A Cryoinjury Model for Studying Skeletal Muscle Regeneration of the Caudal Peduncle in Adult Zebrafish
In This Article
Summary
This protocol describes a cryoinjury model to induce profound damage of several caudal myomeres in adult zebrafish. This method provides a new approach for studying skeletal muscle regeneration after severe loss of tissue in non-mammalian vertebrates.
Abstract
Skeletal muscle undergoes renewal and restoration after minor injury through the activation of satellite-like stem cells. Severe injuries of the musculature often lead to fibrosis in humans. In comparison to mammals, zebrafish possess a higher innate capacity for organ regeneration, providing a powerful model for studying tissue restoration after extensive damage to the organ. Here, a cryoinjury model is described to induce profound damage to four myomeres of the caudal peduncle in adult zebrafish. A custom-made cryoprobe was designed to fit the body shape and reproducibly injure the lateral musculature from the skin to the midline. Importantly, the body integrity remained intact, and the fish continued their swimming activity. Changes to the skeletal muscle were assessed by histological staining and fluorescence staining of sarcomeric proteins on tissue sections. This method will open up new avenues of research aiming to understand how the degeneration of the skeletal muscle induces reparative responses and, thus, the reactivation of the myogenic program in adult zebrafish.
Introduction
In vertebrates, damaged parts of various tissues undergo homeostatic renewal and restoration during the lifespan. This capacity for renewal and restoration typically depends on the presence of competent stem cells or the proliferative capacity of the mature cells1,2. Skeletal muscle comprises post-mitotic myofibers, which are associated with local stem cells, called satellite cells3,4,5,6. Thus, this tissue contains cellular sources for the efficient sealing of areas of interrupted continuity or for the repair of minor wounds. However, larger volumetric losses in mammalian skeletal muscle are often followed by non-regenerative repair, such as fibrosis7. Animal models could provide new insights into the biological mechanisms that promote the regeneration of extensively damaged organs.
The zebrafish is a well-established model organism with a high regenerative capacity. Adult zebrafish can regenerate an amputated part of their caudal fin or the resected apex of the cardiac ventricle8,9,10,11. In addition, a cryoinjury method has previously been applied to study fin and heart regeneration in zebrafish12,13,14,15. In the case of inner organs, the cryoinjury method has the advantage of inducing cell death without disrupting the organ integrity, thus mimicking physiological conditions16,17. Tissue debris is disintegrated by natural clearance during wound healing, followed by the reparative processes. However, whether this method could be applied to skeletal muscle remains to be established.
In fish, the lateral musculature enables the side-to-side bending of the trunk during swimming18. The skeletal muscles are organized into metameric units, called myomeres, which are separated by connective tissue5,19. Zebrafish can regenerate their muscle after minor tissue disruptions, such as those caused by laser ablation or a stab wound20,21,22,23,24, but whether whole myomeres can regenerate after extensive injury remains unknown. This gap in knowledge is probably due to the lack of a suitable injury model. This protocol establishes a new approach to induce extensive injury of the skeletal muscle, spanning multiple myomeres. The described cryoinjury method is based on the rapid freezing and thawing of the myofibers with a precooled stainless-steel instrument. Despite the extensive damage, the well-being of the fish was not severely impaired. Entire myomeres could be restored, and, thus, this work provides a new model system for studying the mechanisms of musculature regeneration in adult zebrafish.
Protocol
This study was conducted in agreement with all the relevant ethical regulations. The zebrafish were bred, raised, and maintained in accordance with the Federation of European Laboratory Animal Science Associations (FELASA) guidelines25. The animal housing and all experimental procedures were approved by the cantonal veterinary office of Fribourg, Switzerland.
1. Equipment and setup
- Arrange the production of a stainless steel cryoprobe in a technical workshop that can manufacture instruments for research.
NOTE: Provide a design of the instrument with the specific dimensions (Figure 1A). - To avoid frostbite while handling the probe during the procedure, insert the handle into a pipette tip, and wrap it with tape.
- Prepare a beaker for the anesthesia working solution, a spoon for handling the fish, a moist sponge, and a tank with system water to let the fish recover after the procedure.
