis of 3D cellular models. Nature Review Drug Discovery. 17 (8), 606 (2018).
- Huang, Y., et al. Longitudinal morphological and physiological monitoring of three-dimensional tumor spheroids using optical coherence tomography. Journal of Visualized Experiments. (144), e59020 (2019).
- Yazdanfar, S., et al. Simple and robust image-baed autofocusing for digital microscopy. Optics Express. 16 (12), 8670-8677 (2008).
- Chen, Z., et al. Automated evaluation of tumor spheroid behavior in 3D culture using deep learning-based recognition. Biomaterials. 22 (272), 120770 (2021).
- Boucherit, N., Gorvel, L., Olive, D. 3D tumor models and their use for the testing of immunotherapies. Frontiers in Immunology. 11, 603640 (2020).
- Anastasiou, D., et al. Microenvironment factors shaping the cancer metabolism landscape. British Journal of Cancer. 116 (3), 277-286 (2017).
- Zhou, H., et al. Functions and clinical significance of mechanical tumor microenvironment: Cancer cell sensing, mechanobiology and metastasis. Cancer Communications. 43 (5), 374-400 (2022).
- Zhu, G. G., et al. Targeting KRAS cancers: From druggable therapy to druggable resistance. Molecular Cancer. 21 (1), 159 (2022).
- Ando, Y., Mariano, C., Shen, K. Engineered in vitro tumor models for cell-based immunotherapy. Acta Biomaterialia. 132, 345-359 (2021).
- Timmins, N. E., Dietmair, S., Nielsen, L. K. Hanging-drop multicellular spheroids as a model of tumor angiogenesis. Angiogenesis. 7 (2), 97-103 (2004).
- Costa, E. C., et al. 3D tumor spheroids: An overview on the tools and techniques used for their analysis. Biotechnology Advances. 34 (8), 1427-1441 (2016).
- Sant, S., Johnston, P. A. The production of 3D tumor spheroids for cancer drug discovery. Drug Discovery Today. Technologies. 23, 27-36 (2017).
- Zanoni, M., et al. 3D tumor spheroid models for in vitro therapeutic screening: A systematic approach to enhance the biological relevance of data obtained. Scientific Reports. 6, 19103 (2016).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved