Rearing the Cabbage White Butterfly (Pieris rapae) in Controlled Conditions: A Case Study with Heavy Metal Tolerance
In This Article
Summary
This paper presents a detailed protocol for rearing the cabbage white butterfly in controlled lab conditions with an artificial diet, which allows precise manipulations of early-life nutrition and toxin exposure. The representative results show how heavy metal toxicity can be assayed with this protocol.
Abstract
The cabbage white butterfly (Pieris rapae) is an important system for applied pest control research and basic research in behavioral and nutritional ecology. Cabbage whites can be easily reared in controlled conditions on an artificial diet, making them a model organism of the butterfly world. In this paper, a manipulation of heavy metal exposure is used to illustrate basic methods for rearing this species. The general protocol illustrates how butterflies can be caught in the field, induced to lay eggs in greenhouse cages, and transferred as larvae to artificial diets. The methods show how butterflies can be marked, measured, and studied for a variety of research questions. The representative results give an idea of how artificial diets that vary in components can be used to assess butterfly performance relative to a control diet. More specifically, butterflies were most tolerant to nickel and least tolerant to copper, with a tolerance of zinc somewhere in the middle. Possible explanations for these results are discussed, including nickel hyper-accumulation in some mustard host plants and recent evidence in insects that copper may be more toxic than previously appreciated. Finally, the discussion first reviews variations to the protocol and directions for troubleshooting these methods, before considering how future research might further optimize the artificial diet used in this study. Overall, by providing a detailed video overview of the rearing and measurement of cabbage whites on artificial diets, this protocol provides a resource for using this system across a wide range of studies.
Introduction
The small cabbage white butterfly (Pieris rapae, hereafter "cabbage white") is a cosmopolitan pest species of mustard crops, such as cabbage, broccoli, and canola1,2,3. At the same time, the cabbage white is a powerful system for research in biology and a commonly used butterfly model, as they can be easily reared and manipulated in controlled lab experiments4,5. Research on cabbage white butterflies has provided critical insights with respect to host searching6,7,8, nectar resource use9,10,11, mate choice and sexual selection12,13,14, wing pattern development and evolution15,16,17, and responses to novel and changing environments18,19. Many of these insights rely on the fact that cabbage whites can be reared on artificial diets4,20,21, which can be precisely manipulated to reflect poor nutritional conditions22,23, ecologically relevant pollutant levels24,25,26,27, or transitions to novel host plants28,29. The present study uses an experiment on exposure to heavy metals to illustrate basic methods for rearing cabbage white butterflies on an artificial diet in the laboratory and key performance measures of larvae and adults. Many aspects of these methods apply to other butterflies30,31 and moths32,33,34 that can be reared on an artificial diet.
In this paper, an experiment on metal tolerance is used to illustrate the general methods of rearing cabbage white butterflies. Heavy metals are a common anthropogenic pollutant stemming from the degradation of human products, industrial processes, and legacy contamination from historical use in pesticides, paints, and other products35,36,37,38. Many heavy metals, including lead, copper, zinc, and nickel, can move from soil and water into plant tissue39,40,41,42, and metals in dust can be deposited on plant leaves43,44,45, resulting in multiple routes of exposure to phytophagous insect larvae. Heavy metal exposure early in life can have negative effects on animal development, especially on neural tissue, and high levels can be lethal35,36,46,47,48. A number of studies have shown the negative effects of metal exposure on developing insects, including both pests and beneficial insects49,50,51. The large number of heavy metal pollutants, and the fact that they often co-occur in human environments52, means that precise lab methods are needed in which researchers can expose developing insects to different levels and combinations of diverse metals to understand and mitigate their environmental effects.
The present work contrasts the impacts of common metals on cabbage white survival and development, focusing on copper (Cu), zinc (Zn), and nickel (Ni), three common pollutants in human environments. For instance, forbs from rural Minnesota roadsides contain up to 71 ppm Zn, 28 ppm Cu, and 5 ppm Ni53. This experiment manipulates the levels of these metals in artificial diets of cabbage white butterflies at levels corresponding to, and exceeding, the levels seen in the environment. An artificial diet is used to contrast the relative toxicity of these metals, predicting that cabbage whites would be more sensitive to metal pollutants that are not an integral part of their physiology (nickel) relative to those that occur, albeit at small levels, in enzymes and tissue (copper and zinc; Figure 1). Throughout, this text provides methodological details and accompanying video visualizations to illustrate the rearing and research methods of this important butterfly model system.
Protocol
This research was conducted under USDA APHIS permit P526P-13-02979.
1. Collection of experimental butterflies
- Catch adult female butterflies with an aerial insect net. Cabbage whites are generally found in open, disturbed habitats with nectar plants and host plants (in the family Brassicaceae) present.
- Search when the sun is out and the temperature is warm. To target females, look for individuals fluttering slowly, close to the ground, and landing on plants to "drum" (taste) the leaves with their foretarsi.
- Set up females in cages to harvest eggs.
NOTE: Field-collected females, on average, have mated with one or two males12, and should start laying fertile eggs shortly after capture. Wild-caught females need natural light to oviposit and mate, so place the cage in a greenhouse or windowsill. - House females with a host plant to harvest eggs.
NOTE: Females will accept a variety of host plants, including green cabbage, radish, kale, collards, and Arabidopsis, but ensure that the plants have not been treated with any pesticides.- Present the host plants either in pots or in containers with water to maintain the leaf pressure, such as stems of kale in floral water tubes.
- If the researcher wishes to collect eggs and transfer them directly to diet, first use a rubber band to attach a host plant leaf to the top of a plastic cup of water, and then stretch a piece of parafilm around the edge-females touching the leaf will oviposit onto the parafilm (see5).
- Ensure that the cage also contains something to keep the relative humidity high, for instance, through daily watering of a potted plant or wetting of a towel, especially in dry conditions. If potted plants are watered in the cage, ensure a towel is under the pot, as butterflies can get stuck in pooling water.
- Feed the butterflies with a 10% diluted honey water solution presented on a yellow sponge, which butterflies quickly learn to use, especially if housed with experienced individuals.
- To encourage butterflies to feed from sponges, place them directly on the feeder, especially after lightly spraying with a water bottle, which often causes them to stick out their proboscis.
- To set up the feeder, first thoroughly rinse the yellow or orange sponges, and then cut them into small squares that fit into 60 mm plastic Petri dishes. Change the feeders daily and clean the sponges in a mild bleach solution, followed by thorough rinsing to prevent mold growth.
2. Making artificial diets
- First, use the recipe in Table 1, or other relevant sources, to determine the relevant recipe for an experiment. Make necessary modifications specific to the focal species or experiment. Print out a recipe to follow while weighing ingredients.
- Weigh all the dry ingredients, except for the agar, into one container. Ensure the ingredients are put back into their respective storage location, noting that several ingredients are stored at 4° C. Place the pre-weighed, dry ingredient mixture into a blender with 5 mL of flaxseed oil.
- For each diet batch, follow the steps detailed below.
- Mix 15 g of fine-mesh agar with 400 mL of distilled water in a beaker at least 1 L in size. Microwave until the agar is close to boiling, with fine bubbles throughout the mixture, stirring the mixture every 30-60 s to prevent boiling over.
- To this hot agar mixture, add 400 mL of faucet-temperature distilled water to bring it to an appropriate temperature to mix with the dry ingredients, as the vitamin mixture is heat-sensitive.
- Add the agar mixture to the blender and thoroughly mix, scraping the edges of the blender if necessary.