- Prepare the working solution freshly before every experiment. To prepare the working solution, add 4 mL of Tricaine stock solution to 100 mL of system water in a beaker.
NOTE: The anesthesia stock solution consists of 4 g of Tricaine dissolved in 980 mL of distilled water. After adjusting the pH to 7 with 1 M Tris-HCl, pH 9, fill up the solution to 1 L with distilled water. The stock solution is light-sensitive and should be stored at 4 ˚C in an amber bottle.
2. Muscle cryoinjury procedure
- Begin the procedure by immersing the probe in liquid nitrogen for a minimum of 3 min. Wet the sponge in system water, and place it on a flat surface.
- Transfer a single adult zebrafish into the Tricaine working solution, and confirm its unresponsiveness by touching the fish gently with the spoon after 1 min or 2 min. If the fish is still reactive, wait a bit longer.
- Place the anesthetized fish onto the wet sponge. Locate the caudal peduncle posterior to the anal fin and anterior to the caudal fin.
- Remove the cryoprobe from the liquid nitrogen. Shake the probe gently to ensure no residual liquid nitrogen remains on the tip.
- Place the edge of the spatula perpendicularly to the body on the caudal peduncle (Figure 1C). Keep the probe in this position for 6 s without applying pressure (Figure 1D).
NOTE: The weight of the cryoprobe is sufficient to ensure contact between the instrument and the tissue. - Release the cryoprobe from the tissue, and transfer the fish into the tank with system water.
- Monitor the fish while it resumes breathing and swimming after waking up from the anesthesia. If opercular movements do not occur after 30 s, stimulate the fish by pipetting system water into the gills until the animal initiates respiration by itself.
NOTE: The fish should resume swimming within a few minutes in the recovery tank.
NOTE: In the following days, the fish can be filmed to monitor their swimming activity (Video 1 and Video 2). Before the video recording, transfer the control and injured fish into a translucent mating tank, and let them habituate for at least 1 min.
3. Caudal peduncle collection and fixation
- Prepare 2 mL of 4% formalin or other fixative that is suitable for the subsequent assays, a Petri dish, forceps, and surgical scissors.
- Euthanize the fish at a selected time point after the cryoinjury according to the legal permission given by the regional veterinary office.
NOTE: Euthanasia is performed by an overdose of tricaine solution (300 mg/L) for approximately 10 min. The loss of gill movement and the tail fin pinch reflex confirms death. The time points of euthanasia should be selected according to the phase of regeneration: for example, from 1 day post-cryoinjury (dpci) to 3 dpci for wound clearance; from 3 dpci to 10 dpci for the initiation of muscle regeneration; from 10 dpci to 30 dpci for progressing regeneration; and after 30 dpci for the completion of tissue restoration. Thus, to assess the different steps and biological processes of muscle degeneration and regeneration, groups of fish need to be euthanized at various time points after cryoinjury. - Place the euthanized fish in a Petri dish containing deionized water. Use scissors to perform a cut through the body anterior and posterior to the caudal peduncle (Figure 1E). Let the tissue bleed out in the deionized water of the Petri dish.
- Collect the caudal peduncle, and transfer it with forceps into the prepared fixative solution in a microcentrifuge tube.
NOTE: Carefully invert the tubes several times, and keep them overnight at 4 ˚C.
4. Mounting the caudal peduncle
- Wash the fixed tissue in 1x PBS for 10 min on a rocker. Then, transfer it into a 2 mL microcentrifuge tube with pre-cooled 30% sucrose in deionized water at 4 ˚C, and gently invert the tube several times. Leave the microcentrifuge tubes for a minimum of 24 h at 4 ˚C in an upright position.
NOTE: The caudal peduncle will float on top of the sucrose solution and begin to sink as the tissue dehydrates. - Fill an embedding mold with a 5 mm layer of O.C.T. mounting medium. Use forceps to adjust the caudal peduncle in the medium. Place it at the bottom of the mold, and adjust its orientation for either transversal or coronal sections (Figure 1F).
