Reproducibility and Harmonization in Research Using Biological Standards: The Example of Platelet Agonist Collagen-Related Peptide
In This Article
Summary
Here we present a method to standardize the platelet agonist collagen-related peptide cross-linked (CRP-XL) using light transmission aggregometry. While the protocol is targeted at platelet function, the experimental process can be applied to most biological molecules and bioassays to ensure scientific rigor and reproducibility.
Abstract
Metrology - the science of measure - is a subject few biological scientists are taught about in their training to their detriment; the application of simple standardization processes to everyday working practices provides confidence in data and reproducibility over distance and time.
This method demonstrates how to standardize a core laboratory experiment used widely in hemostasis research and clinical practice, specifically, measuring responses to the platelet collagen receptor (glycoprotein [GP]VI) agonist collagen-related peptide, cross-linked (CRP-XL) by light transmission aggregometry (LTA). Using this approach will ensure intra-lab reproducibility and inter-lab harmonization, regardless of agonist stock or supplier. Importantly, this method is applicable to other platelet agonists and, indeed, many other biological molecules and bioassays.
The process outlined below involves making a 6-8 point dilution series of the 'standard' and the 'test' (the material you are checking) and running them side by side in a chosen assay (in this case, LTA). CRP-XL is used at mass/volume concentrations, but not every material gives the same biological activity at a given concentration, so a dilution series is made to compare the standard and test material and determine what concentration is needed to give equivalent activity. The dilution series must span 0-100% aggregation. Data is plotted using non-linear regression, and the EC50 value of each sample (standard and test) is determined. To assign activity, divide the EC50 value of the standard by that of the test to determine how much more or less potent it is and adjust the concentration accordingly. This approach will ensure that the same biological 'activity' is added to the assay time and time again.
Introduction
Many of us use biological agonists and antagonists in our experiments, most often in a biological assay that quantifies their effect on cell function under specific conditions. The method described here is for the platelet agonist collagen-related peptide, cross-linked (CRP-XL), a glycoprotein VI agonist that activates platelets and whose activity can be measured in a variety of assays (light transmission aggregometry, microscopy, flow cytometry, Ca2+ release, etc.), but the agonist standardization protocol is applicable to any biological agonist/antagonist and/or bioassay. The physical sciences are well versed in the use of standards in their day-to-day working practices, and companies developing biotherapeutic medicines in the commercial sector understand the value of using reference standards1, yet they remain underutilized by many biological scientists, especially in the academic research community, and their lack of use negatively impacts the quality of scientific output.
CRP-XL is a specific GPVI-specific agonist that the platelet field has used for decades since its description in 19952, helping the community to delineate the role of GPVI from that of other collagen-binding platelet proteins ever since3,4. This agonist can be procured from a variety of commercial sources or produced in-house. The peptide monomer can be purchased from a supplier and then cross-linked in-house or this can be done by the supplier for an extra fee. Further cost-saving can be made by reducing the required purity upon delivery (70% versus 95%, for example). There is also no consensus on how CRP-XL should be stored, with some opting for cryostorage and others refrigeration, some keeping the material lyophilized until use, and others storing it in solution (water or buffer, depending on preference). Therefore, the quality and composition of laboratory stocks are non-standardized or harmonized. Because this agonist is used at a defined concentration (mass/volume) rather than units of activity, the potency of CRP-XL in one lab, e.g., at 1 μg/mL, will not be the same as another. It is worth noting that other platelet agonists used in the field are already standardized (e.g., thrombin, which is supplied and used in units of activity rather than mass/volume), so the movement away from mass/volume to units of biological should not be too challenging for the community. It should also be noted that patient responses to platelet agonists, including CRP-XL, are variable in terms of their sensitivity to agonists as well as their capacity to respond (extent of response)5, so it is even more important to use consistent amounts of agonist activity in the assays.
If platelet agonists can be harmonized by using them at a defined activity rather than weight/volume, it can be ensured that an experiment in one lab is directly comparable to that in other labs and can be accurately reproduced with confidence. Until such time as the field reaches an agreement on how to store and standardize CRP-XL activity, it is essential to perform regular checks on the material to ensure its consistency over the months/years in which it is being used.