- As the agar is heating, place at least seventy four-ounce diet cups on the counter with the edges touching. After thoroughly mixing the diet, pour the mixture from the blender into diet cups, ensuring the diet covers the bottom of each cup.
- After the diet cools, place lids on the cups, label the diet cups with diet type, stack them on trays, and store for up to 1 month at 4 °C until use.
3. Transfer and rearing on artificial diets
- House host plant leaves with butterfly eggs in 30 oz deli cups with a mesh cover in a 24 °C climate chamber. After 1 week, check the cups-ensuring that the larvae are hatched and in the late first or early second instar stage, a good time for transfer to the artificial diet.
- Transfer the larvae to the artificial diet with a paintbrush, disinfecting it with bleach spray and a water rinse in between containers of larvae. Transfer three larvae into each 4 oz cup. While the artificial diet is energy dense and can support high densities of larvae, avoid packing larvae into cups, as diseases and mold can spread in cups with high larval density.
- Place the cups into a plastic bin on their sides so that frass falls to the bottom of the cups and away from the diet, reducing mold and disease risk.
- House the diet cups in controlled temperature conditions with low to moderate light levels. Monitor the cups for mold and disease every 1-2 days by peeking through the clear cup lids.
- Cups with mold or disease can be quarantined or frozen to prevent spreading to other cups.
4. Adult emergence and handling
- Allow the larvae to pupate and emerge in the diet cups. When adults emerge, give them a few hours for their wings to harden before removing them for marking. Remove adult butterflies from the cups with clean hands by gently grasping their wings, noting that grabbing all four wings closer to their body is a more stable hold.
- To mark the butterflies, hold dry individuals by the head and thorax and use a fine-tipped sharpie to lightly mark a number on their hindwing.
- Sex individuals using a combination of wing markings and genitalia; females generally have two black spots on their dorsal forewing and darker, more yellowish hindwings, while males generally have one smaller black spot on the dorsal forewing on a brighter white background54.
- Given that this coloration shows individual and seasonal variation, confirm the sex using abdominal traits-males have two claspers at the distal end of their abdomen and a narrower abdomen in general, while females have a single genital opening.
- Transfer the adults to wax glassine envelopes by opening the envelope with one hand, holding the butterfly by the head and thorax, sliding it into the envelope, and grabbing the wings through the envelope with the other hand.
- Make sure all four wings are closed normally within the envelope.
- Maintain the butterflies in cold conditions (5-6 °C) for up to 1 week prior to experimentation, but allow at least 1 day to acclimate when taken out of the fridge.
5. Performance measures
- To measure wing traits on dead individuals, remove the wings of the butterfly by holding the thorax in one hand and using forceps to remove each wing at its base. Place the wings flat in a lightbox and take photographs for later measurements.
- To get estimates of fecundity, house the adults in mating cages, allowing at least 1 day for reproductive maturation of males and 1 day for mating. Sacrifice females at set time points for egg counts through dissection, or collect eggs each day on host plants.
- To estimate egg loads, remove the abdomen of the female, place it in 1x PBS buffer, and cut a slit along the ventral side.
- Use forceps to separate the innards from the cuticle, then pull the ovaries away from the gut, trachae, and other contents of the abdomen.
- Uncurl the four curled ovarioles within each of the two ovaries, noting where mature, yolked, and shelled eggs transition to immature follicles. Use a counter to help tally the total mature eggs, generally ranging from 0-200 eggs.
- To determine the mating status of a dissected female, open the bursa copulatrix and separate the spermatophores within. As spermatophores are digested, they generally develop a "tail" and are nested within each other.
6. Case study
NOTE: Adult female cabbage white butterflies were collected from the wild in 2014 to found the experimental populations. Adult females originated from near Davis, California (N = 8 founding females).
- Housing the butterflies
- House the females in "BugDorm" mesh cages (61 cm x 61 cm x 61 cm) under natural light in a greenhouse. Provide an organic leaf of the host plant cabbage (Brassica oleracea) for ovipositioning.
- To maintain humidity in the cages, include a small potted plant (Cosmos), watered daily, placed on top of a towel within each cage.
- Collect eggs daily by transferring leaves with new eggs to 473 mL plastic cups with holes in the lid and place in a climate chamber.
- Provide butterflies with ad libitum access to a 10% honey water solution (made by diluting organic honey with distilled water), accessible through a yellow sponge in a small Petri dish that is changed daily.
- Preparing artificial diets
- Prepare artificial diets for cabbage white larvae using modifications of previously developed Lepidoptera diets4. One batch of diet contained 50 g of wheat germ, 27 g of casein, 10 g of cellulose, 24 g of sucrose, 15 g of cabbage flour, 9 g of Wesson salt mix, 12 g of Torula yeast, 3.6 g of cholesterol, 10.5 g of Vanderzant vitamin mix, 1.1 g of methyl paraben, 1.5 g of sorbic acid, 3 g of ascorbic acid, and 0.175 g of streptomycin (see Table of Materials).
- Pre-weigh the dry ingredients for multiple diet batches (Table 1) and mix thoroughly to increase the homogeneity across diet types before being subdivided into separate batches for mixing with metal solutions.
- Place the dry ingredients in a blender with linseed oil and the corresponding metal mixture.
NOTE: Linseed oil was used in the present experiment as it was sold by a past provider of insect diets. Now, organic flaxseed oil is used exclusively, which is made from the same plant, but is less likely to contain any additives as commercial providers of linseed oil. - Pour the prepared diet into 118 mL (4 oz) plastic deli cups. Use soluble metal salts to add focal metals to artificial diets. Aim for metal concentrations based on prior observations of the metal content of plants (e.g., nickel accumulation55,56,57 or roadside contamination of plants58,59,60) and tolerance of metals in other Lepidoptera49,50,51.
- Dissolve metal salts in 500-1,000 mL of distilled water prior to taking the corresponding amounts to add to artificial diets. For example, to make the 100 ppm nickel diet, add 317.6 mL of 1 M NiCl2 solution to the artificial diet before blending to give a final diet concentration of 100 mg/g Ni dry weight (approximately 53 mg/g wet weight). This amount translates into an average measured concentration of 109.6 ppm (Table 2) based on inductively-coupled plasma atomic emission spectroscopy.
NOTE: The levels of metals were estimated by the University of Minnesota's Research Analytical Labs with six samples.
- Maintenance
- Maintain the eggs harvested on host plants in climate chambers at 23 °C on 14:10 photoperiods for 7 days. After this, transfer the early second instar larvae to the artificial diet.
- At transfer, evenly divide the larvae from a given plant across the four diet types to avoid confounding batches of larvae with diet type. Transfer the larvae (N = 346 total) as two individuals per 118 mL diet cup to reduce disease incidence from overcrowding and allow for ample space for adults to eventually eclose.
- Punch holes (three per lid) in the lids of the rearing cups. Place the cups in shoebox-sized plastic bins for rearing, with the different diets interspersed to avoid any systematic effects of location in the rearing chamber.
- House the cups of larvae in climate chambers at 23 °C on 14:10 photoperiods (with bins of water at the bottom of the chamber to keep the humidity around 50%-60%, monitored with a home humidity sensor). In the event that the cups became moldy (about eight total cups in this case study), remove the cups from the chamber and remove those individuals from the experiment.
- Allow larvae to pupate and emerge in the rearing cups (N = 162 total).