- Let the medium freeze in a box of dry ice. As soon as the tissue is stabilized in the desired position, fill up the rest of the mold before the O.C.T. completely freezes over. Store the mold for a minimum of 1 h at −80 ˚C.
NOTE: Under these conditions, the tissues can be stored for many months.
5. Cutting the sections with a cryostat
- Set up a cryostat with a cutting thickness of 25 µm. Adjust the chamber temperature to −26˚C, the specimen temperature to −24 ˚C, and the cutting angle to 12˚.
- Place the frozen block with the specimen in the cryostat, and fix its orientation in order to cut parallel to the bottom of the block. Cut until you reach the specimen, and then trim the block to ease the sample collection.
- Prepare six adhesion slides. Label the slides with consecutive numbers in order to prepare replicates of the sample.
- Start cutting, and collect the tissue sections on the labeled adhesion slides. Arrange the sections on the slides as per the further experimental demands.
- Let the slides dry for 1 h at room temperature. Store them in closed boxes at −20 °C.
NOTE: The slides can be safely stored in this condition for up to 1 year.
Representative Results
Monitoring of the fish after cryoinjury
To determine the effect of myomere cryoinjury on animals, a video recording of control and cryoinjured fish at 1 days post-cryoinjury (dpci) and 5 dpci was performed. Each group contained five fish. At 1 dpci, the cryoinjured fish were swimming less actively, but they did not display any abnormal movements, such as swirling, convolution, or reduced equilibrium (Video 1). In the husbandry system, their position in the tank and food intake were similar to those of the uninjured fish. Normal behavior persisted throughout the following days, as exemplified by the video at 5 dpci (Video 2). In conclusion, the cryoinjury procedure of the caudal peduncle did not severely affect the well-being of the animals.
Histological analysis of the caudal peduncle sections
To assess the extent of the injury, the time point of 4 dpci was selected, as this is when the myofiber debris has been completely resorbed in the wound. To analyze the effects of cryoinjury along the dorso-ventral and anterior-posterior axes of the body, two groups of fish were used (i.e., coronal and transversal sections of the caudal peduncle, respectively) (Figure 1F).
The sections were analyzed by trichrome staining composed of Aniline Blue, Acid Fuchsin, and Orange G (AFOG). Using this combination of reagents, intact muscles were shown in orange, the spinal cord in dark red, and the collagenous matrix in blue. To determine the number of damaged myomeres, which are the metameric units of the fish musculature, a series of sections were analyzed (Figure 2). Myomere boundaries, called myocommata, were identified by collagen deposition, as detected by blue coloration. The damaged areas were determined by the absence of orange staining. A closer examination of specimens with evident myocommata revealed that approximately four consecutive myomeres were damaged, as inferred from the lack of orange staining (n, number of fish = 4; Figure 3A,A'). The uninjured side of the same fish served as the internal reference.
To examine the depth of the wound perpendicular to the body axis, transversal sections were prepared using zebrafish at 4 dpci and 7 dpci. The latter time point corresponds to the activation of the myogenic program and, thus, the onset of muscle regeneration. AFOG staining of these specimens displayed an extensive lack of orange staining in the cryoinjured flank of the body, demarcating the zone of degenerated skeletal muscle (Figure 3B,C). At 4 dpci and 7 dpci, the wound area spanned tissues from the skin toward the vertical septum. This demonstrates that the cryoinjury method deeply targeted one lateral half of the caudal peduncle, which remained devoid of functional muscle for 7 days after the procedure. Taken together, four myomeres were profoundly damaged on one side of the caudal peduncle.
Immunofluorescence analysis of the transversal sections
To assess the dynamics of muscle regeneration, experimental groups of fish were euthanized at 4 dpci, 7 dpci, 10 dpci, and 30 dpci. The transversal sections of the caudal peduncle were labeled by multicolor fluorescence staining using Phalloidin (which binds to filamentous actin [F-actin]), Tropomyosin-1 antibody, which detects a sarcomere protein, and DAPI, which labels nuclei. At all the time points, the uninjured half of the body provided an internal control; both F-actin and Tropomyosin 1 were strongly detected in the uninjured control parts, indicating undamaged tissue (Figure 4).
