Simultaneous Detection of Different Antibody Classes in a Multiplexed Serological Test
* These authors contributed equally
In This Article
Summary
A three-channel dual-reporter fluorescence flow analysis system was used to develop a bead-based multiplex immunoassay that simultaneously evaluates serum samples for IgG and IgM elicited against multiple antigens of different Borrelia species that cause Lyme borreliosis in Europe and North America.
Abstract
To monitor the progression of infectious diseases, it is useful to assess immunoreactivity against various antigenic determinants, and measure different antibody isotypes because they appear at different stages of the host immune response. With Lyme borreliosis, the pathogenic agent can be one of the multiple members of the Borrelia species. Therefore, correct sample classification requires evaluating the immunoreactivity against different antigens of different Borrelia species. Additionally, anti-pathogen IgG and IgM responses can have different elicitation time courses during disease progression. Here we demonstrate the development of a two-reporter multiplex immunoassay that has utility in identifying Borrelia-specific immune response in human serum samples by simultaneously evaluating both IgG and IgM immunoreactivity against different bacterial antigens in the same reaction well. This dual-reporter approach retains the analytical performance of single-reporter methods while conserving time and resources and reducing sample size requirements. This assay allows essentially double the serological information to be generated from a blood sample in half the time.
Introduction
Lyme borreliosis is the most common tick-borne infectious disease in moderate climates of the northern hemisphere1. It is caused by spirochete bacteria of the genus Borrelia, with five known human pathogens that vary in geographical distribution2. The main pathogenic Borrelia species in Europe are B. afzelii and B. garinii, with B. burgdorferi s.s., B. spielmanii and B. bavariensis implicated less frequently. In North America, B. burgdorferi s.s. is the sole causative agent of Lyme borreliosis2,3. Borrelia pathogens are transmitted by members of the tick genus Ixodes, with transmission able to occur within 24 hours of tick bite4.
Lyme borreliosis diagnosis is typically made by clinical symptoms and subsequently confirmed by serology. In both Europe and North America, diagnostic guidelines recommend a two-step testing series consisting of an enzyme-linked immunosorbent assay (ELISA) with a reflex immunoblot to evaluate antibody response against Borrelia specific antigens1,5,6,7,8,9. However, this approach lacks sensitivity and is suboptimal, particularly in the early phase of infection when seroconversion may be incomplete and anti-Borrelia IgG and IgM titers are too low6.
Multiplex immunoassays improve upon traditional immunoassays that measure only one target at a time, and can simultaneously evaluate multiple antibody isotype responses against one or more antigens10,11,12. Assays such as ELISAs are limited to identifying and quantifying a single analyte per reaction, in the current case either circulating IgG or IgM induced against a single bacterial antigen after infection with Borrelia. This report illustrates using bead-based analyte profiling technology for the development of a multiplex immunoassay that simultaneously detects both IgG and IgM antibodies against any of the herein chosen Borrelia antigens in human serum samples. We selected four antigens that together cover the most common pathogenic Borrelia species native in Europe (B. garinii, B. afzelii, B. burgdorferi s.s.) and North America (B. burgdorferi s.s.) (Table 1)2,3. This allows decisive pathogen identification and the ability to discern early IgM and later, more durable, IgG immunoreactivity in patient samples.
| Target Isotype | Reporter Channel | Antigen | Borrelia Species | Strain | Antigen Coupling Concentration |
| IgM | PE | OspC | B. garinii | 20047 | 5.0 µg/106 beads |
| IgG | BV421 | VlsE | B. burgdorferi s.s | B31 | 1.25 µg/106 beads |
| IgG | BV421 | DbpA | B. burgdorferi s.s. | ZS7 | 10.0 µg/106 beads |
| IgG | BV421 | DbpA | B. afzelii | PKo | 5.0 µg/106 beads |
Table 1: Representative Borrelia antigens used for multiplex assay development.
We initially developed a single-reporter immunoassay that detected either anti-Borrelia antigen IgG or IgM antibodies in two separate reactions and then merged these assays into a dual-reporter multiplex assay that measures both antibody isotypes in the same reaction mixture. Magnetic beads are coupled to a pathogen target antigen of interest, and then incubated with patient serum samples. The bead-coupled antigen gets recognized by, and captures, both circulating IgG and IgM in serum that is generated in an immune response against that pathogen antigen. Assay IgG versus IgM specificity is determined by the selection of a secondary antibody that binds to either IgG or IgM, each with a distinct fluorophore signal associated with the two secondary antibodies. Each fluorescent signal is detected in one of the instrument's two Reporter Channels (i.e., dual-reporter) that has a different excitation laser and emission capture specific for the single fluorophore used to detect either IgG or IgM (here Brilliant Violet 421 or phycoerythrin, respectively). The instrument Classification Channel identifies the color-coded dye that is inherent to different bead sets. Thus, multiple target antigens can be coupled to differently dyed beads, and the bead sets mixed together and used to comprehensively assess diverse anti-pathogen immunoreactivity in serum samples. The Classification Channel identifies each individual bead set (i.e., specific antigen) and measures the fluorescence associated with IgG or IgM against that antigen. The resulting multiplex assay saves time and resources versus less comprehensive classical testing and accurately catalogs Lyme immunoreactivity in limited sample volumes. Whereas a similar dual-reporter approach has been previously used to categorize immune responses in other pathologies such as SARS-CoV-2 infection13, this report details the application of multiplex fluorescent assay technology for characterizing immunoreactivity in Lyme borreliosis.