NOTE: For the rearing conditions in this study, the development time from egg collection to adult emergence averaged around 25-30 days (ranging from 20-40 days, e.g.,25,28). - As the pupae approach adult emergence, check the cups daily for newly-eclosed individuals and remove adults with dried wings. Label the adults on their hindwings with their corresponding individual number (assigned at larval transfer) using a fine-tipped black sharpie. Determine the sex of each individual and mark on a glassine envelope along with their number and emergence date. Place the adult butterflies in glassine envelopes and store at -20° C until further processing.
NOTE: A small fraction of emerging adults show wing deformities that would interfere with flight and adult survival (5%-8%); these individuals are excluded from survival analyses for these experiments.
- Measurement and data analysis
- Measure the survival as survival from second instar (when the caterpillars were placed on diet) to adult emergence.
NOTE: The present study focused on survival and development time as measures of performance on the different diets. - Measure the development time as the number of days between transfer to diets and adult emergence in the climate chamber.
- For data analysis, run two sets of models that included interactions between the metal and concentration.
NOTE: As both interactions were significant (F2,194 = 4.56, p = 0.01 for development time and X2 = 12.1, p = 0.002 for survival), the study proceeded with separate analysis of each metal. - To analyze survival, run chi-square tests for each metal to test the effects of metal dose (treated as four categories) on survival to adulthood with wings fully intact.
- When a significant effect of dose is detected, perform a follow-up chi-square to compare each level to the control diet. To analyze the development time (from time of transfer to emergence as an adult), test for effects of sex on development time.
NOTE: As there was no effect of sex on development time, (p > 0.10) for any metal in this experiment, we dropped it from consideration in the model. - Run a separate ANOVA for each metal to test for the effect of the four concentrations on development time. Additionally, run t-tests for each concentration relative to the control to determine the minimum concentration where a performance effect is seen.
NOTE: In this study, JMP v16 was used for all analyses. All raw data are available on Mendeley61.
- Measure the survival as survival from second instar (when the caterpillars were placed on diet) to adult emergence.
Representative Results
Overview
Artificial diet can be used to raise cabbage white butterflies in standard conditions to test the effects of certain diet ingredients on butterfly performance. In the present work, artificial diets were used to study the toxicity of different metals found in host plants growing in polluted areas (Figure 1). Larvae were raised on diets containing increasing concentrations of three different metals (Figure 2; specific methodological details presented in section 6 of the protocol). Butterfly survival and development were more impacted by copper and zinc and least impacted by nickel (Figure 3 and Figure 4), with a sensitivity comparable to other studies with butterflies and moths raised on artificial diets (Figure 5).
Survival
Butterfly larvae were transferred to artificial diets containing copper, nickel, zinc, or control, where each metal type varied in concentration at three levels (Table 3). A representative image of larvae at an increasing dosage of toxin is shown in Figure 2. There was no effect of metal concentration on survival for nickel, but there was a significant effect for both copper and zinc (Table 3 and Figure 3). Post-hoc chi-square comparisons demonstrated that zinc showed a decline in survival relative to the control diet at only the highest level of zinc (1,000 ppm, post-hoc comparison X12 = 8.41, p = 0.004; Figure 1). Copper also showed a significant decline in survival only at the highest levels used (500 ppm, X12 = 7.00, p = 0.008), although there was a non-significant beneficial increase in survival at the two lowest levels (50 ppm and 100 ppm; Figure 3).
Development time
There was a significant effect of copper and zinc concentration on development time (Table 4 and Figure 4). As copper concentration increased, there was an increase in development time, with a significant deviation from the control starting at 50 ppm (p = 0.027; Figure 3). As zinc concentration increased, there was an increase in development time, with a significant deviation from the control starting at 100 ppm (p = 0.03; Figure 4). There was a trend for increasing nickel to result in longer developmental times (p = 0.08; Table 4), and comparisons of each diet with the control showed significant effects starting at 100 ppm (p = 0.022; Figure 4).
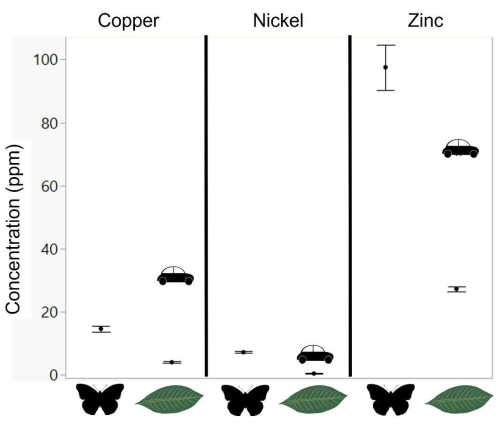
Figure 1: Observed levels of focal metals in butterfly tissue and host plants. (Data from62.) Levels of copper, nickel, and zinc are shown for Pieris butterfly tissue (reared on bok choy in the lab) and wild-collected mustards (Bertorea sp.). Cars indicate the levels seen in plant leaves along high-traffic roads53. The levels of metals in artificial diets used in this study are reported in Table 1; points represent means, and error bars represent standard error. Please click here to view a larger version of this figure.

Figure 2: Image of cabbage white larvae transferred on the same day to artificial diets of increasing concentration of a toxin. This image shows larvae from a dose-response study (presented in 28 using dried plant material for the toxic plant Aristolochia). Photo by ESR. Please click here to view a larger version of this figure.
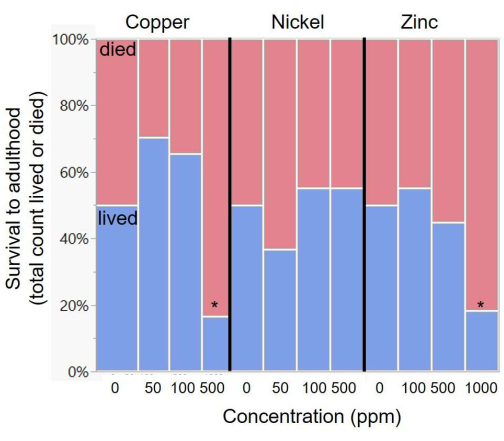
Figure 3: Variation in survival across metal diets of increasing concentrations. Asterisks indicate significant deviation in survival relative to the control diet. The exact metal concentrations in the diets are listed in Table 2. Please click here to view a larger version of this figure.
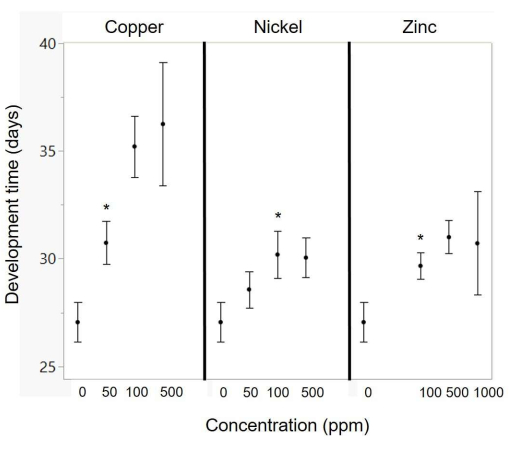
Figure 4: Effects of metal concentration on development time. The asterisks indicate the lowest metal concentration for which there is a significant difference relative to the control (using a t-test). The exact metal concentrations in the diets are listed in Table 2. Points represent means, and error bars represent standard error. Please click here to view a larger version of this figure.