At 4 dpci, the injured side of the caudal peduncle contained abundant DAPI-positive cells, but little to no F-actin and Tropomyosin 1 immunofluorescence was observed, indicating the wound zone with degenerated muscles (Figure 4A-B'). At 7 dpci, Tropomyosin 1 and F-actin could be detected in a part of the wound close to the vertical body midline (Figure 4C-D'). This expression pattern demarcates the position where the formation of new myofibers begins in the caudal peduncle. At 10 dpci, both muscle markers expanded toward the surface of the body, suggesting progressing regeneration of the skeletal muscle (Figure 4E-F'). At 30 dpci, both sides of the body displayed a similar distribution of F-actin staining (Figure 4G-H'). This finding indicates that the skeletal muscle was efficiently restored after the cryoinjury of the caudal peduncle.
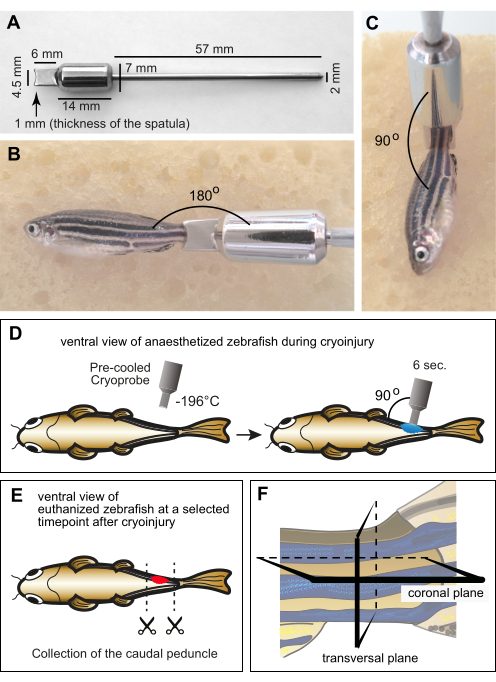
Figure 1: Experimental setup for myomere cryoinjury. (A) Dimensions of the custom-manufactured cryoprobe made of stainless steel. The distal part of the instrument consists of a spatula with a concave edge at the depth of 1 mm to account for the curvature of the zebrafish body. The middle part of the tool comprises a cylinder that functions as a weight and a reservoir to maintain the low temperature of the spatula during the procedure. The proximal end of the instrument is in the form of a thin metal handle. (B,C) Anesthetized adult fish on a moist sponge with the cryoprobe on the caudal peduncle. The probe was at room temperature. (B) The margin of the probe is placed horizontally in the vicinity of the caudal peduncle to display the relative size between the fish and the tool. (C) For cryoinjury, the tip of the tool is positioned perpendicular to the fish. (D) Schematic illustration of the cryoinjury procedure from the ventral side of the fish to show the manipulations in a comprehensive manner. The cryoprobe was precooled in liquid nitrogen and immediately placed on one side of the fish for 6 s. (E) At a specific time point after cryoinjury, the fish were euthanized, and their caudal peduncles were collected for fixation. (F) The fixed material was histologically processed and sectioned along the coronal or transversal planes. Please click here to view a larger version of this figure.
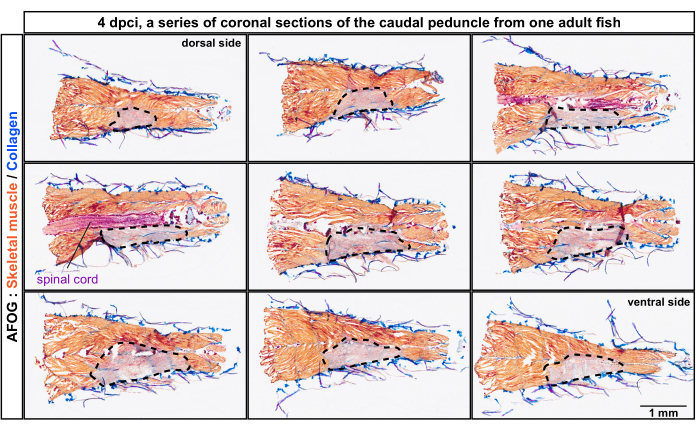
Figure 2: Histological analysis of damaged myomeres in the caudal peduncle from the dorsal to the ventral position of the body. AFOG staining of a series of coronal sections at 4 days post-cryoinjury (dpci). The sections are from the dorsal toward the ventral side, as indicated on the top of the first and the last panel. The sections are non-adjacent, with an interval of approximately 150 µm between them. The uninjured muscle is detected by orange staining of the muscle, whereas the injured tissue lacks this staining and appears grayish (area encircled with a dashed line). Collagen-containing tissues, such as the skin, are stained in blue. The spinal cord appears as a rod-like structure and is stained in red. Number of fish, n = 4. Please click here to view a larger version of this figure.
