Determining Surface Areas and Pore Volumes of Metal-Organic Frameworks
In This Article
Summary
This article describes the use of nitrogen porosimetry to characterize metal-organic frameworks, using UiO-66 as a representative material.
Abstract
The surface area and pore volume of a metal-organic framework (MOF) can provide insight into its structure and potential applications. Both parameters are commonly determined using the data from nitrogen sorption experiments; commercial instruments to perform these measurements are also widely available. These instruments will calculate structural parameters, but it is essential to understand how to select input data and when calculation methods apply to the sample MOF. This article outlines the use of the Brunauer-Emmett-Teller (BET) method and Barrett-Joyner-Halenda (BJH) method for the calculation of surface area and pore volume, respectively. Example calculations are performed on the representative MOF UiO-66. Although widely applicable to MOFs, sample materials and adsorption data must meet certain criteria for the calculated results to be considered accurate, in addition to proper sample preparation. The assumptions and limitations of these methods are also discussed, along with alternative and complementary techniques for the MOF pore space characterization.
Introduction
Relevance of surface area and pore volume
The accurate characterization of porous materials is imperative to understanding their potential applications. Surface area and pore volume are important quantitative metrics that provide insight into metal-organic framework (MOF) performance in a variety of applications, including gas adsorption, separation, catalysis, and sensing1.
The surface area of a MOF is a parameter that quantifies the amount of surface available for interactions with guest molecules and can affect its performance in various applications2,3. In gas adsorption applications, the surface area of a MOF reflects binding site availability and affinity, which is directly related to its separation performance4. In catalysis applications, MOF surface area can affect the number of active sites and their accessibility to reactant molecules and, thus, their catalytic activity5. The quantity and accessibility of active sites are also relevant in sensing applications, as more guest interactions with active sites lead to improved sensitivity (and potentially selectivity)6. The surface area can also affect the stability of the MOF under extreme conditions, as a larger surface area can indicate a higher number of surface defects7.
The pore volume of a MOF is a parameter that quantifies the amount of void space within the porous structure. It is defined as the total volume of the pores in the MOF, which includes both the open (accessible) and closed (inaccessible) pores. The pore volume of a MOF can affect its performance in various applications, including gas adsorption, separation, and catalysis. Like surface area, the pore volume of a MOF is directly related to its capacity for gas uptake and storage and its ability to allow guest molecules to reach adsorptive or catalytic sites8.
Using nitrogen sorption to determine surface area and pore volume
Both surface area and pore volume are typically measured using gas adsorption techniques, most commonly nitrogen sorption. Nitrogen is chosen as the adsorbate in Brunauer-Emmett-Teller (BET) analysis due to its quadrupole moment, where the orientation of the nitrogen molecule is dependent on the surface chemistry of the adsorbent, allowing for the formation of a monolayer. The plot of nitrogen uptake as a function of pressure can be used to obtain information about the surface and pore sizes of the MOF. The material surface area and total pore volume can be calculated using the sorption data9. The overall goal of the method detailed here is to obtain nitrogen sorption data and use that data to calculate MOF surface area and pore volume.