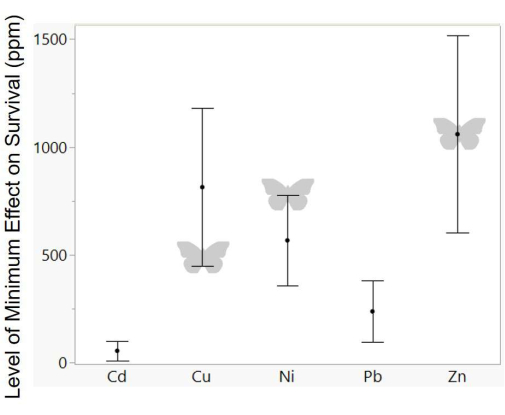
Figure 5: Summary of metal tolerance in other Lepidoptera. Shown are composite survival data plotted from 11 existing studies49,50,51,56,63,64,65,66,67,68. The response variable is the level (in ppm) of metal concentration where negative effects on survival are first seen. Butterflies indicate results from this study, noting that the tolerance values for nickel were higher than those measured in this study. Points represent means, and error bars represent standard error. Please click here to view a larger version of this figure.
| Ingredient | Weigh as | g | mL |
| Wheat Germ | dry ingredients | 50 | |
| Cellulose | dry ingredients | 10 | |
| Cabbage flour | dry ingredients | 15 | |
| Casein | dry ingredients | 27 | |
| Sucrose | dry ingredients | 24 | |
| Wesson Salt Mix | dry ingredients | 9 | |
| Torula Yeast | dry ingredients | 12 | |
| Cholesterol | dry ingredients | 3.6 | |
| Vitamin Mix | dry ingredients | 10.5 | |
| Methyl Paraben | dry ingredients | 0.75 | |
| Sorbic Acid | dry ingredients | 1.5 | |
| Ascorbic Acid | dry ingredients | 3 | |
| Streptomycin | dry ingredients | 0.175 | |
| Flaxseed oil | wet ingredients | 5 | |
| Agar | agar | 15 |
Table 1: Recipe for artificial diet. Shown are the weights (and volumes) of ingredients in one batch of cabbage white butterfly diet. The dry ingredients (and flaxseed oil) are prepared separately from the agar mixture (dissolved in 400 mL of boiling water, then brought to a cooler temperature with 400 mL of room temperature water).
| Diet type | Copper (ppm) | Nickel (ppm) | Zinc (ppm) |
| Copper-“100 ppm” | 96.1 | 1.75 | 69.9 |
| Nickel-“100 ppm” | 7.29 | 109.6 | 68.9 |
| Zinc-“100 ppm” | 7.96 | 1.06 | 186.2 |
| Zinc-“500 ppm” | 6.51 | 1.16 | 708 |
| Control | 5.89 | 0.59 | 59.3 |
Table 2: Measures of metals in diets. Shown are the mean levels of copper, nickel, and zinc in a subset of the artificial diets used in the study. The diet name ("type" in the analysis) is shown on the left, with values in quotes being the calculated level. The target concentration is shown in quotation marks. A subset of diets used in the study was analyzed to ensure the calculated values were on target with realized values; it should be noted that there is often some small degree of variation in the composition of diet components, and each line reported represents only one replicate.
| Metal | Pearson X32 | P |
| Copper (N = 118) | 17.82 | 0.0005 |
| Nickel (N = 152) | 3.45 | 0.33 |
| Zinc (N = 152) | 12.52 | 0.006 |
Table 3: Effects of metal concentration on survival. Shown are the results of a chi-square test for each metal, contrasting three concentrations of metal relative to a control diet.
| Metal | F | P |
| Copper (N = 61) | F3,57 = 9.84 | <0.0001 |
| Nickel (N = 75) | F3,71 = 2.35 | 0.079 |
| Zinc (N = 64) | F3,60 = 3.79 | 0.015 |
Table 4: Effects of metal concentration on development time. Shown are the results of individual ANOVAs for each metal.
Data Availability:
All data are available on Mendeley61.
Discussion
In this research, cabbage white butterflies (Pieris rapae) were raised on an artificial diet to examine differences in heavy metal toxicity. In doing so, this study provides general methods for rearing and laboratory studies of this easy-to-manipulate butterfly system. This discussion first considers more general questions about the methods reviewed here, then reviews our scientific findings before concluding with reflections on the components of the artificial diet.
The protocol reviewed here gives steps of a general rearing method for cabbage white butterflies, but there are many points within this protocol that can be tweaked. For instance, while the case study presented here uses sponges for feeding, other researchers have had luck with dental wicks and silk flowers filled with honey water5. While the present study uses honey water as food, other researchers have used sugar solutions and even Gatorade. If pupae need to be weighed, or moved to other conditions for emergence (e.g., inducing diapause and needing to cold store for 1 month), the researcher can easily remove them from the cups by spritzing them with water to moisten their silk attachments and grab them with feather forceps, then re-hanging them using double-sided tape. If researchers need more flexibility in terms of when adult butterflies are moved into cages for adult behavior, they can be held in the refrigerator for several weeks, but they need to be fed. Every several days, the butterflies should be taken out to be fed a dilute honey water solution. Under indoor lighting, this can be done by using a pin to unroll their proboscis into the food. On the adult performance end, a wide range of fitness measures can be taken on cabbage white butterflies. Body size can be measured as the wet or dry mass of larvae at certain stages, pupae, or adults (sacrificed, or held in glassine envelopes), or through the measurement of wing length in the program ImageJ (see12,24,25,28). The lifetime fecundity of females can be measured through daily egg collections on host plants25,69,70, and the size of specific traits can be measured as a metric of performance; for instance, the mass or volume of the brain or individual brain regions62,71,72, or the mass or protein content of the thorax or flight muscle62,70. Finally, adults can be used in behavioral studies to test any number of questions examining the effect of diet manipulation on foraging or oviposition choice27,73.
If the rearing protocol is not working as expected, there are a few aspects to troubleshoot. First, one can ask whether the light levels are high enough to elicit normal adult behavior. While lab-adapted lines of Pieris will lay eggs under fluorescent light, the only artificial light that works for wild-type lines are powerful broad-spectrum greenhouse lights. Natural light in greenhouses, windowsills, or outdoors works best to elicit mating and egg-laying behavior. Second, if eggs are not hatching or if larvae are dying early in development, there are a few things to consider. The host plant material must be organic, noting that "organic" plants from stores are sometimes treated with chemicals that can kill larvae, so raising one's own host plants is often best. If the host acceptance rate is lower, younger leaves with higher nitrogen content can be attempted, presenting potted plants instead of individual leaves and ensuring females are mated. Females will accept seeding Brassica, even small sprouts that are 2 weeks of age. The paraffin method works well to transfer eggs to different conditions, but it should be noted that the acceptance rate tends to be lower than whole plants. Third, all the components of the diet must be of high quality and not expired. Flaxseed oil should be replaced annually and stored in the fridge24,25. Wheat germ, the vitamin mix, and antibiotics should also be kept cool. Fourth, one can consider tweaking the diet cup setup. Any number of disposable plastic cup types can be used for rearing, from 1 oz to 15 oz. We have found that 4 oz is a good size to allow for adult emergence and packs nicely into our climate chambers. Holes poked in the lids allow for airflow, but too many holes can dry the diet in low humidity conditions, so this number may need to be adjusted. Fifth, the conditions in the climate chamber may need to be adjusted in combination with the cup conditions. If the conditions are too dry, host plants with eggs may dry out before larvae can be transferred, and cups with diet may dry out before butterflies emerge. On the other hand, if the conditions are too wet, the cups can harbor mold and disease. Researchers may need to adjust the airflow in cups through the use of mesh lids, or more or less holes in the lids. Another common issue is chamber lights that are bright enough to cause temperature swings in the cups and a build-up of condensation; using dimmer lights is an easy option for larval rearing.