Activity value assignment for biological standards must be done through collaborative, multi-center studies (for example6,7) and require specialist expertise. The need for established biological standards for platelet agonists has been recently highlighted by a collaborative multi-center study8 supported by the International Society for Thrombosis and Haemostasis (ISTH); until an international CRP-XL standard is available, researchers can standardize their own material locally to ensure consistency over time, and that new material sourced commercially or in-house is of comparable activity to previous preparations, as well as that of the rest of the community.
Protocol
Blood should be collected from consenting healthy volunteers and processed in accordance with local rules. This protocol measures agonist-induced platelet aggregation in platelet-rich plasma (PRP) by light transmission aggregometry (LTA). The ISTH provides standardized protocols, including a 2013 method for PRP preparation and LTA9. Collect whole blood into 4% sodium citrate as per ISTH recommendations in accordance with local ethics committee rules. Exclude donors that have taken any NSAID within the past 2 weeks.
1. Assay procedure
- Take an aliquot of stock CRP-XL and dilute it in assay (Tyrodes10) buffer (Table 1) to a starting concentration that will cause maximum aggregation (see the NOTE below step 1.3).
NOTE: In the literature, a concentration of 1 μg/mL will induce maximum aggregation, but some labs need higher concentrations to reach the same effect - adjust the dilution series accordingly - Make a corresponding dilution series with the standard (see the NOTE below step 1.3 and Figure 1) such that the concentrations are the same as the test reagent.
- Produce a 6- to 8-point dilution series for the test and the standard, again in assay buffer, that takes the aggregation response from 100% to 0% aggregation. An example showing an 8-point dilution series concentration curve is shown in Table 2.
NOTE: Some labs add agonist at a total of 10% assay volume, e.g., 50 μL of agonist to 450 μL of platelets, such that they dilute a 10x concentration to a final 1x concentration in the assay, while others add smaller (more concentrated) volumes of agonist, e.g., 5 μL of agonist to 495 μL of platelets - just make sure that the dilution series is appropriate for the assay bearing in mind how the volume displacement affects platelet concentration - again, be consistent. In the example, 30 μL of 10x agonist is added to 270 μL of platelet rich plasma (PRP). - Once the platelets are rested and ready to use, aliquot them into LTA cuvettes, add a stir bar, and allow them to reach 37 °C in the holding wells.
- Once at temperature, place the cuvette(s) into the aggregometer and begin recording the trace(s). Add the agonist (with stirring) and record the data.
- Repeat step 1.4 for each concentration of the test and standard until the data needed to plot the curves has been collected (example curves shown in Figure 2).
- Calculate the potency of the test reagent relative to that of the standard; again, use any metric - maximum aggregation or area under the curve - as long as there is consistency and the analysis model appropriately fits the data.
2. Checking the concentration curves
- First, check that the concentration response curves are parallel. There are many software packages that do this, but here the process is described for Graphpad Prism (see Figure 3).
- Enter the data so that log(concentration) is on the X axis and response (% maximum aggregation/AUC) is on the Y axis (Figure 3A)
- Transform CRP-XL concentrations (Figure 3B)
- Select Non-Linear Regression from the Analysis functions and use the equation for dose-response stimulation/inhibition.
- Select log (agonist) vs. response (variable slope) - use 3- or 4-way fit depending on which is best for the data.
- Determine whether the curves are parallel by using the Compare tab (as shown in Figure 3C) and click on the button to compare the datasets to ensure that the slope (Hill slope) and asymptotes (the Top and Bottom of the curves) are equivalent. In this example, the Hill slope for the test is 2.279, and that of the standard is 2.025 giving a ratio of 1.125.
- If the slopes are parallel (for example, an acceptance criterion of slope ratio between 0.8-1.25) and the asymptotes are equivalent, proceed with potency (EC50) calculation using steps 2.1.3 and 2.1.4. In the example shown here, the asymptotes have been constrained as the evaluation in step 2.1.5 confirmed that the asymptotes are equivalent.