Protocol
Appropriate Institutional Review Board/Ethics Committee approval was received for the use of human serum samples in this experimental series. Samples were anonymized residual materials from a German national study of SARS-CoV-2 seroprevalence14. Approval for the use of human samples was granted by the Ethics Committee of the Hannover Medical School, Germany (9086_BO_S_2020). A total of 21 human serum samples were used in the current study.
1. Ethical approval for the use of human samples
- Obtain appropriate ethical approvals for the use of human samples.
2. Reagents and equipment
- Human serum samples: Perform technical assay validation and quality control using serum samples from persons previously diagnosed with Lyme borreliosis or from demonstrated Borrelia-negative immune status12,14.
- Single-Reporter Instrumentation. Use a two-channel single-reporter instrument (Table of Materials) for the initial assay development and technical validation.
NOTE: The single-reporter system has two lasers: 1) identifies and quantifies bead set-specific fluorescence to discriminate different bead sets, if used (Classification Channel), and 2) detects and quantifies bead-bound target-specific phycoerythrin (PE) fluorescence (Reporter Channel; 532 nm excitation, "orange" 565-585 nm emission). Thus, only a single antibody isotype class (e.g., either IgG or IgM) can be analyzed at one time using the single Reporter Channel.- Perform initial validation studies to assess anti-Borrelia IgG and IgM separately, in a single-reporter 96-well format that uses PE-labeled detection reagents for both antibody classes.
NOTE: The current assay evaluates circulating IgGs specific for Borrelia antigen VlsE and two variants of DbpA, and circulating IgM specific for antigen OspC, as representative examples. The assay can be expanded to evaluate serum immunoreactivity against other anti-Borrelia antigens, as desired12.
- Perform initial validation studies to assess anti-Borrelia IgG and IgM separately, in a single-reporter 96-well format that uses PE-labeled detection reagents for both antibody classes.
- Dual-Reporter Instrumentation. Use a three-channel dual-reporter instrument (Table of Materials) that allows concurrent IgG/IgM analysis in the same reaction, for the development and technical validation of the dual IgM/IgG assay (detailed below). This instrument has 3 total fluorescence-detecting channels, of which 2 are Reporter Channels that evaluate targeted Ig-associated signals.
NOTE: The dual-reporter system has three lasers: 1) identifies and quantifies bead set-specific fluorescence (Classification Channel); 2) detects and quantifies target-specific phycoerythrin (PE) fluorescence (Reporter Channel 1; 532 nm excitation, "orange" 565-585 nm emission), and 3) evaluates target-specific Brilliant Violet 421 (BV421) fluorescence of a second target analyte (Reporter Channel 2; 405 nm excitation, "blue" 421-441 nm emission). The dual-reporter system is compatible with both 96-well and 384-well microtiter plate (semi-automated handling) assay formats. The general assay scheme is shown in Figure 1. The protocol steps are detailed below.
3. Antigen coupling
- Couple the Borrelia antigens to magnetic, carboxylated and fluorescent color-coded 6.5µm microspheres (beads; Table of Materials) using 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC)/sulfo-N-hydroxysuccinimide (sNHS) chemistry.
NOTE: A detailed description can be found in reference11and reference12. Find the coupling concentration of each antigen in Table 1. Coupled beads should be stored in the dark at 4 °C until use. All buffers used in coupling and detection reactions are detailed in the Supplemental Materials.
4. Assay procedure: Single-reporter IgG or IgM serological assay: 96-well format
- Dilute the Sample in 2 steps (2 steps, both in 96-well plates; 200-fold serum sample dilution in assay buffer).
NOTE: The assay buffer composition is a 1:4 mixture of Low Cross Buffer: PBS containing 1% (w/v) bovine serum albumin. Tween-20 is added to the mixture to a final concentration of 0.05 % (v/v).- Sample dilution step 1: Mix 5 µL of the serum sample with 120 µL of the assay buffer (25-fold dilution).
- Sample dilution step 2: Dilute the 25-fold sample dilution additionally 8-fold by mixing 10 µL of the dilution with 70 µL of the assay buffer to make the final sample dilution 200-fold.
- Magnetic bead mix dilution
- Prepare a bead mix containing 1.0 × 106 beads/mL per bead population (i.e., per unique target antigen).
- Dilute the prepared bead mix 25-fold in assay buffer to make the final bead concentration in this diluted suspension is now 4 × 104 beads/mL per bead population (i.e., per unique target antigen).
- Serum sample incubation with beads
- Pipette 25 µL of diluted coupled multiplex bead suspension (containing 4.0 × 104 beads/set/mL) into assigned wells of a 96-well half-area microtiter plate (Table of Materials).
