A subscription to JoVE is required to view this content. Sign in or start your free trial.
Gas Chromatography-Mass Spectrometry-Based Targeted Metabolomics of Hard Coral Samples
In This Article
Summary
Here, we present the extraction and preparation of polar and semi-polar metabolites from a coral holobiont, as well as separated coral host tissue and Symbiodiniaceae cell fractions, for gas chromatography-mass spectrometry analysis.
Abstract
Gas chromatography-mass spectrometry (GC-MS)-based approaches have proven to be powerful for elucidating the metabolic basis of the cnidarian-dinoflagellate symbiosis and how coral responds to stress (i.e., during temperature-induced bleaching). Steady-state metabolite profiling of the coral holobiont, which comprises the cnidarian host and its associated microbes (Symbiodiniaceae and other protists, bacteria, archaea, fungi, and viruses), has been successfully applied under ambient and stress conditions to characterize the holistic metabolic status of the coral.
However, to answer questions surrounding the symbiotic interactions, it is necessary to analyze the metabolite profiles of the coral host and its algal symbionts independently, which can only be achieved by physical separation and isolation of the tissues, followed by independent extraction and analysis. While the application of metabolomics is relatively new to the coral field, the sustained efforts of research groups have resulted in the development of robust methods for analyzing metabolites in corals, including the separation of the coral host tissue and algal symbionts.
This paper presents a step-by-step guide for holobiont separation and the extraction of metabolites for GC-MS analysis, including key optimization steps for consideration. We demonstrate how, once analyzed independently, the combined metabolite profile of the two fractions (coral and Symbiodiniaceae) is similar to the profile of the whole (holobiont), but by separating the tissues, we can also obtain key information about the metabolism of and interactions between the two partners that cannot be obtained from the whole alone.
Introduction
Metabolites represent the end products of cellular processes, and metabolomics - the study of the suite of metabolites produced by a given organism or ecosystem - can provide a direct measure of organismal functioning1. This is particularly critical for exploring ecosystems, symbiotic interactions, and restoration tools, as the goal of most management strategies is to preserve (or restore) specific ecosystem service functions2. Coral reefs are one aquatic ecosystem that demonstrates the potential value of metabolomics for elucidating symbiotic interactions and linking coral physiological responses to community-level and ....
Protocol
NOTE: The experimental design, sample collection and storage have been described in detail elsewhere2,30,31. Permit approval for the collection of wild corals must be obtained prior to collection and experimentation. The samples here were collected from colonies of Montipora mollis (green colour-morph) imported from Batavia Coral Farms (Geraldton, WA), originally collected from a reef off the Abrohlos Islands (Western Australia; 28°52'43.3"S 114°00'17.0"E) at a depth of 1 m under the Aquaculture License AQ1643. Prior to sampling, the colonie....
Representative Results
All the data produced during this work are available in the supplementary information.
Host-symbiont separation
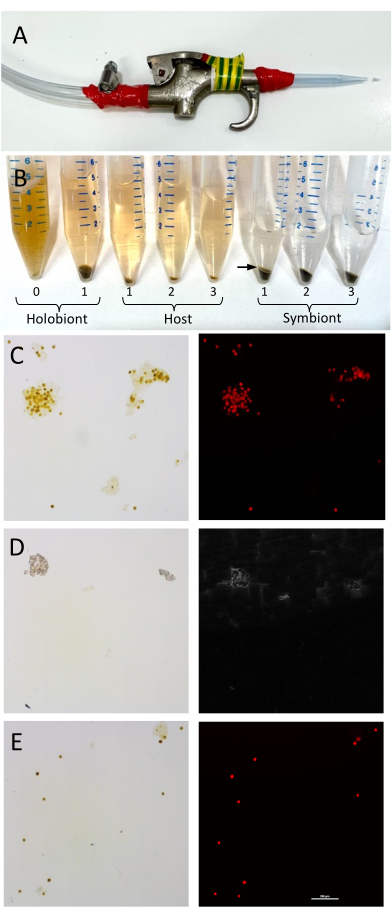
Figure 1: Setup and validation of the separation of coral host tissues and Symbiodiniaceae cells. (A) The air gun setup for the removal of coral.......
Discussion
The separation of the host and symbiont is easily and rapidly achievable via simple centrifugation, and the results here show that separating the fractions can provide valuable information indicative of specific holobiont member contributions, which can contribute toward the functional analysis of coral health. In adult corals, lipid synthesis is primarily performed by the resident algal symbiont40, which supplies lipids (e.g., triacylglycerol and phospholipids)41 .......
Disclosures
The authors have no conflict of interests to disclose.
Acknowledgements
J.L.M. was supported by a UTS Chancellor's Research Fellowship.
....Materials
| Name | Company | Catalog Number | Comments |
| 100% LC-grade methanol | Merck | 439193 | LC grade essential |
| 2 mL microcentrifuge tubes, PP | Eppendorf | 30121880 | Polypropylene provides high resistance to chemicals, mechanical stress and temperature extremes |
| 2030 Shimadzu gas chromatograph | Shimadzu | GC-2030 | |
| 710-1180 µm acid-washed glass beads | Merck | G1152 | This size is optimal for breaking the Symbiodiniaceae cells |
| AOC-6000 Plus Multifunctional autosampler | Shimadzu | AOC6000 | |
| Bradford reagent | Merck | B6916 | Any protein colourimetric reagent is acceptable |
| Compressed air gun | Ozito | 6270636 | Similar design acceptable. Having a fitting to fit a 1 mL tip over is critical. |
| DB-5 column with 0.25 mm internal diameter column and 1 µm film thickness | Agilent | 122-5013 | |
| DMF | Merck | RTC000098 | |
| D-Sorbitol-6-13C and/or 13C5–15N Valine | Merck | 605514/ 600148 | Either or both internal standards can be added to the methanol. |
| Flat bottom 96-well plate | Merck | CLS3614 | |
| Glass scintillation vials | Merck | V7130 | 20 mL, with non-plastic seal |
| Immunoglogin G | Merck | 56834 | if not availbe, Bovine Serum Albumin is acceptable |
| Primer | v4 | ||
| R | v4.1.2 | ||
| Shimadzu LabSolutions Insight software | v3.6 | ||
| Sodium Hydroxide | Merck | S5881 | Pellets to make 1 M solution |
| tidyverse | v1.3.1 | R package | |
| TissueLyser LT | Qiagen | 85600 | Or similar |
| TQ8050NX triple quadrupole mass spectrometer | Shimadzu | GCMS-TQ8050 NX | |
| UV-96 well plate | Greiner | M3812 | |
| Whirl-Pak sample bag | Merck | WPB01018WA | Sample collection bag; Size: big enough to house a ~5 cm coral fragment, but not too big that the water is too spread |
References
- Bundy, J. G., Davey, M. P., Viant, M. R. Environmental metabolomics: A critical review and future perspectives. Metabolomics. 5 (1), 3-21 (2008).
- Matthews, J. L., Beale, D. J., Hillyer, K. E., Warden, A. C., Jones, O. A. H., et al.
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
ABOUT JoVE
Copyright © 2025 MyJoVE Corporation. All rights reserved