With respect to the research questions in this paper, this study found that cabbage whites were relatively more sensitive to copper than to nickel or zinc. Copper had significant negative impacts on development time at concentrations as low as 50 ppm (Figure 3 and Table 3) and on survival at 500 ppm (Figure 4, Table 4). In contrast, there were no negative effects of nickel on survival (up to 500 ppm; Figure 3) or negative effects on development time at 100 ppm (Figure 4). Cabbage whites were fairly tolerant of zinc, with survival effects seen only at 1,000 ppm (Figure 3) and negative effects on development time starting at 100 ppm (Figure 4). Based on the relatively greater concentrations of zinc in butterfly tissue and mustards (their host plant; Figure 1), it was expected that a relatively greater tolerance to zinc would be seen. However, the sensitivity to copper and the tolerance of nickel were somewhat unexpected given the very low levels of nickel in butterfly tissue (Figure 1) and the necessity of copper as a micronutrient. These unexpected findings are discussed below after considering the tolerance of these metals in other butterflies and moths.
To compare the present data with metal sensitivity measured in other Lepidoptera, data from existing studies were compiled on the minimum concentration, where heavy metals negatively impacted survival49,50,51,56,63,64,65,66,67,68; these studies focused on moths, especially pest species (Galleria mellonella, Lymantria dispar, Plutella xylostella, Spodoptera sp.). All of the measured sensitivity values in this study fall close to the range measured for these other species (Figure 5). However, the measure of nickel tolerance in this study does seem to be higher than expected-while there was not a significant effect of survival at 500 ppm, the previous study on Pieris rapae also found a very high tolerance for nickel (significant effects starting at 1,000 ppm56), despite low levels in their tissue naturally (Figure 1). The measure of copper sensitivity in this study also seems to be at the low end for studies of Lepidoptera. While the use of an artificial diet allows a convenient and controlled comparison of relative metal sensitivity, it is important to note that components of the diet could alter the measurement of absolute metal sensitivity. For instance, vitamin C in the diet could offset metal-induced oxidative stress74, or antibiotics in the diet could alter any effects of microbes on the processing of metals75. An interesting line of future research would be to systematically manipulate such diet components to test effects on metal toxicity, especially given questions about the functional role of lepidopteran gut microbes76,77 and nectar components that may have antioxidant properties78. In addition, variation in dietary requirements across species can make interspecific comparisons challenging, and artificial diet-based methods should be complemented with manipulations of host plants.
These butterflies are particularly tolerant of nickel and sensitive to copper. Previous research has noted that many plants in the mustard family, which includes plants favored by Pieridae, hyper-accumulate nickel as a defensive mechanism against herbivores55,56,63,79,80,81. This hyper-accumulation is over 1,000 ppm in plant tissue, which is orders of magnitude greater than what is seen in most plants (Figure 1). It is possible that Pieris have a particularly high tolerance for nickel due to past selection by such nickel accumulators, as previously speculated26. While copper has been less frequently studied as a micronutrient in insect diets, there is some evidence that it plays a small role in reproduction and immunity, although primarily in blood-feeding insects (e.g.,82,83). It is possible that copper plays a less important physiological role in butterflies than in other animals84,85,86, consistent with recent work highlighting how copper may be as concerning of a pollutant for insects as lead, cadmium, and mercury (e.g.,87,88,89). While Pieris have been shown to avoid copper contamination at low levels90, the mobility of copper in plants (e.g., moving into leaves and flowers) has also flagged it as a metal contaminant of concern91.
While these results provide interesting data on the relative toxicity of these metals to cabbage white butterflies, this paper also aims to be of general use as a detailed visual illustration of methods for rearing this powerful system. Cabbage whites are easy to rear and manipulate in controlled lab experiments4,5 facilitating studies of host searching6,7,8, foraging9,10,11, and sexual selection12,13,14. The ability to rear these butterflies on an artificial diet is key in creating common garden conditions for comparisons and to manipulate nutrients, toxins, and even novel host plants. However, it is important to note that this artificial diet is not necessarily the optimal artificial diet for this species, and could likely be improved with future manipulations. For instance, the salt mix in this diet (and other lepidopteran diets) was originally developed for vertebrates and has higher calcium levels than what most insects need92,93. Thus, some of our rearing efforts have made custom salt mixes with lower calcium levels (e.g.,62), and others make use of "Beck's salt mix", which may be more appropriate for many insect species94. In our own manipulations, we also found that butterflies performed better with relatively less wheat germ and relatively more cellulose compared to original concentrations4. One area in need of further attention is the lipid source and concentration in the diet. For instance, past work has shown that shifting from linseed oil (used in this study) to phospholipids increased the mating rates and growth rates of Pieris on artificial diets95. Supplementation of specific fatty acids in artificial diets may have additional positive effects96,97. Optimizing the artificial diet of Pieris98,99 creates opportunities for addressing interesting questions about nutritional ecology100,101,102, evolutionary ecology, and ecotoxicology. These artificial diet approaches allow researchers to address questions about the role of specific lipids in cognitive evolution103, pre-adaptation to toxins28, dietary components that reduce the toxicity of pollutants104, or stoichiometric interactions between nutrients105.
Acknowledgements
We are grateful for the support from undergraduate assistants during the rearing for this work, in particular Regina Kurandina and Rhea Smykalski. Carolyn Kalinowski helped compile literature on metal toxicity in other Lepidoptera. This work was made possible by a University of Minnesota Department of Ecology, Evolution, and Behavior summer research grant.
Materials
| Name | Company | Catalog Number | Comments |
| 1-L Pyrex beaker | Fisher Scientific | 07-250-059 | |
| 500 mL graduated cylinder | Fisher Scientific | 03-007-43 | |
| 60-mm plastic petri dish lid | Fisher Scientific | 08-757-100B | |
| Ascorbic Acid | Frontier | 6015 | |
| Blender | Amazon - Ninja Store | BL610 Professional | |
| Cabbage Flour | Frontier | 1086 | |
| Casein | Frontier | 1100 | |
| Celluose | Frontier | 3425 | |
| Cholsterol | Sigma | C3045 | |
| Cups for rearing (4 oz) | Wasserstrom | 6094583 | purchase with matching lids |
| Fine Mesh Agar | Sigma | ||
| Flaxseed Oil | amazon | B004R63VI6 | |
| Floral water tubes, 2.8 x 0.8inch | Amazon - Yimaa Direct | B08BZ969DK | |
| Glassine envelopes (1 3/4 x 2 7/8 INCHES) | Amazon - Wizard Coin Supply | B0045FG90G | |
| Mesh Cages (15.7 x 15.7 x 23.6") | Amazon | B07SK6P94S | |
| Methyl Paraben | Frontier | 7685 | |
| Ohaus Portable Scale | Fisher Scientific | 02-112-228 | |
| Organic Honey | Amazon | B07DHQQFGM | |
| Photo studio portable lightbox | Amazon | B07T6TNYJ1 | |
| Plastic bin, shoebox size | Amazon | B09L3B3V1R | |
| Plastic disposable transfer pipets | Fisher Scientific | 13-680-50 | |
| Sorbic Acid | Sigma | S1626 | |
| Spatulas | Fisher Scientific | 14-357Q | |
| Streptomycin | Sigma | S9137 | |
| Sucrose | Target | ||
| Torula Yeast | Frontier | 1720 | |
| Vanderzant vitamin mix | Frontier | F8045 | |
| Weigh boats | Fisher Scientific | 01-549-750 | |
| Wesson Salt Mix | Frontier | F8680 | |
| Wheat Germ | Frontier | G1659 | |
| Wooden handled butterfly net, 12" hoop | Amazon - Educational Science | B00O5JDLVC | |
| Yellow sponges | Amazon-Celox | B0B8HTHY5B |
References
- Snyder, L. D., Gomez, M. I., Mudrak, E. L., Power, A. G. Landscape-dependent effects of varietal mixtures on insect pest control and implications for farmer profits. Ecological Applications. 31 (2), 2246 (2021).