The BET method10 is a widely used technique for determining the specific surface area of a porous material, based on the principle that the adsorption of a gas onto a solid surface is a function of the surface area, the properties of the gas molecule, and the system. A known amount of an adsorbate gas (such as nitrogen) is introduced to the sample material over a given pressure range, and the amount of gas adsorbed onto the surface is measured at each pressure increment. The data is used to calculate the specific surface area by relating the adsorbate uptake, pressure, and monolayer capacity, which is represented by the BET equation9:
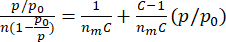 (equation 1; eq. 1)
(equation 1; eq. 1)
where:
p = equilibrium pressure of adsorbate (Pa)
p0 = adsorbate saturation pressure (Pa)
n = adsorbate uptake amount (m3/g)
nm = monolayer capacity (m3/g)
C = BET constant (unitless)
The monolayer capacity is related to the total surface area by the following equation:
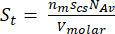 (equation 2; eq. 2)
(equation 2; eq. 2)
where:
St = total MOF surface area (m2)
nm = monolayer capacity (m3/g)
NAv = Avogadro's number (molecule/mol)
scs = cross sectional area of adsorbate molecule (m2/molecule)
Vmolar = adsorbate molar volume (m3/mol)
The Barrett-Joyner-Halenda (BJH) method11 is a common procedure that utilizes desorption data to calculate the total pore volume. Like the BET method, a known amount of adsorbate gas (often nitrogen) is introduced to the sample. The partial pressure of the adsorbate is then incrementally decreased, and the volume of gas desorbed at each step is measured. Under the assumption that desorption in each pore first occurs in the capillary volume, followed by a reduction in adsorbed layer thickness, the BJH equation relates the volume desorbed to the adsorbed layer thickness, pore radius, and pore volume. This relationship can be represented with a BJH pore size distribution plot, which plots pore radius against pore volume. The distribution is integrated with respect to pore size to determine the total pore volume. The BJH equation12 is written as:
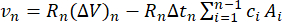 (equation 3; eq. 3)
(equation 3; eq. 3)
where:
n = desorption step (unitless)
vn = volume of pores emptied of capillary condensate (m3)
ΔVn = volume of adsorbate removed from pores (m3)
Δtn = change in adsorbed layer thickness (m)
A = surface area of pores involved in desorption (m2)
Rn = BJH constant dependent on average pore size (unitless)
c = BJH constant, dependent on average adsorbed layer thickness (unitless)
Protocol
1. Sample preparation
- Sample synthesis
- Dissolve 0.35 mM terephthalic acid and 0.35 mM ZrCl4 in 4 mL of dimethyl formamide (DMF). Seal in a PTFE liner and heat at 120 °C for 24 h. Allow to cool to room temperature.
- Centrifuge solution at 120 x g for 30 min. Decant remaining liquid and allow powder to dry in ambient air overnight.
- Sample loading
- Measure the mass of an empty sample tube. Load 30-50 mg of the MOF UiO-66 into the sample tube. Measure the new mass.
- Activation
- Attach the sample tube to the sample preparation system, securing the seal with a 0.5 inch O-ring. Place the tube inside the heating mantle.
- Set the temperature controller to the designated activation temperature, 120 °C here, and wait for the temperature to stabilize.
NOTE: The activation temperature should be above the synthesis solvent's (or the solvent used in solvent exchange) boiling point under vacuum. - Open the valve connecting the system to the vacuum and wait for the pressure to stabilize. Wait for the designated activation time, 24 h.
- Remove the tube from the heating mantle and allow the sample to cool to room temperature. Backfill the sample tube with nitrogen. Remove the tube from the preparation system.
- Take the mass of the activated sample and tube. Calculate the mass of the activated sample as described in equation 4 (eq. 4).
(sample mass) = (mass of activated sample and tube) - (mass of empty sample tube) (eq. 4)
2. Experiment file setup
- Create a sample file
- Open instrument software, click File, then click New Sample. Under the Sample Description tab, enter the sample name, sample mass, and sample density.
- Input analysis parameters
- Open the Analysis Conditions tab and select the adsorptive gas (nitrogen) and analysis conditions (BET).
- Select the Free Space button. Enter whether the free space is to be measured by the instrument, entered by the user, or calculated. If the free space will be measured, enter the duration of evacuation prior to the measurement.
- Select whether the nitrogen dewar will be lowered during the measurement and whether the system will perform a test for sample outgassing. If the free space will be entered, specify both the ambient free space and the analysis free space. Click OK.
NOTE: At 77 K, helium can become trapped inside micropores. For microporous materials, the helium free space may be measured after N2 adsorption analysis. - Select p0 and T. Enter whether p0 will be measured by the po tube, entered by the user, or calculated. Typically, the P0 of the adsorbate is measured by the instrument. Input the analysis temperature (77K), and the p0 value if applicable. Click OK.
- Select Backfill. Select whether the sample will be backfilled before and after analysis. If either is chosen, select the identity of the backfill gas (N2). Click OK.
- In the Isotherm Collection section, select Target Pressures. Click Pressures, then input the isotherm pressure values from a p/p0 between 0 and 1 in intervals of 0.005, then click OK. Click Options and input the relative pressure tolerance of 5%. Click OK.
- Open the Report Options tab and select the data analysis plots to be reported. Click Save As, name the file, and select a folder destination.
3. Performing adsorption measurement
- Physical setup
- Slide the sample tubes into the isothermal sleeves. Attach the sample tube to adsorption instrument, securing the seal with O-rings.