- Add 25 µL of 200-fold diluted serum sample (from Step 4.1 above) into appropriate wells containing 25 µL of coupled bead suspension.
NOTE: This produces an additional 2-fold dilution, so the final serum sample dilution in the well is 400-fold and the final bead count is 1000 beads/bead set/50-µL reaction. - Cover the 96-well reaction plate with a microplate seal (adhesive foil or plastic plate covers).
- Incubate plate for 2 h at 20 °C, at 750 rpm on a plate shaker.
- Bead washing
- Remove the 96-well reaction plate from the plate shaker and carefully remove the adhesive plate seal.
- Put the 96-well reaction plate into a plate washer.
- Wash the beads three times with 100 µL wash buffer (1× PBS + 0.05% v/v Tween 20) using the automated magnetic plate washer.
- Resuspend washed beads in 100 µL of wash buffer, cover the plate with adhesive foil or plastic plate cover, and shake the plate on a plate shaker for 1 min at 20 °C at 1000 rpm.
- Split the washed beads
- Remove the 96-well reaction plate from the plate shaker and carefully remove the adhesive plate seal.
- Mix reactions by pipetting up and down five times and transfer 50 µL of each 100-µL reaction volume to each of two new 96-well reaction plates, one plate for IgG detection and one for IgM detection.
- Detection antibody/reagent incubation
NOTE: At this point, target antigen-coupled beads have been incubated with serum samples to extract circulating antibodies in serum (if present) that react with the antigen(s). Reacted beads with bound immunoglobulin have been divided into two plates, and IgG and IgM are now detected and quantified in their separate plates using isotype-specific secondary antibodies with a PE label. Stagger plate handling by 20 min.- Aspirate the supernatant from the wells containing magnetic beads using the magnetic plate washer.
- For each plate, make the appropriate Ig Detection Reagent mix (either anti-IgG or anti-IgM), containing PE-conjugated goat anti-human IgG (3 µg/mL) or PE-conjugated donkey anti-human IgM (5 µg/mL) in the assay buffer. For each bead-containing well, 30 µL of Detection Reagent is used. Prepare sufficient IgG and IgM Detection Reagent volumes for reaction wells, with sufficient extra to accommodate pipetting losses.
- Pipette 30 µL of IgG Detection Reagent into each well containing reacted beads on the IgG plate and cover the 96-well reaction plate with a microplate seal.
- Pipette 30 µL of IgM Detection Reagent into each well containing reacted beads on the IgM plate and cover the 96-well reaction plate with a microplate seal.
- Incubate for 45 min at 20 °C while shaking at 750 rpm on a plate shaker.
- Bead washing
- Remove the 96-well reaction plate from the plate shaker and carefully remove the adhesive plate seal.
- Put the 96-well reaction plate into a magnetic plate washer.
- Wash the beads three times with 100 µL wash buffer using the magnetic plate washer.
- Resuspend beads in 100 µL of wash buffer.
- Analyze the results on the single reporter instrument.
- Cover the 96-well reaction plate with a microplate seal.
- Shake the 96-well reaction plate for 3 min at 20 °C, at 1000 rpm on a plate shaker.
- Transfer the 96-well reaction plate from the plate shaker and put in a single-reporter flow analyzer instrument (Table of Materials).
- For each sample, analyze the median fluorescent intensity (MFI) using the following instrument settings: Uptake volume = 80 µL, Count = 50/bead population, Timeout = 60s, Gating = 7,500-15,000)12,13.
5. Assay procedure: Dual-reporter IgG and IgM serological assay: 96-well format
- Sample dilution (2 steps, both in 96-well plates; 200-fold serum sample dilution in assay buffer)
- Sample dilution step 1: Mix 5 µL serum sample with 120 µL assay buffer (25-fold dilution).
- Sample dilution step 2: Dilute the 25-fold sample dilutions an additional 8-fold by mixing 10 µL of the first dilution (25-fold diluted sample from Section 5.1.1) with 70 µL assay buffer (8-fold dilution), to obtain final sample dilution to 200-fold.
- Magnetic bead mix dilution
- Prepare a bead mix containing 1.0 × 106 target antigen-coupled beads/mL per bead population (i.e., per unique target antigen).
NOTE: In this experimental series, we evaluated both IgG and IgM immunoreactivity against four unique Borrelia antigens in each reaction. - Dilute the prepared bead mix 50-fold in assay buffer. The bead concentration in this diluted suspension is now 2.0 × 104 beads/mL per bead population.
NOTE: The number of beads in the duplex assay reaction is halved from that used in the single-reporter reaction to allow for direct comparability between the two assays and to conserve resources, after preliminary studies confirmed that the fluorescence signal was maintained within the linear range of quantification when using half the number of beads.
- Prepare a bead mix containing 1.0 × 106 target antigen-coupled beads/mL per bead population (i.e., per unique target antigen).
- Serum sample incubation with beads
- Pipette 25 µL of diluted target antigen-coupled bead suspension (containing 2.0 × 104 beads/set/mL) into pre-assigned wells of a 96-well half-area microtiter plate. The exact number of reaction wells and their placement on the plate depend upon the operator-determined total number of samples tested and whether single or replicate wells will be run per sample.