- Divide the EC50 value of the standard by that of the test to determine the relative potency. In the example shown here, test/standard (0.07259/0.1498) = 0.4846, i.e., the 'test' is half as potent as the standard.
- Adjust mass/volume concentrations to account for the difference in potency, i.e., if the standard was used previously at 1 µg/mL, the new material would be used at 1/0.4846 = 2.063 µg/mL) to ensure comparable amounts of active material are being used in experiments
Representative Results
Figure 1 is a diagrammatic representation of a dilution series and meant to highlight that a dilution series is needed for both the standard and the test. Figure 2A shows aggregation traces for the standard, while the test data is shown in Figure 2B. At 0.075 µg/mL, there is a clear response to the standard, while for the test, the corresponding concentration (0.075 µg/mL) is flat, i.e., no response. These data are used to plot the subsequent graphs to give the EC50. In the example shown here, 100% maximum aggregation was used to plot the concentration-response curve and determine EC50, as shown in Figure 3.
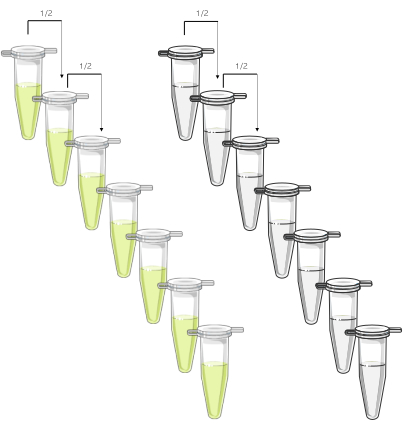
Figure 1: Graphical description of the two dilutions series of the test (left) and the standard (right). Starting with the top concentration (suggested concentration: 10 µg/mL CRP-XL), sequential 1 in 2 dilutions are made in parallel over 8 points (>3 log units). Please click here to view a larger version of this figure.
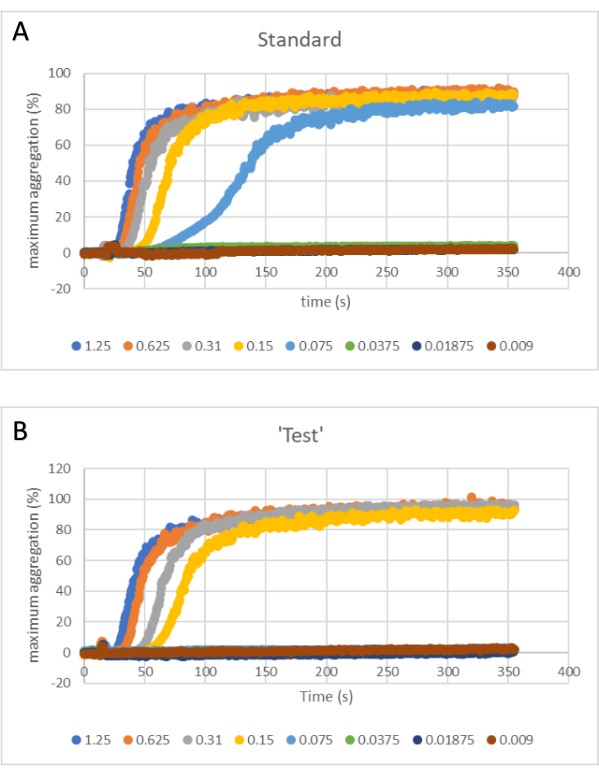
Figure 2: Aggregation traces for the standard (left) and test dilution series (right). As platelet aggregation proceeds (x-axis), the amount of light passing through the sample and reaching the sensor increases (y-axis). (A) Standard. (B) Test. Please click here to view a larger version of this figure.