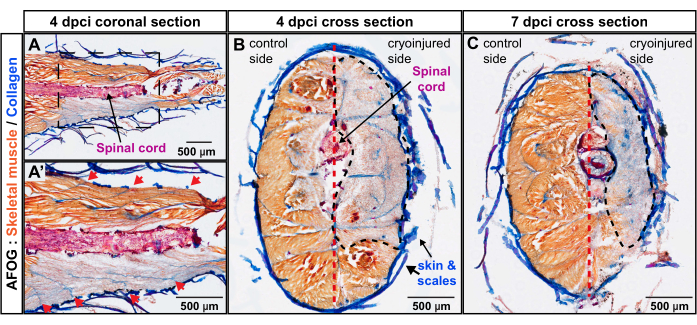
Figure 3: Assessment of injury depth in the caudal peduncle using AFOG staining. (A,A') The coronal section is at the level of the spinal cord (a red-stained horizontal rod). The bottom image shows a magnified area encompassed with a frame in the upper image. The sequential myomere boundaries appear as collagenous stripes (blue) positioned obliquely to the spinal cord (red arrows in the magnified image A').(B,C) The cross-sections display the uninjured flank with orange-stained muscles and the cryoinjured flank with grayish staining. The damaged area is encircled with a black dashed line. The vertical septum (depicted with a red dashed line) subdivides the body into the control and cryoinjured sides. Number of fish, n = 4 per time point. Please click here to view a larger version of this figure.
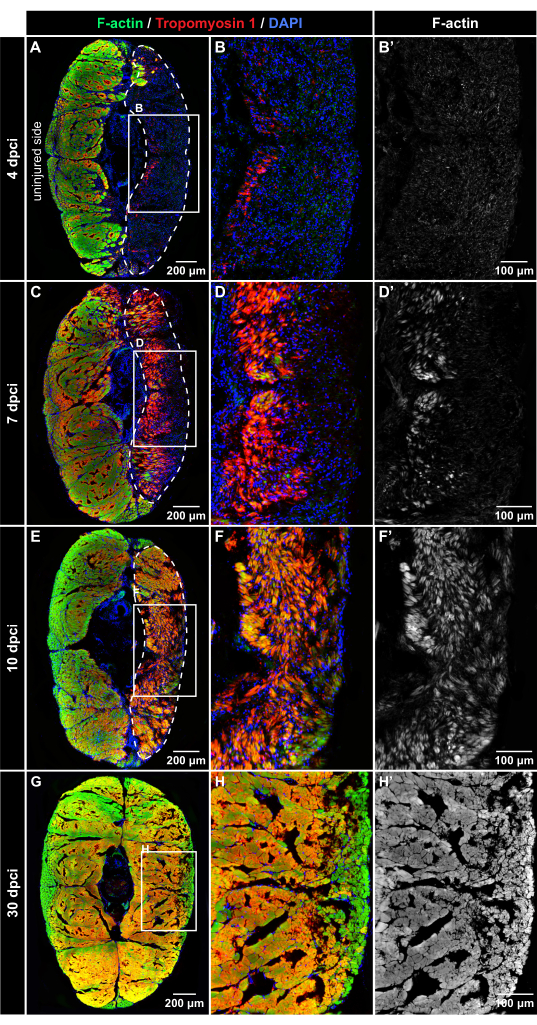
Figure 4: Immunofluorescent detection of muscle proteins after cryoinjury. Fluorescence staining of cross-sections at 4 dpci, 7 dpci, 10 dpci, and 30 dpci, as labeled on the left side and the top of the panels (A-B'). At 4 dpci, the injured tissue (encircled with the dashed line) is DAPI-positive (blue) but devoid of Phalloidin staining (green) or Tropomyosin-1 immunoreactivity (red), suggesting degeneration of the muscle fibers after cryoinjury. (C-D') At 7 dpci, both muscle markers progressively emerge in the wounded area, indicating the regenerative process. Tropomyosin-1 appears more intense than F-actin in the newly formed fibers. (E-F') At 10 dpci, the injury zone is filled with new myofibers that display a higher intensity of Tropomyosin-1 immunoreactivity in comparison to F-actin. (G-H') At 30 dpci, a similar pattern of myofibers is detected in both sides of the body. The frames in panels A, C, E, and H encompass the areas that are magnified in the adjacent images to the right. Dermal scales, emanating fluorescence outside of the myomere, were erased from the images using Adobe Photoshop. Number of fish, n = 4 per time point. Please click here to view a larger version of this figure.