NOTE: The target antigen-coupled magnetic beads will be reacted with human serum samples. Both anti-Borrelia antigen immunoreactive IgG and IgM, if present in samples, will bind to and be immobilized on the same antigen-coupled beads. Secondary antibody quantification of bound IgG and IgM will then be performed on the same beads in the same reactions, using different fluorophores for IgG and IgM identification that allows their differentiation. - Add 25 µL of 200-fold diluted serum (from Step 5.1 above) into each well containing 25 µL of mixed target antigen-coupled bead suspension (Step 5.2).
NOTE: This produces an additional 2-fold dilution, so the final serum sample dilution in the well is 400-fold, and the final bead count is 500 beads/bead set/50-µL reaction. - Cover the 96-well reaction plate with a microplate seal (adhesive foil or plastic plate cover).
- Incubate plate with beads for 2 h at 20 °C, at 750 rpm on a plate shaker.
- Pipette 25 µL of diluted target antigen-coupled bead suspension (containing 2.0 × 104 beads/set/mL) into pre-assigned wells of a 96-well half-area microtiter plate. The exact number of reaction wells and their placement on the plate depend upon the operator-determined total number of samples tested and whether single or replicate wells will be run per sample.
- Bead washing (to remove excess serum sample)
- Remove the 96-well reaction plate from the plate shaker and remove the adhesive plate seal.
- Put the 96-well reaction plate into a magnetic plate washer.
- Wash the beads three times with 100 µL wash buffer using the magnetic plate washer.
- Aspirate final wash volume from beads after the last washing step.
- Detection antibody (Incubation - Part I)
- Make fresh IgG and IgM Dual Detection Reagent containing biotinylated goat anti-human IgG (1 µg/mL) together with PE-conjugated donkey anti-human IgM (5 µg/mL) in assay buffer. For each bead-containing well, 30 µL of Dual Detection Reagent is used. Prepare sufficient IgG and IgM Dual Detection Reagent volumes for reaction wells, with sufficient extra to accommodate pipetting losses.
- Pipette 30 µL of IgG and IgM Dual Detection Reagent into each assigned well and cover the 96-well reaction plate with a microplate seal.
- Incubate the plate for 45 min at 20 °C while shaking at 750 rpm on a plate shaker.
- Bead washing (to remove excess detection antibodies)
- Remove the 96-well reaction plate from the plate shaker and carefully remove the adhesive plate seal.
- Put the 96-well reaction plate into an automated magnetic plate washer.
- Wash the beads three times with 100 µL wash buffer using the automated magnetic plate washer.
- Aspirate final wash volume from beads after the last washing step.
- Streptavidin-conjugated reporter (Incubation - Part II)
- Dilute fresh BV421-labeled Streptavidin in assay buffer to a concentration of 0.2 µg/mL. For each bead-containing well, 30 µL of BV421-labeled Streptavidin is used. Prepare sufficient BV421-labeled Streptavidin volume for reaction wells, with sufficient extra to accommodate pipetting losses.
- Pipette 30 µL of diluted BV421-Streptavidin into each reaction well and cover the 96well reaction plate with the microplate seal.
- Incubate the plate for 30 min at 20 °C while shaking at 750 rpm on a plate shaker.
- Wash beads (to remove excess BV421-Streptavidin)
- Remove the 96-well reaction plate from the plate shaker and carefully remove the adhesive plate seal.
- Put the 96-well reaction plate into the magnetic plate washer.
- Wash the beads three times with 100 µL wash buffer using the automated magnetic plate washer.
- Resuspend beads within wells to a final volume of 100 µL in wash buffer.
- Analyze results on the dual-reporter instrument
- Cover the 96-well reaction plate with a microplate seal.
- Incubate the 96-well reaction plate for 3 min at 20 °C, at 1000 rpm on a plate shaker.
- Transfer the 96-well reaction plate from the plate shaker to the dual-reporter flow analyzer instrument (Table of Materials).
- Evaluate sample median fluorescent intensity (MFI) according to manufacturer instructions (Instrument Settings: Dual-Reporter Mode, Uptake volume = 80 µL, Count = 50/bead population, Timeout = 60s, Gating = 7,500-15,000).
6. Dual reporter IgG and IgM serological assay: 384-well semi-automated format
- Perform the dual-reporter assay in 384-well plate format using the assay steps detailed in section 5, but process samples using a pipetting robot and automated magnetic plate washer (Table of Materials). In 384-well format, shake plates during incubations and washes at 1450 rpm, and before measuring fluorescence, shake the plate for 5 min at 21 °C and 1800 rpm.
Representative Results
Experimental overview
The general schemes for the single-reporter and dual-reporter bead-based Borrelia assays are shown in Figure 1. For single antibody targets (i.e., IgG or IgM) generated against a given Borrelia antigen, both antibody classes were evaluated independently in human serum samples using a PE-conjugated anti-isotype antibody for reporting. For the dual-reporter assay with simultaneous analysis of both IgG and IgM immunoreactivity, Borrelia antigen-specific IgG detection used a biotinylated anti-human IgG + BV421-labeled Streptavidin (blue fluorescence) reporter system, while retaining the PE-conjugated reporter (orange fluorescence) for IgM detection. The workflow of the dual-reporter assay is similar to the single-reporter assay, with the exception of an additional 30-minute detection system incubation with the second-channel fluorescence detection reagent BV421-Streptavidin.