- Fill dewar with liquid nitrogen using appropriate safety/personal protection equipment. Place the dewar on the elevator below the sample. If using p0 tube, attach it and ensure it is configured to sit inside the dewar once the elevator is raised.
- Close the shield doors.
- Running the experiment
- In the instrument software, click the name of the instrument then click Sample Analysis.
- Click Browse, then select the sample file. Ensure to match the analysis number with the number of the port where the sample is loaded. Click Start.
4. Nitrogen adsorption measurement
- Adsorption: Inject nitrogen into the sample tube until the first target pressure (± the pressure tolerance range) is reached. Leave the sample to equilibrate until the pressure is stable for the designated equilibration time. Repeat this until nitrogen's saturation pressure is reached.
- Desorption: Open the vacuum valve to desorb nitrogen until the first desorption target pressure (± the pressure tolerance range) is reached. Leave the sample to equilibrate until the pressure is stable for the designated equilibration time. Repeat this until the nitrogen in the sample has been fully desorbed.
- Backfill the sample tube with designated backfill gas (N2). The instrument will automatically backfill the tubes if that option was selected when inputting the analysis parameters.
NOTE: A diagram of the adsorption apparatus is shown in Figure 1.
5. Data analysis
- Once all data points have been collected, select File, then Export, and choose the experiment file. Enter the file destination and save the file as a spreadsheet. Click OK.
- Use the isotherm data to create a BET plot, with p/p0 on the x-axis and (p/p0)/[n(1-p/p0)] on the y-axis according to equation 1.
- To apply the BET method to a given isotherm, take the linear range of the knee. For mesoporous materials this is typically in a P/P0 range of 0.05-0.30, while for microporous materials it is taken from a P/P0 range of 0.005-0.03.
- Make sure the linear range meets the Rouquerol criteria discussed below. There are tools available to automatically detect the linear range for MOF materials13. The linear range is:
Slope = (C-1)/(nmC)
Y-intercept = 1/nmC - Use the values of the BET plot's slope and the y-intercept to calculate the BET constant (C) and the monolayer capacity (nm)
- Use the monolayer capacity and adsorbate properties to calculate the total surface area using the relation presented in equation 3.
Representative Results
After following the protocol, the obtained isotherm can be analyzed, and critical material properties can be derived. The results from a nitrogen adsorption experiment gives critical information into the surface area, pore volume, and pore structure of a given sorbent. The aim of this experiment was to investigate the use of nitrogen adsorption to measure the surface area and pore volume of a nanoporous MOF, UiO-66. UiO-66 is an archetypal zirconium-based MOF that has a high surface area and remarkable stability. While many MOFs possess weak thermal, mechanical, and chemical stability, UiO-66 is very robust due to the zirconium oxide cuboctahedral metal node, allowing for 12 extension points in the BDC linker coordination. The structure is comprised of 7.5 Å tetrahedron cages and 12 Å octahedral cages14,15.
Defect-free UiO-66 exhibits a type 1 isotherm shape16. Type 1 isotherms are indicative of microporous solids that have relatively small external surfaces. The amount adsorbed in a type 1 isotherm quickly approaches a limiting value, indicating that the nitrogen uptake is governed by the micropore volume that is accessible to the adsorbate, rather than the internal surface area. The sharp uptake at a low P/P0 indicates a strong interaction in the narrow micropores between the adsorbent and adsorbate17. Hysteresis loops are not commonly seen in type 1 isotherms as they are seen in the multilayer range of physisorption and are associated with capillary condensation in the pores. The monolayer formation of nitrogen on the adsorbent in the low P/P0 range is related to the surface area of the adsorbent, while pore filling at a P/P0 close to unity relates to the total pore volume of the material17.
The application of the BET method is often done in the adsorption instrument software. However, the analysis and calculation can easily be done manually, or with other computational programs and methods that can be adapted to give critical results. To apply the BET model to the obtained nitrogen isotherm, there are two critical steps. First, the nitrogen isotherm must be transformed into a BET plot, and from there the BET monolayer capacity can be derived. Next, the BET surface area is calculated from the monolayer capacity and by selecting an appropriate value of the molecular cross-sectional area17. This is typically done in nitrogen adsorption instrument software. Figure 2 shows the nitrogen isotherm obtained for UiO-66. The isotherm is type 1, indicating a microporous structure and a nitrogen monolayer formation. The sharp step at high relative pressures, resulting in a slight type 2 isotherm, is indicative of multilayer formation as well as the formation of larger meso- or macro-pores due to defect engineering in UiO-66. The hysteresis observed at high relative pressures indicates larger meso- and macro-pore formation. Table 1 shows the values obtained from the BET analysis.