Discussion
The zebrafish provides an anamniote vertebrate model organism to study the mechanisms of muscle regeneration. Most of the existing injury methods, such as laser ablation or stab wounding, result in relatively minor tissue disruption20,21,22,23. Major resections have been conducted on extraocular muscle26. However, this surgical approach would probably be less appropriate for the lateral musculature due to the health hazards of cutting the body wall. To avoid such invasive procedures, this protocol describes a milder form of injury that, nevertheless, results in profound damage to the caudal peduncle. This approach relies on a superficial manipulation that allows for the very precise targeting of a few myomeres on one side of the body. The strengths of the cryoinjury model lie in its reproducibility and ability to produce extensive muscle degeneration; based on these strengths, this model provides a new path to study how the body reacts to significant muscle loss.
The application of extreme cold leads to a thermal shock, which destroys the plasma membrane and organelles in the affected muscle tissue27. As a result, the injured myofibers undergo "accidental" cell death28. Consequently, the damaged tissue can be resorbed by natural mechanisms of wound clearance. Zebrafish tolerate the cryoinjury procedure well, as the survival rate in this study was nearly 100%, given the precooled probe was correctly positioned on the body for the exact duration. However, if the wound is too extensive (e.g., if too much pressure is applied or the duration of cryoinjury is too long), the fish may display aberrant swimming movements shortly after the procedure, and the animal should be euthanized as a humane endpoint. For other fish species, the exposure time to the cryoprobe should be adjusted according to the size of the body.
After cryoinjury, the fish can resume their swimming activity without any symptoms of abnormal movement. However, cryoinjured fish swim less dynamically than control fish, which indicates some mild impairments. Further quantification of the fish behavior at different time points after cryoinjury will be necessary to determine temporal changes in the swimming performance.
The effect of the cryoinjury method on other non-muscle tissues of the caudal peduncle remains to be elucidated. Obviously, the outer-most body layer (i.e., the skin) is damaged by the procedure. In this context, the cryoinjury method can provide a new strategy to study wound healing, scale regeneration, and the restoration of the pigmentation pattern. Furthermore, the vasculature and innervation of the myomeres could also be affected by cryoinjury, and these topics require further investigation.
The cryoinjury model has been previously used to investigate zebrafish heart regeneration13,14,15,29. This method showed some advantages compared to the ventricular resection method10 due to the transient deposition of a collagen-rich scar, which better mimics the infarct healing response in humans30. Remarkably, zebrafish can regenerate their heart after multiple cryoinjuries31. Interestingly, cryoinjury has also been applied to the zebrafish fin, resulting in histolytic processes12. As opposed to the classical fin amputation, the remaining cryoinjured stump contains a distorted margin with a mixture of dead material and healthy cells. Studies with both zebrafish organs, the heart and the fin, have revealed the powerful capacity of zebrafish to restore their original functional components even after extensive tissue damage. Whether the cryoinjured skeletal muscle activates an interplay between reparative and regenerative processes warrants future studies.