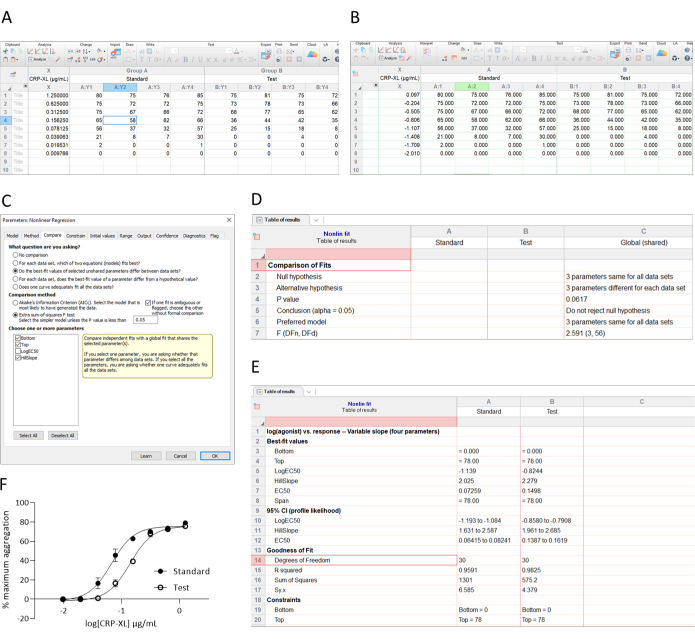
Figure 3: Data entry and analysis. (A) Data is entered, (B) transformed, and (C,D) analyzed for parallelism (ask the software to tell if the Hill slopes and asymptotes are comparable [C]). (E) If the curves are parallel, determine the potency (EC50) using non-linear regression curve fitting. (F) Plotted transformed data. Please refer to the method for more detail. Please click here to view a larger version of this figure.
| Tyrodes buffer9 | ||
| Constituents | Concentration | Comments |
| NaCl | 134 mM | |
| Na2HPO4 | 0.34 mM | |
| KCl | 2.9 mM | |
| NaHCO3 | 12 mM | |
| 4-(2- hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) | 20 mM | |
| Glucose | 5 mM | |
| MgCl2 | 1 mM | pH 7.3 |
Table 1: Composition of Tyrodes buffer.
| 8 point dilution series | |
| Dilution 1 | 10 µg/mL |
| Dilution 2 | 5 µg/mL |
| Dilution 3 | 2.5 µg/mL |
| Dilution 4 | 1.25 µg/mL |
| Dilution 5 | 0.625 µg/mL |
| Dilution 6 | 0.3125 µg/mL |
| Dilution 7 | 0.15625 µg/mL |
| Dilution 8 | 0.073125 µg/mL |
Table 2: An example showing an 8-point dilution series.
Discussion
As shown here, the process of standardizing the materials is very straightforward. Although it requires a little bit of time to evaluate new batches of material and/or to check that the current batch is stable and not losing activity over time, the reward is that experiments are reproducible time and time again and comparable over years rather than just the lifetime of a batch of reagent. It also means that other researchers can accurately recreate assay conditions and that the community is harmonized at the global level. Critical steps in the protocol include whole blood collection and PRP preparation9, as well as precision when making the dilution series. It is also important to ensure the test and standard curves are parallel before proceeding with the EC50 comparison. If the lines are not parallel, or the asymptotes are not equivalent, it could indicate that the test and standard material are different to some degree.
Everyone that works in the biological sciences knows that biological assays do not always perform consistently, so one needs to be mindful of this when evaluating the data. In the example shown here, even though we are using the same donors to measure the potency of two very similar, if not identical, materials, the hill slopes and asymptotes are not identical. Yet we accept a degree of variability and conclude that the curves are, in this case, parallel, and therefore suitable for comparing and adjusting potency. Biological molecules are large and complex, and post-translational modifications during manufacture can influence their potency in bioassays. Standardizing biomolecules and bioassays cannot be done using just mass or volume and must be done by quantifying and comparing biological activity in a bioassay11. One must use his/her training and experience to evaluate the data in the context of the assay.
Limitations of the technique are that while the data may indicate an issue with the reagent(s), it cannot tell you what the issue is. Further work will be needed to determine why the materials are not comparable. In an ideal world, any material critical for experiment(s) and subsequent scientific conclusions would be verified by mass spectroscopy, but this is not always an option. However, if the data are not comparable further investigation is still warranted in some capacity. Another limitation of this approach is the time and resources needed to do it. Ideally, the test and standard should be compared in 3-6 donors to provide some confidence in assigning biological activity to the material, but we feel this is justified by supporting reproducibility and reliability. Standardizing biological material is not needed every time experiments are run, but at a minimum, it should be done for every new batch of agonists, preferably more frequently, depending on stability and use. Indeed, should a standard be made available, this is something that international standardization organizations can provide clarity on.