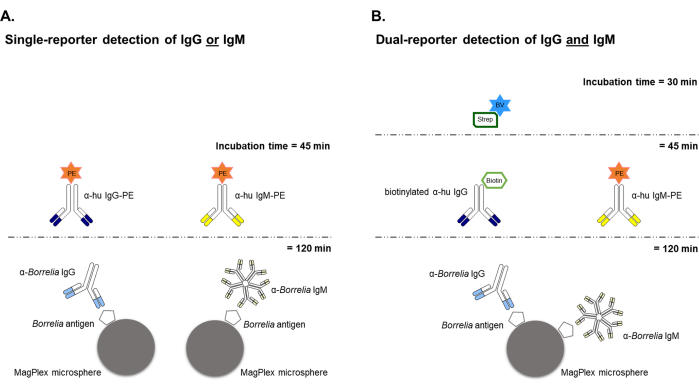
Figure 1: Schematic of the single-reporter and dual-reporter bead-based Borrelia assays. (A) The single-reporter instrument was used to develop and initially validate the bead-based assay that characterized either anti-Borrelia IgG or IgM individually, both using a phycoerythritin (PE) fluorescent reporter label that emits signal in the "orange" spectra. Any desired Borrelia antigen can be coupled to the beads and used to capture and quantify either IgG or IgM present in serum samples. Two separate immunoassays are needed to evaluate both IgG and IgM reactivity against a given antigen. (B) The dual-reporter system allowed simultaneous analysis of serum samples for both IgG and IgM antibodies against any individual Borrelia antigen in the same well. This approach uses the same PE-conjugated detection antibody to target anti-Borrelia IgM, but replaces the IgG detection from a PE-based system to using a biotinylated primary detection antibody and BV421-labeled Streptavidin (a "blue" emitter) that needs an additional detection system incubation step of 30 minutes. Please click here to view a larger version of this figure.
Intra-assay precision
Spearman correlation analysis for three representative Borrelia antigens (Figure 2) demonstrated uniform reproducibility of MFI values when detecting anti-Borrelia antibodies present in human sera.
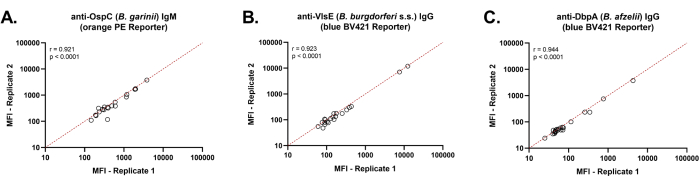
Figure 2: Intra-assay Precision of the dual-reporter bead-based Borrelia assay. Spearman's correlation analysis showed a high intra-assay precision of the dual-reporter assay when a PE detection system was used to detect Borrelia-specific IgM antibodies (A) and a BV421 detection system was used to detect Borrelia specific IgG antibodies (B, C). A total of 21 serum samples were analyzed in duplicates using a semi-automated procedure. In each graph, MFI signals were plotted against each other and analyzed by linear regression. A linear curve (x=y) shown as a red dashed line indicates identical MFI signals for detection systems. Please click here to view a larger version of this figure.
Inter-assay precision
Good assay-to-assay reproducibility was observed with the dual-reporter assay. Levey-Jennings charts (Figure 3) with MFI values of a quality control sample measured over seven independent runs demonstrated the high inter-assay precision for all representative antigens. The average percent Coefficient of Variation (CV% = standard deviation/mean × 100) of the four representative Borrelia antigens was 5.3%.
Dilution linearity
Because the original single-reporter assay and the new dual-reporter assay employ different flow cytometric platforms, we compared median fluorescence outputs of the same antigen immunoassay between the two flow cytometry instruments (Figure 4). Sample dilution series provided linear dilution curves for both IgM and IgG evaluation, with good parallelism of dose-response between instruments through the entire dilution range evaluated (1:100-1:12,800).