- Shelton, A., Andaloro, J. T., Barnards, J. Effects of cabbage looper, imported cabbageworm, and diamondback moth on fresh market and processing cabbage. Journal of Economic Entomology. 75 (4), 742-745 (1982).
- Cartea, M. E., Padilla, G., Vilar, M., Velasco, P. Incidence of the major Brassica pests in northwestern Spain. Journal of Economic Entomology. 102 (2), 767-773 (2009).
- Troetschler, R. G., Malone, C. M., Bucago, E. R., Johnston, M. R. System for rearing Pieris rapae (Lepidoptera: Pieridae) on a noncruciferous artificial diet developed for Manduca sexta (Lepidoptera: Sphingidae). Journal of Economic Entomology. 78 (6), 1521-1523 (1985).
- Webb, S., Shelton, A. Laboratory rearing of the imported cabbageworm. New Yorks Food and Life Sciences Bulletin. 122, 1-6 (1988).
- Root, R. B., Kareiva, P. M. The search for resources by cabbage butterflies (Pieris rapae): ecological consequences and adaptive significance of Markovian movements in a patchy environment. Ecology. 65 (1), 147-165 (1984).
- Hern, A., Edwards-Jones, G., McKinlay, R. G. A review of the pre-oviposition behaviour of the small cabbage white butterfly, Pieris rapae (Lepidoptera: Pieridae). Annals of Applied Biology. 128 (2), 349-371 (1996).
- Renwick, J. A. A., Radke, C. D. Sensory cues in host selection for oviposition by the cabbage butterfly, Pieris-Rapae. Journal of Insect Physiology. 34 (3), 251-257 (1988).
- Lewis, A. C. Memory constraints and flower choice in Pieris rapae. Science. 232 (4752), 863-865 (1986).
- Kandori, I., Ohsaki, N. The learning abilities of the white cabbage butterfly, Pieris rapae, foraging for flowers. Researches on Population Ecology. 38, 111-117 (1996).
- Alm, J., Ohmeiss, T. E., Lanza, J., Vriesenga, L. Preference of cabbage white butterflies and honey-bees for nectar that contains amino-acids. Oecologia. 84 (1), 53-57 (1990).
- Espeset, A., et al. Anthropogenic increases in nutrients alter sexual selection dynamics: a case study in butterflies. Behavioral Ecology. 30 (3), 598-608 (2019).
- Tigreros, N. Linking nutrition and sexual selection across life stages in a model butterfly system. Functional Ecology. 27 (1), 145-154 (2013).
- Morehouse, N. I., Rutowski, R. L. In the eyes of the beholders: female choice and avian predation risk associated with an exaggerated male butterfly color. American Naturalist. 176 (6), 768-784 (2010).
- Stoehr, A. M., Goux, H. Seasonal phenotypic plasticity of wing melanisation in the cabbage white butterfly, Pieris rapae L. (Lepidoptera: Pieridae). Ecological Entomology. 33 (1), 137-143 (2008).
- Stoehr, A. M., Walker, J. F., Monteiro, A. Spalt expression and the development of melanic color patterns in pierid butterflies. Evodevo. 4 (1), 6 (2013).
- Stoehr, A. M., Wojan, E. M. Multiple cues influence multiple traits in the phenotypically plastic melanization of the cabbage white butterfly. Oecologia. 182 (3), 691-701 (2016).
- Ryan, S. F., et al. Global invasion history of the agricultural pest butterfly Pieris rapae revealed with genomics and citizen science. Proceedings of the National Academy of Sciences. 116 (40), 20015-20024 (2019).
- Snell-Rood, E. C., Papaj, D. R. Patterns of phenotypic plasticity in common and rare environments: a study of host use and color learning in the cabbage white butterfly Pieris rapae. American Naturalist. 173 (5), 615-631 (2009).
- Kono, Y. Rearing Pieris rapae crucivora Boisduval (Lepidoptera: Pieridae) on artificial diets. Applied Entomology and Zoology. 3 (2), 96-98 (1968).
- Parra, J. R. . The Evolution of Artificial Diets and their Interactions in Science and Technology. Insect Bioecology and Nutrition for Integrated Pest Management. , (2012).
- Morehouse, N. I., Rutowski, R. L. Developmental responses to variable diet composition in a butterfly: the role of nitrogen, carbohydrates and genotype. Oikos. 119 (4), 636-645 (2010).
- Rotem, K., Agrawal, A. A., Kott, L. Parental effects in Pieris rapae in response to variation in food quality: adaptive plasticity across generations. Ecological Entomology. 28 (2), 211-218 (2003).
- Shephard, A. M., et al. Assessing zinc tolerance in two butterfly species: consequences for conservation in polluted environments. Insect Conservation and Diversity. 13 (2), 201-210 (2020).
- Shephard, A. M., Zambre, A. M., Snell-Rood, E. C. Evaluating costs of heavy metal tolerance in a widely distributed, invasive butterfly. Evolutionary Applications. 14 (5), 1390-1402 (2021).
- Kobiela, M. E., Snell-Rood, E. C. Nickel exposure has complex transgenerational effects in a butterfly. Integrative and Comparative Biology. 58 (5), 1008-1017 (2018).
- Philips, K. H., Kobiela, M. E., Snell-Rood, E. C. Developmental lead exposure has mixed effects on butterfly cognitive processes. Animal Cognition. 20 (1), 87-96 (2017).
- Sikkink, K. L., et al. Tolerance of novel toxins through generalized mechanism simulating gradual host shifts of butterflies. American Naturalist. 195 (3), 485-503 (2020).
- Pentzold, S., et al. excretion and avoidance of cyanogenic glucosides in insects with different feeding specialisations. Insect Biochemistry and Molecular Biology. 66, 119-128 (2015).
- Lampert, E. C., Dyer, L. A., Bowers, M. D. Dietary specialization and the effects of plant species on potential multitrophic interactions of three species of nymphaline caterpillars. Entomologia Experimentalis et Applicata. 153 (3), 207-216 (2014).
- Genc, H., Nation, J. L. An artificial diet for the butterfly Phyciodes phaon (Lepidoptera: Nymphalidae). Florida Entomologist. 87 (2), 194-198 (2004).
- Brinton, F., Proverbs, M., Carty, B. Artificial diet for mass production of the codling moth, Carpocapsa Pomonella (Lepidoptera: Olethreutidae)1. The Canadian Entomologist. 101 (6), 577-584 (1969).
- Metwally, H. M., et al. Low cost artificial diet for rearing the greater wax moth, Galleria mellonella L. (Lepidoptera: Pyralidae) as a host for entomopathogenic nematodes. Egyptian Journal of Biological Pest Control. 22 (1), 15 (2012).
- Carpenter, J. E., Bloem, S. Interaction between insect strain and artificial diet in diamondback moth development and reproduction. Entomologia Experimentalis et Applicata. 102 (3), 283-294 (2002).