While the example shown here is for CRP-XL in the context of measuring platelet aggregation, the protocol of comparing the biological activity of biomolecules in bioassays is widely applicable across the sector.
Acknowledgements
None.
Materials
| Name | Company | Catalog Number | Comments |
| 4% sodium citrate | Sigma-Aldrich | S1804 | Anticoagulant dissolved in water9 |
| 4-(2- hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) | Sigma-Aldrich | 54457 | |
| CRP-XL | Peptide Protein Research Ltd. | A crosslinked, triple-helical peptide2,4 with the sequence GCP*(GPP*)GCP*G (single letter amino acid code: P* = hydroxyproline was obtained as a custom order from Peptide Protein Research Ltd. | |
| Cuvettes and stir bars | Stago | 86921 | Consumables for aggregometer |
| Glucose | Sigma-Aldrich | G7021 | |
| KCl | Sigma-Aldrich | P5405 | |
| MgCl2 | Sigma-Aldrich | M4880 | |
| Na2HPO4 | Sigma-Aldrich | S5136 | |
| NaCl | Sigma-Aldrich | Sigma-Aldrich | |
| NaHCO3 | Sigma-Aldrich | S6014 | |
| Prism | Graphpad | Analysis software package | |
| Platelet rich plasma | Isolated from whole blood from healthy volunteers free of non-steroidal anti-inflammatory drugs for a minimum of 10 days8. |
References
- Faya, P., et al. Potency assignment of biotherapeutic reference standards. Journal of Pharmaceutical and Biomedical Analysis. 191, 113577 (2020).
- Morton, L. F., et al. Integrin alpha 2 beta 1-independent activation of platelets by simple collagen-like peptides: collagen tertiary (triple-helical) and quaternary (polymeric) structures are sufficient alone for alpha 2 beta 1-independent platelet reactivity. The Biochemical Journal. 306 (2), 337-344 (1995).
- Pugh, N., et al. Synergism between platelet collagen receptors defined using receptor-specific collagen-mimetic peptide substrata in flowing blood. Blood. 115 (24), 5069-5079 (2010).
- Farndale, R. W., et al. Cell-collagen interactions: the use of peptide Toolkits to investigate collagen-receptor interactions. Biochemical Society Transactions. 36 (Pt 2), 241-250 (2008).
- Dunster, J. L., et al. Multiparameter phenotyping of platelet reactivity for stratification of human cohorts. Blood Advances. 5 (20), 4017-4030 (2021).
- Hubbard, A. R., et al. Establishment of the WHO 2nd International Standard Factor V, plasma (16/374): communication from the SSC of the ISTH. Journal of Thrombosis and Haemostasis. 17 (4), 695-697 (2019).
- Sergaki, C., et al. Developing whole cell standards for the microbiome field. Microbiome. 10 (1), 123 (2022).
- Alessi, M. C., et al. Multi-center evaluation of light transmission platelet aggregation reagents: Communication from the ISTH SSC Subcommittee on Platelet Physiology. Journal of Thrombosis and Haemostasis. (23), (2023).
- Cattaneo, M., et al. Recommendations for the standardization of light transmission aggregometry: A consensus of the working party from the platelet physiology subcommittee of SSC/ISTH. Journal of Thrombosis and Haemostasis. , (2013).
- Taylor, L., et al. Discovery of novel GPVI receptor antagonists by structure-based repurposing. PLoS One. 9 (6), e101209 (2014).
- Coxon, C. H., Longstaff, C., Burns, C. Applying the science of measurement to biology: Why bother. PLoS Biology. 17 (6), e3000338 (2019).
This article has been published
Video Coming Soon
ABOUT JoVE
Copyright © 2024 MyJoVE Corporation. All rights reserved