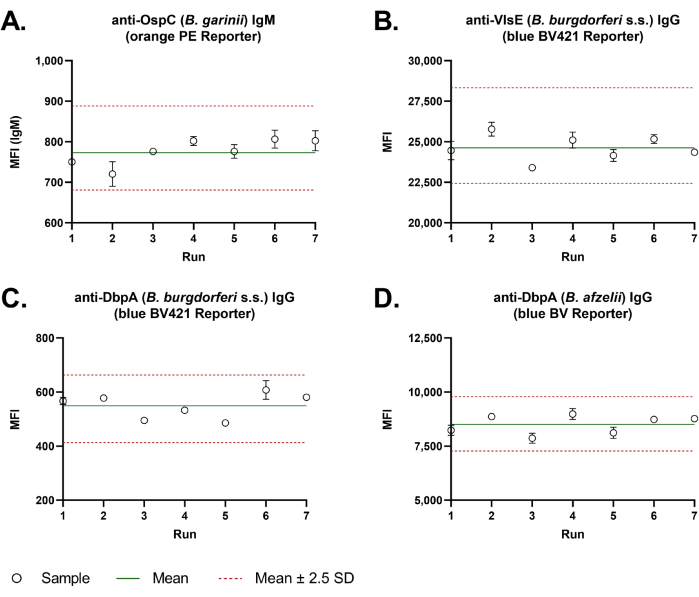
Figure 3: Inter-assay precision of the dual-reporter bead-based Borrelia assay. For the evaluation of the inter-assay precision, Borrelia-specific IgM (A) and IgG (B-D) antibody response of a quality control sample was analyzed over seven independent runs, in duplicate wells each time. Assays were performed manually. MFI values were plotted in a Levey-Jennings chart. A green line gives the mean of all values. Two red dashed lines indicate the tolerance range of the inter-assay precision. This was calculated from the mean value ± 2.5 times the standard deviation (2.5 S.D.). Please click here to view a larger version of this figure.
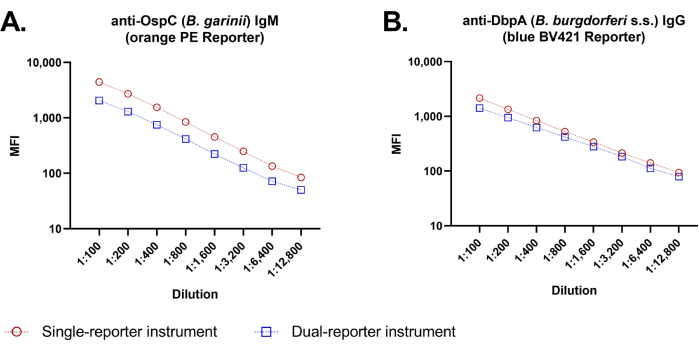
Figure 4: Dilution linearity of the dual-reporter bead-based Borrelia assay. At sample dilutions of 100-fold to 12,800-fold, the dilution curves for both IgM detection (A) and IgG detection (B) were similar when performed manually with the single-reporter instrument and the dual-reporter instrument. At all tested dilutions, MFI values were slightly higher using the single-reporter instrument (red symbols) than the dual-reporter instrument (blue symbols). Shown are dilution curves for 2 representative antigens, with each dilution point representing the average measurement of triplicate wells with standard deviation (SD *) indicated by error bars. Note: Small SDs are not visible at this scale. Please click here to view a larger version of this figure.
Supplemental Materials. Please click here to download this File.
Discussion
This report highlights the development of a two-reporter bead-based Borrelia immunoassay that reproducibly and sensitively determines anti-Borrelia immunoreactivity in serum samples12. The various pathogenic Borrelia species that cause Lyme borreliosis can be differentiated by variant-specific antigen heterogeneity6,7,8,9. The multiplexed assay concurrently evaluates IgG- and IgM-mediated anti-Borrelia immunoreactivity within the same reaction well, thereby conserving reagents, labor, and sample material otherwise needed to perform two singleplex assays separately. Comparing IgM and IgG responses over time may allow better tracking of disease progression as IgM-to-IgG seroconversion occurs after infection6.
The dual-reporter system uses two different detection antibody systems13,15. Related experiments demonstrated no significant detectable cross-reactivity between Borrelia anti-IgM and anti-IgG detection systems when both antibody classes were analyzed together in the same reaction well12. Considering the lower binding affinity of IgM versus IgG antibodies16,17, we chose a PE-conjugated detection antibody for IgM detection (the first reporter channel of the dual-reporter instrument) because PE is one of the strongest-emitting fluorophores routinely used in immunoassays18. For IgG evaluation, we used a biotinylated detection antibody that was subsequently illuminated with BV421-conjugated streptavidin (the second reporter channel of the instrument)19. Despite the additional 30 min incubation step compared to the single-reporter system, the dual-reporter system yields twice the information per reaction. Overall, the dual-reporter multiplexed assay requires less cumulative time and material inputs than running two single-reporter assays.
Strong performance and stability of the multiplexed Borrelia assay was exemplified by high reproducibility in intra- and inter-assay precision studies, and by demonstration of dilution linearity and dilution parallelism over a wide range of sample concentrations for both IgG and IgM assessment. We observed higher absolute fluorescence emission levels for the same fluorophores using the single-channel instrument versus the dual-channel system (≈1.7× higher with PE), attributable to differences in the optics and calibration settings between the two instruments (Figure 4). Nonetheless, fluorescence emission curves with both fluorophores remained within the linear range of both instruments at high and low sample dilution extremes, and any discrepancy in absolute fluorescence measured did not affect the classification of Borrelia exposure status.12
A major advantage of this bead-based Borrelia multiplex assay is the ease with which the assay can be modified or expanded to evaluate different or additional analytes, e.g., to detect antibodies against antigens of further Borrelia species. xMAP magnetic bead sets contain different dye combinations that can be distinguished in the instrument's Classification Channel and can theoretically be implemented into multiplex assays that can concurrently evaluate up to 500 unique analytes within the same sample. While the current study highlights four representative Borrelia antigens to demonstrate assay functionality and stability and to compare the single- and dual-reporter system, the final assay interrogates eight antigens that together can identify all five clinically-relevant Borrelia pathogens circulating throughout Europe and North America12.