- Jaiswal, A., Verma, A., Jaiswal, P. Detrimental effects of heavy metals in soil, plants, and aquatic ecosystems and in humans. Journal of Environmental Pathology Toxicology and Oncology. 37 (3), 183-197 (2018).
- Kumar, A., et al. Lead toxicity: Health hazards, influence on food chain, and sustainable remediation approaches. International Journal of Environmental Research and Public Health. 17 (7), 2179 (2020).
- Gall, J. E., Boyd, R. S., Rajakaruna, N. Transfer of heavy metals through terrestrial food webs: a review. Environmental Monitoring and Assessment. 187, 201 (2015).
- Mohammed, A. S., Kapri, A., Goel, R. . Heavy Metal Pollution: Source, Impact, and Remedies. Biomanagement of Metal-Contaminated Soils. , 1-28 (2011).
- Mitra, A., Chatterjee, S., Voronina, A. V., Walther, C., Gupta, D. K. Lead toxicity in plants: a review. Lead in Plants and the Environment. , 99-116 (2020).
- Perugini, M., et al. Heavy metal (Hg, Cr, Cd, and Pb) contamination in urban areas and wildlife reserves: Honeybees as bioindicators. Biological Trace Element Research. 140 (2), 170-176 (2011).
- Rycewicz-Borecki, M., McLean, J. E., Dupont, R. R. Bioaccumulation of copper, lead, and zinc in six macrophyte species grown in simulated stormwater bioretention systems. Journal of Environmental Management. 166, 267-275 (2016).
- Zulfiqar, U., et al. Lead toxicity in plants: Impacts and remediation. Journal of Environmental Management. 250, 109557 (2019).
- Spliethoff, H. M., et al. Estimated lead (Pb) exposures for a population of urban community gardeners. Environmental Geochemistry and Health. 38, 955-971 (2016).
- Li, C. C., et al. Foliar dust as a reliable environmental monitor of heavy metal pollution in comparison to plant leaves and soil in urban areas. Chemosphere. 287, 132341 (2022).
- Ram, S. S., et al. Plant canopies: bio-monitor and trap for re-suspended dust particulates contaminated with heavy metals. Mitigation and Adaptation Strategies for Global Change. 19, 499-508 (2014).
- Karri, V., Schuhmacher, M., Kumar, V. Heavy metals (Pb, Cd, As and MeHg) as risk factors for cognitive dysfunction: A general review of metal mixture mechanism in brain. Environmental Toxicology and Pharmacology. 48, 203-213 (2016).
- Tong, S., von Schirnding, Y. E., Prapamontol, T. Environmental lead exposure: a public health problem of global dimensions. Bulletin of the World Health Organization. 78 (9), 1068-1077 (2000).
- Liu, J. H., Lewis, G. Environmental toxicity and poor cognitive outcomes in children and adults. Journal of Environmental Health. 76 (6), 130-138 (2014).
- Gintenreiter, S., Ortel, J., Nopp, H. J. Effects of different dietary levels of cadmium, lead, copper, and zinc on the vitality of the forest pest insect Lymantria-Dispar L (Lymantriidae, Lepid). Archives of Environmental Contamination and Toxicology. 25, 62-66 (1993).
- Cheruiyot, D. J., Boyd, R. S., Moar, W. Testing the joint effects hypothesis of elemental defense using Spodoptera exigua. Journal of Chemical Ecology. 41 (2), 168-177 (2015).
- Coleman, C. M., Boyd, R. S., Eubanks, M. D. Extending the elemental defense hypothesis: Dietary metal concentrations below hyperaccumulator levels could harm herbivores. Journal of Chemical Ecology. 31 (8), 1669-1681 (2005).
- Kaushal, S. S., et al. Making 'chemical cocktails'-Evolution of urban geochemical processes across the periodic table of elements. Applied Geochemistry. 119, 104632 (2020).
- Shephard, A. M., et al. Traffic patterns, more than adjacent land use, influence element content of roadside forbs for insect pollinators. Ecological Solutions and Evidence. 3 (4), 12195 (2022).
- Scott, J. A. . The Butterflies of North America: A Natural History and Field Guide. , (1992).
- Boyd, R. S. High-nickel insects and nickel hyperaccumulator plants: A review. Insect Science. 16 (1), 19-31 (2009).
- Boyd, R. S., Martens, S. N. Nickel hyperaccumulated by thlaspi-montanum var montanum is acutely toxic to an insect herbivore. Oikos. 70 (1), 21-25 (1994).
- Cempel, M., Nickel, G. A review of its sources and environmental toxicology. Polish Journal of Environmental Studies. 15 (3), 375-382 (2006).
- Aslam, J., Khan, S. A., Khan, S. H. Heavy metals contamination in roadside soil near different traffic signals in Dubai, United Arab Emirates. Journal of Saudi Chemical Society. 17 (3), 315-319 (2013).
- Mitchell, T. S., et al. Traffic influences nutritional quality of roadside plants for monarch caterpillars. Science of the Total Environment. 724, 138045 (2020).
- Voegborlo, R., Chirgawi, M. Heavy metals accumulation in roadside soil and vegetation along a major highway in Libya. Journal of Science and Technology. 27 (3), 86-97 (2007).
- Snell-Rood, E., Kobiela, M. . Data for: Rearing the Cabbage White Butterfly (Pieris rapae) in Controlled Conditions: A Case Study with Heavy Metal Tolerance. , (2023).
- Snell-Rood, E. C., Espeset, A., Boser, C. J., White, W. A., Smykalski, R. Anthropogenic changes in sodium affect neural and muscle development in butterflies. Proceedings of the National Academy of Sciences. 111 (28), 10221-10226 (2014).
- Boyd, R. S., Moar, W. J. The defensive function of Ni in plants: response of the polyphagous herbivore Spodoptera exigua (Lepidoptera: Noctuidae) to hyperaccumulator and accumulator species of Streptanthus (Brassicaceae). Oecologia. 118 (2), 218-224 (1999).
- Cheruiyot, D. J., Boyd, R. S., Moar, W. J. Exploring lower limits of plant elemental defense by cobalt, copper, nickel, and zinc. Journal of Chemical Ecology. 39 (5), 666-674 (2013).
- Davis, M. A., Boyd, R. S., Cane, J. H. Host-switching does not circumvent the Ni-based defence of the Ni hyperaccumulator Streptanthus polygaloides (Brassicaceae). South African Journal of Science. 97 (11-12), 554-557 (2001).
- Dubovskiy, I. M., Grizanova, E. V., Ershova, N. S., Rantala, M. J., Glupov, V. V. The effects of dietary nickel on the detoxification enzymes, innate immunity and resistance to the fungus Beauveria bassiana in the larvae of the greater wax moth Galleria mellonella. Chemosphere. 85 (1), 92-96 (2011).
- Jhee, E. M., Boyd, R. S., Eubanks, M. D. Nickel hyperaccumulation as an elemental defense of Streptanthus polygaloides (Brassicaceae): influence of herbivore feeding mode. New Phytologist. 168 (2), 331-343 (2005).
- Zhou, J. L., Shu, Y. H., Zhang, G. R., Zhou, Q. Lead exposure improves the tolerance of Spodoptera litura (Lepidoptera: Noctuidae) to cypermethrin. Chemosphere. 88 (4), 507-513 (2012).
- Snell-Rood, E. C., Davidowitz, G., Papaj, D. R. Reproductive tradeoffs of learning in a butterfly. Behavioral Ecology. 22 (2), 291-302 (2011).