Acknowledgements
We thank V. Zimmermann for fish care, as well as Dr. Thomas Bise, Dr. Catherine Pfefferli, and Lea Gigon for the initiation of this project and their preliminary results. This work was supported by the Swiss National Science Foundation, grant number 310030_208170.
Materials
| Name | Company | Catalog Number | Comments |
| Program | |||
| ImageJ | National Institutes of Health (NIH) | ||
| Photoshop Version 23.5.3 | Adobe | ||
| Material/ Equipment | |||
| 35/10 mm Petri Dish | Greiner Bio-one | Item No.: 627102 | |
| Camera | Sony | / | HDR-PJ410 |
| Cryostat | Histcom | HRA C50 | |
| Formaldehyde ~36% | Sigma-Aldrich | 47630 | |
| Macro 50 mm f/2.8 EX DG lens | Sigma | / | Discontinued lense |
| Peel-A-Way Embedding Truncated Molds T8 | Polyscience, Inc. | 18985 | |
| Slides Superfrost Plus | Fisher Scientific | 12-550-15 | |
| Sponge | any | any | flat sponge, c.a. 7cm x 3 cm x 1 cm |
| Stainless steel cryoprobe | Custom-made | / | specifics in the article |
| Sucrose | Sigma-Aldrich | 84100 | |
| Surgical scissors | Any | / | |
| TCS SP2 | Leica | / | Discontinoued product |
| Tissue-Tek O.C.T. compound | Sakura Finetek | 4583 | |
| Tricaine (Anestethic) | Sigma | E10521 | |
| Dyes and Antibodies | |||
| Dapi | Sigma | 10236276001 | Concentration: 1/2000 |
| Phalloidin-Atto-565 (F-actin) | Sigma | 94072 | Concentration: 1 / 500 |
| Tropomyosin (TPM1) | DHSB | CH1 | Concentration: 1 / 50 |
| Recipies/Solutions | |||
| 1x PBS | 123 mM NaCl | Sigma | |
| 2.7 mM KCl | Sigma | ||
| 10 mM Na2HPO4 | Sigma | ||
| 1.8 mM KH2PO4 | Sigma | ||
| AFOG solution | 3 g Fuchsin | Fisher Scientific | |
| 2 g Orange G | Sigma | ||
| 1 g Anilin blue | Fulka AG | ||
| 200 ml acifidied distilled H2O (pH 1.1) |
References
- Muneoka, K., Allan, C. H., Yang, X., Lee, J., Han, M. Mammalian regeneration and regenerative medicine. Birth Defects Research Part C: Embryo Today: Reviews. 84 (4), 265-280 (2008).
- Carlson, B. M. Some principles of regeneration in mammalian systems. Anatomical Record. Part B, New Anatomist. 287 (1), 4-13 (2005).
- Dumont, N. A., Bentzinger, C. F., Sincennes, M. -. C., Rudnicki, M. A. Satellite cells and skeletal muscle regeneration. Comprehensive Physiology. 5 (3), 1027-1059 (2015).
- Relaix, F., et al. Perspectives on skeletal muscle stem cells. Nature Communications. 12 (1), 692 (2021).
- Tulenko, F. J., Currie, P., Cartner, S. C. Zebrafish myology. The Zebrafish in Biomedical Research. , 115-121 (2020).
- Siegel, A. L., Gurevich, D. B., Currie, P. D. A myogenic precursor cell that could contribute to regeneration in zebrafish and its similarity to the satellite cell. The FEBS Journal. 280 (17), 4074-4088 (2013).
- Corona, B. T., Wenke, J. C., Ward, C. L. Pathophysiology of volumetric muscle loss injury. Cells Tissues Organs. 202 (3-4), 180-188 (2016).
- Pfefferli, C., Jaźwińska, A. The art of fin regeneration in zebrafish. Regeneration. 2 (2), 72-83 (2015).
- Sehring, I. M., Weidinger, G. Recent advancements in understanding fin regeneration in zebrafish. WIREs Developmental Biology. 9 (1), 367 (2020).
- Poss, K. D., Wilson, L. G., Keating, M. T. Heart regeneration in zebrafish. Science. 298 (5601), 2188-2190 (2002).