High-throughput performance is possible using standard 384-well microtiter plates in a semi-automated assay format. Assay and instrument compatibility with both 96- and 384-well plates allows the Borrelia multiplex assay to be used as an efficient screening tool for rapidly analyzing large sample sets, such as national studies20. Manual performance of the assay remains possible for smaller sample sets using smaller 96-well plates.
Study limitations include the comparative evaluation of only a few Borrelia immunoreactivity targets in a small number of human serum samples. However, the original study did confirm that assay performance for both IgG and IgM was maintained when analyzing eight antigens from all five known Borrelia species within a larger sample set12. Also, the dual-reporter instrument can only assess two antibody isotypes simultaneously within each reaction, so complete isotype profiling would necessitate performing additional assay reactions13.
In conclusion, this report details the successful merger and conversion of bead-based single-reporter immunoassays into dual-reporter assays that can simultaneously evaluate pathogenic Borrelia-specific IgG and IgM antibodies in human serum samples. This combined approach saves total time, material, and labor inputs to generate the same data volume as two independent single-reporter assays. The multiplex assay can be scaled from 96-well to 384-well microtiter plate format and can be semi-automated by the use of robotic plate and liquid handling instrumentation, making it suitable for high-throughput applications such as large population surveys. Bead-based dual-reporter assay systems have previously demonstrated utility in evaluating, for instance, immune responses to other viral and bacterial pathogens13,21, assessing allogeneic antibody responses against HLA epitopes in organ transplantation22, and exploring mechanisms of autoimmune disease23. The current report detailed the use of multiplex technology to identify exposure to the Borrelia pathogens that cause Lyme disease, as an example of how laboratories can adapt this approach for exploring complex immune mechanisms in diverse pathologies.
Acknowledgements
This report was funded by Luminex (Austin, TX). The authors thank Matthew Silverman PhD (Biomedical Publishing Solutions, Panama City, FL; mattsilver@yahoo.com) for analytical and scientific editing assistance. The authors also thank Harald Klein and Christoph von Eichel-Streiber of tgcBIOMICS GmbH (Bingen, Germany) for providing the Borrelia antigens used in the study. Human serum samples for technical assay validation and quality control were obtained from: 1) the Multilocal and Serial Prevalence Study on Antibodies against SARS-CoV-2 in Germany via the Department of Epidemiology, Helmholtz Centre for Infection Research, Braunschweig, Germany; and 2)the Department of Neurology, Sächsisches Krankenhaus Rodewisch (Rodewisch, Germany). Approval for use of human samples was granted by the Ethics Committee of the Hannover Medical School, Germany (9086_BO_S_2020).
Materials
| Name | Company | Catalog Number | Comments |
| Antibodies and Detection Reagents | Source | Catalog Number | |
| Biotinylated Goat Anti-Human IgG | Jackson ImmunoResearch (Dianova) | 109-066-098 | |
| Brilliant Violet 421-Streptavidin | BD Biosciences | 563259 | |
| Donkey Anti-Human IgM | Jackson ImmunoResearch (Dianova) | 709-116-073 | |
| Borrelia Antigens | tgcBIOMICS (Bingen, Germany) | ||
| Coupling Reagents | |||
| 1-Ethyl-3-[3-dimethylaminopropyl]carbodiimide hydrochloride (EDC) | Thermo Scientific Pierce | 77149 ProteoChem (100 mg) | |
| 10x PBS | Fisher Scientific | BP399-4 | |
| BSA | Carl Roth | T844.3 | |
| MES (2-ethanesulfonic acid; zwitterionic buffer) | Carl Roth | 4256.2 | |
| Na2HPO4 | Carl Roth | 4984.1 | |
| ProClin300 | Sigma | 48914-U | |
| Sulfo-NHS (N-hydroxysulfosuccinimide) | Thermo Scientific Pierce | 24510 (500 mg) | |
| Triton X-100 | Thermo Scientific | 85111 | |
| Instrumentation and Ancillary Lab Supplies | Source | ||
| 384-well plate | Corning, Cat# 3570 | ||
| 96-well deep-well plates | ThermoFisher Scientific, Cat# 95040450 | ||
| 96-well half-area plates | Corning, Cat# 3690 | ||
| BioTek 405 TS Plate Washer | BioTek Instruments/Agilent Technologies, Santa Clara, CA | ||
| BioTek MultiFlo FX Plate Washer | BioTek Instruments/Agilent Technologies, Santa Clara, CA | ||
| DynaMag Spin Magnet (for isolating beads in microcentrifuge tubes) | ThermoFisher, Cat# 12320D | ||
| Flexmap 3D (two-channel, single-reporter instrument) | Luminex Corp., Austin, TX | ||
| KingFisher Magnetic Particle Processor (for isolating beads in 96-well plates) | ThermoFisher, Cat# A31508 | ||
| MagPlex Microspheres (magnetic, fluorescent, 6.5-µm-diameter beads) | Luminex Corp., Austin, TX | ||
| SmartBlock Plates | Eppendorf, Cat# 5363000039 | ||
| ThermoMixer C | Eppendorf, Cat# 5382000015 | ||
| ThermoTop | Eppendorf, Cat# 5308000003 | ||
| xMAP Intelliflex (three-channel, dual-reporter instrument) | Luminex Corp., Austin, TX |
References
- Stanek, G., Strle, F. Lyme borreliosis-from tick bite to diagnosis and treatment. FEMS Microbiology Reviews. 42 (3), 233-258 (2018).