- Snell-Rood, E. C., Davidowitz, G., Papaj, D. R. Plasticity in learning causes immediate and trans-generational changes in allocation of resources. Integrative and Comparative Biology. 53 (2), 329-339 (2013).
- Snell-Rood, E. C., Papaj, D. R., Brain Gronenberg, W. size: A global or induced cost of learning. Brain Behavior and Evolution. 73 (2), 111-128 (2009).
- Snell-Rood, E. C., et al. Nutritional constraints on brain evolution: Sodium and nitrogen limit brain size. Evolution. 74 (10), 2304-2319 (2020).
- Jaumann, S., Snell-Rood, E. C. Adult nutritional stress decreases oviposition choosiness and fecundity in female butterflies. Behavioral Ecology. 30 (3), 852-863 (2019).
- Sahiti, H., Bislimi, K., Bajgora, A., Rexhepi, A., Dalo, E. Protective effect of vitamin C against oxidative stress in common carp (Cyprinus carpio) induced by heavy metals. International Journal of Agriculture and Biosciences. 7 (2), 71-75 (2018).
- Rothman, J. A., Leger, L., Graystock, P., Russell, K., McFrederick, Q. S. The bumble bee microbiome increases survival of bees exposed to selenate toxicity. Environmental Microbiology. 21 (9), 3417-3429 (2019).
- Hammer, T. J., Janzen, D. H., Hallwachs, W., Jaffe, S. P., Fierer, N. Caterpillars lack a resident gut microbiome. Proceedings of the National Academy of Sciences. 114 (36), 9641-9646 (2017).
- Hammer, T. J., Sanders, J. G., Fierer, N. Not all animals need a microbiome. FEMS Microbiology Letters. 366 (10), (2019).
- Baker, H. G., Baker, I. . Coevolution of Animals and Plants. , 100-140 (1975).
- Boyd, R. S., Davis, M. A., Wall, M. A., Balkwill, K. Metal concentrations of insects associated with the South African Ni hyperaccumulator Berkheya coddii (Asteraceae). Insect Science. 13 (2), 85-102 (2006).
- Boyd, R. S., Wall, M. A., Jaffre, T. Nickel levels in arthropods associated with Ni hyperaccumulator plants from an ultramafic site in New Caledonia. Insect Science. 13 (4), 271-277 (2006).
- Kramer, U. Metal hyperaccumulation in plants. Annual Review of Plant Biology. 61, 517-534 (2010).
- Cardoso-Jaime, V., Broderick, N. A., Maya-Maldonado, K. Metal ions in insect reproduction: a crosstalk between reproductive physiology and immunity. Current Opinion in Insect Science. 52, 100924 (2022).
- Rivera-Perez, C., Clifton, M. E., Noriega, F. G. How micronutrients influence the physiology of mosquitoes. Current Opinion in Insect Science. 23, 112-117 (2017).
- Lee, J. H. Micronutrient deficiency syndrome: zinc, copper and selenium. Pediatric Gastroenterology, Hepatology & Nutrition. 15 (3), 145-150 (2012).
- Nube, M., Voortman, R. L. Human micronutrient deficiencies: linkages with micronutrient deficiencies in soils, crops and animal nutrition. Combating Micronutrient Deficiencies: Food-Based Approaches. , 289-311 (2011).
- Wysocka, D., Snarska, A., Sobiech, P. Copper-An essential micronutrient for calves and adult cattle. Journal of Elementology. 24 (1), 101-110 (2019).
- Oliveira, C. S., et al. Toxic metals that interact with thiol groups and alteration in insect behavior. Current Opinion in Insect Science. 52, 100923 (2022).
- Mogren, C. L., Trumble, J. T. The impacts of metals and metalloids on insect behavior. Entomologia Experimentalis et Applicata. 135 (1), 1-17 (2010).
- Hladun, K. R., Di, N., Liu, T. X., Trumble, J. T. Metal contaminant accumulation in the hive: Consequences for whole-colony health and brood production in the honey bee (Apis mellifera L.). Environmental Toxicology and Chemistry. 35 (2), 322-329 (2016).
- Elbassiouny, S. A. Changes in food-related behavioral-patterns of some phytophagous insect species following exposures to an antifeedant. Acta Phytopathologica et Entomologica Hungarica. 26 (3-4), 483-496 (1991).
- Hladun, K. R., Parker, D. R., Trumble, J. T. Cadmium, copper, and lead accumulation and bioconcentration in the vegetative and reproductive organs of Raphanus sativus: Implications for plant performance and pollination. Journal of Chemical Ecology. 41, 386-395 (2015).
- Nation, J., Robinson, F. Concentration of some major and trace elements in honeybees, royal jelly and pollens, determined by atomic absorption spectrophotometry. Journal of Apicultural Research. 10 (1), 35-43 (1971).
- Herbert Jr, E. W., Shimanuki, H. Mineral requirements for brood-rearing by honeybees fed a synthetic diet. Journal of Apicultural Research. 17 (3), 118-122 (1978).
- Beck, S. D., Chippendale, G., Swinton, D. Nutrition of the European corn borer, Ostrinia nubilalis. VI. A larval rearing medium without crude plant fractions. Annals of the Entomological Society of America. 61 (2), 459-462 (1968).
- Junnikkala, E. Rearing Pieris-Brassicae (L.) on a phospholipid and vitamin-supplemented semi-artificial diet. Annales Zoologici Fennici. 17 (1), 39-42 (1980).
- Hixson, S. M., et al. Long-chain omega-3 polyunsaturated fatty acids have developmental effects on the crop pest, the Cabbage White Butterfly Pieris rapae. PLoS One. 11 (3), e0152264 (2016).
- Turunen, S. Lipid utilization in adult Pieris brassicae with special reference to the role of linolenic acid. Journal of Insect Physiology. 20 (7), 1257-1269 (1974).
- Vanderzant, E. S. Development, significance, and application of artificial diets for insects. Annual Review of Entomology. 19, 139-160 (1974).
- Cohen, A. C. . Insect Diets: Science and Technology. , (2003).
- Balluffi-Fry, J., Leroux, S. J., Champagne, E., Vander Wal, E. In defense of elemental currencies: can ecological stoichiometry stand as a framework for terrestrial herbivore nutritional ecology. Oecologia. 199 (1), 27-38 (2022).
- Coogan, S. C. P., Raubenheimer, D., Zantis, S. P., Machovsky-Capuska, G. E. Multidimensional nutritional ecology and urban birds. Ecosphere. 9 (4), 02177 (2018).
- Raubenheimer, D., Simpson, S. J., Mayntz, D. Nutrition, ecology and nutritional ecology: toward an integrated framework. Functional Ecology. 23 (1), 4-16 (2009).
- Arien, Y., Dag, A., Zarchin, S., Masci, T., Shafir, S. Omega-3 deficiency impairs honey bee learning. Proceedings of the National Academy of Sciences. 112 (51), 15761-15766 (2015).
- Man, K. Y., et al. Use of biochar as feed supplements for animal farming. Critical Reviews in Environmental Science and Technology. 51 (2), 187-217 (2021).
- Shephard, A. M., Knudsen, K., Snell-Rood, E. C. Anthropogenic sodium influences butterfly responses to nitrogen-enriched resources: implications for the nitrogen limitation hypothesis. Oecologia. 201 (4), 941-952 (2023).
This article has been published
Video Coming Soon
ABOUT JoVE
Copyright © 2024 MyJoVE Corporation. All rights reserved