- Sanz-Morejón, A., Mercader, N. Recent insights into zebrafish cardiac regeneration. Current Opinion in Genetics & Development. 64, 37-43 (2020).
- Chassot, B., Pury, D., Jaźwińska, A. Zebrafish fin regeneration after cryoinjury-induced tissue damage. Biology Open. 5 (6), 819-828 (2016).
- Chablais, F., Veit, J., Rainer, G., Jaźwińska, A. The zebrafish heart regenerates after cryoinjury-induced myocardial infarction. BMC Developmental Biology. 11, 21 (2011).
- Schnabel, K., Wu, C. C., Kurth, T., Weidinger, G. Regeneration of cryoinjury induced necrotic heart lesions in zebrafish is associated with epicardial activation and cardiomyocyte proliferation. PLoS One. 6 (4), e18503 (2011).
- Gonzalez-Rosa, J. M., Martin, V., Peralta, M., Torres, M., Mercader, N. Extensive scar formation and regression during heart regeneration after cryoinjury in zebrafish. Development. 138 (9), 1663-1674 (2011).
- Ryan, R., Moyse, B. R., Richardson, R. J. Zebrafish cardiac regeneration-Looking beyond cardiomyocytes to a complex microenvironment. Histochemistry and Cell Biology. 154 (5), 533-548 (2020).
- Jaźwińska, A., Sallin, P. Regeneration versus scarring in vertebrate appendages and heart. Journal of Pathology. 238 (2), 233-246 (2016).
- Alexander, R. The orientation of muscle fibres in the myomeres of fishes. Journal of the Marine Biological Association of the United Kingdom. 49, 163-290 (1969).
- Morin-Kensicki, E. M., Melancon, E., Eisen, J. S. Segmental relationship between somites and vertebral column in zebrafish. Development. 129 (16), 3851-3860 (2002).
- Berberoglu, M. A., et al. Satellite-like cells contribute to pax7-dependent skeletal muscle repair in adult zebrafish. Developmental Biology. 424 (2), 162-180 (2017).
- Montandon, M., Currie, P. D., Ruparelia, A. A. Examining muscle regeneration in zebrafish models of muscle disease. Journal of Visualized Experiments. (167), e62071 (2021).
- Pipalia, T. G., et al. Cellular dynamics of regeneration reveals role of two distinct Pax7 stem cell populations in larval zebrafish muscle repair. Disease Models & Mechanisms. 9 (6), 671-684 (2016).
- Ratnayake, D., et al. Macrophages provide a transient muscle stem cell niche via NAMPT secretion. Nature. 591 (7849), 281-287 (2021).
- Kaliya-Perumal, A. -. K., Ingham, P. W. Musculoskeletal regeneration: A zebrafish perspective. Biochimie. 196, 171-181 (2022).
- Aleström, P., et al. Zebrafish: Housing and husbandry recommendations. Laboratory Animals. 54 (3), 213-224 (2019).
- Saera-Vila, A., et al. Myocyte dedifferentiation drives extraocular muscle regeneration in adult zebrafish. Investigative Ophthalmology & Visual Science. 56 (8), 4977-4993 (2015).
- Baust, J. G., Gage, A. A. The molecular basis of cryosurgery. BJU International. 95 (9), 1187-1191 (2005).
- Galluzzi, L., et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death and Differentiation. 25 (3), 486-541 (2018).
- Chablais, F., Jaźwińska, A. Induction of myocardial infarction in adult zebrafish using cryoinjury. Journal of Visualized Experiments. (62), e3666 (2012).
- Chablais, F., Jaźwińska, A. The regenerative capacity of the zebrafish heart is dependent on TGFbeta signaling. Development. 139 (11), 1921-1930 (2012).
- Bise, T., Sallin, P., Pfefferli, C., Jaźwińska, A. Multiple cryoinjuries modulate the efficiency of zebrafish heart regeneration. Scientific Reports. 10 (1), 11551 (2020).
This article has been published
Video Coming Soon
ABOUT JoVE
Copyright © 2024 MyJoVE Corporation. All rights reserved