- Stanek, G., Wormser, G. P., Gray, J., Strle, F. Lyme borreliosis. The Lancet. 379 (9814), 461-473 (2012).
- Rizzoli, A., et al. Lyme borreliosis in Europe. Eurosurveillance. 16 (27), 19906 (2011).
- Cook, M. J. Lyme borreliosis: a review of data on transmission time after tick attachment. International Journal of General Medicine. 8, 1-8 (2015).
- Strle, F., Stanek, G., Lipsker, D., Jaulhac, B. Clinical Manifestations and Diagnosis of Lyme Borreliosis. Lyme Borreliosis: Biological and Clinical Aspects. , 51-110 (2009).
- Wilske, B., Fingerle, V., Schulte-Spechtel, U. Microbiological and serological diagnosis of Lyme borreliosis. FEMS Immunology and Medical Microbiology. 49 (1), 13-21 (2007).
- Dessau, R. B., et al. To test or not to test? Laboratory support for the diagnosis of Lyme borreliosis: a position paper of ESGBOR, the ESCMID study group for Lyme borreliosis. Clinical Microbiology and Infection. 24 (2), 118-124 (2018).
- Eldin, C., et al. Review of European and American guidelines for the diagnosis of Lyme borreliosis. Médecine et Maladies Infectieuses. 49 (2), 121-132 (2019).
- Lantos, P. M., et al. Clinical Practice Guidelines by the Infectious Diseases Society of America (IDSA), American Academy of Neurology (AAN), and American College of Rheumatology (ACR): 2020 Guidelines for the Prevention, Diagnosis and Treatment of Lyme Disease. Clinical Infectious Diseases. 72 (1), e1-e48 (2021).
- Gerritzen, A., Brandt, S. Serodiagnosis of Lyme borreliosis with bead based immunoassays using multiplex technology. Methods. 56 (4), 477-483 (2012).
- Embers, M. E., et al. Five-antigen fluorescent bead-based assay for diagnosis of Lyme disease. Clinical Vaccine Immunology. 23 (4), 294-303 (2016).
- Häring, J., et al. Borrelia multiplex: a bead-based multiplex assay for the simultaneous detection of Borrelia specific IgG/IgM class antibodies. BMC Infectious Diseases. 22 (1), 859 (2022).
- Angeloni, S., Cameron, A., Pecora, N. D., Dunbar, S. A rapid, multiplex dual reporter IgG and IgM SARS-CoV-2 neutralization assay for a multiplexed bead-based flow analysis system. Journal of Visualized Experiments. 170, (2021).
- Gornyk, D., et al. SARS-CoV-2 Seroprevalence in Germany. Deutsches Ärzteblatt International. 118 (48), 824-831 (2021).
- Angeloni, S., Das, S., De Jager, W., Dunbar, S. . xMAP Cookbook. , (2022).
- Racine, R., Winslow, G. M. IgM in microbial infections: Taken for granted. Immunology Letters. 125 (2), 79-85 (2009).
- Ehrenstein, M. R., Notley, C. A. The importance of natural IgM: scavenger, protector and regulator. Nature Reviews Immunology. 10 (11), 778-786 (2010).
- Kovaleski, G., et al. Extraction and purification of phycobiliproteins from algae and their applications. Frontiers in Chemistry. 10, 1065355 (2022).
- Chattopadhyay, P. K., et al. Brilliant violet fluorophores: A new class of ultrabright fluorescent compounds for immunofluorescence experiments. Cytometry Part A. 81 (6), 456-466 (2012).
- Coors, A., et al. Regional seropositivity for Borrelia burgdorferi and associated risk factors: findings from the Rhineland Study, Germany. Parasites & Vectors. 15 (1), 241 (2022).
- Gürsoy, M., et al. Salivary IgA and IgG antibody responses against periodontitis-associated bacteria in Crohn's Disease. International Journal of Molecular Science. 24 (3), 2385 (2023).
- Argani, H. Anti-HLA Antibody: The role of epitopes in organ transplantation. Experimental and Clinical Transplantation. 17 (Suppl 1), 38-42 (2019).
- Laman, J. D., Huizinga, R., Boons, G. J., Jacobs, B. C. Guillain-Barré syndrome: expanding the concept of molecular mimicry. Trends in Immunology. 43 (4), 296-308 (2022).
This article has been published
Video Coming Soon
ABOUT JoVE
Copyright © 2024 MyJoVE Corporation. All rights reserved