When using the BET method, the Rouquerol criteria must hold true. The Rouquerol criteria state that a linear fit to the transformed BET data must be obtained, the C value should always be positive if the method is within the proper range for analysis, the Rouquerol transform must increase with increasing relative pressure, and the monolayer capacity must be within the limits of data used to fir the BET parameters18. To apply the BET method to a given isotherm, the linear range of the knee must be taken. For mesoporous materials this is typically in a P/P0 range of 0.05-0.30, while for many microporous materials it is typically taken from a P/P0 range of 0.005-0.03. However, the actual linear range is often more restricted as it is dependent on the material and the analysis temperature. Thus, the selection of the linear range will require qualitative assessment, similar to the parameters displayed in Table 1 (positive C and correlation coefficient close to unity indicating a proper analysis range). Similarly, there must be a sufficient number of experimental data points in the linear range (minimum of 10) for reliable analysis. These considerations also indicate inherent limitations with the BET method. C is a constant that relates to the relative pressure at which a monolayer is formed. C is a metric used to define the fraction of the surface uncovered by a monolayer as the BET method assumes a statistical monolayer formation. Thus, a larger C value correlates to a higher degree of surface coverage and a more uniform monolayer formation. When the C value is less than 2, the isotherm is type 3 or 5 and BET is not applicable. When C is less than 50 there is appreciable overlap of the monolayer and multilayer formation. A coefficient C of at least 80 indicates a sharp isotherm knee where monolayer adsorption is completed, and multilayer adsorption begins. A parameter C greater than 150 is typically associated with the filling of narrow micropores or adsorption on high energy surface sites17.
UiO-66 is a microporous MOF that commonly exhibits defects which can increase the surface area and improve certain desirable adsorption properties, but can result in a lower stability and crystallinity15. A defective UiO-66 framework can have a BET surface area anywhere from 1000-1800 m2/g and a pore volume from 0.40-0.90 cm3/g, depending on the degree of defect engineering15,16.
For the measured UiO-66, when using the linear P/P0 range 0.01-0.05, the BET surface area is 1211 m2/g and the C value is 457. The theoretical surface area of a simulated, defect-free UiO-66 is 1200 m2/g14. In a type 1 isotherm, as seen in UiO-66, the BET surface area should be treated as an apparent surface area since the BET model does not confirm the validity of the BET monolayer capacity17. The measured surface area falls within the expected range for UiO-66, and combined with the C value, indicates a microporous structure with uniform monolayer formation and pore filling.
The pore volume of a material is typically analyzed at a P/P0 of 0.80-0.95. If there are macropores present in the material, the nitrogen adsorption isotherm will not be nearly horizontal at P/P0 close to unity, and thus the total pore volume cannot be evaluated17. The pore volume measured in this case would only be the pore volume of the micro and mesopores.
The measured pore volume, taken at a P/P0 of 0.80, of UiO-66 is 0.86 cm3/g. The theoretical pore volume of UiO-66 is 0.77 cm3/g15. The higher pore volume for the UiO-66 sample measured is most likely due to defects present within the UiO-66 structure. Rather than having solely micropores, there are defects present resulting in larger meso- or macro-pores, giving a larger pore volume. This is corroborated with the shape of the nitrogen isotherm where there is a sharp increase and hysteresis at high relative pressures and a type 1-2 isotherm shape.
Often, the measured BET surface area and pore volume of a given material will be within a given range. It has been shown that the repeatability of nitrogen adsorption isotherms and surface area measurements vary widely across the literature19. This is due to variations in the BET range selected, material defects, forgoing repeat experiments, and intrinsic characteristics of the model. Tools like the BET surface identification (BETSI) program can be utilized for an unambiguous assessment of the BET surface area by an automatic selection of the linear range based on extended selection criteria. The BET model was not developed for adsorption in microporous materials, despite it being the standard in material characterization. This is due to the idea of monolayer coverage and idealized adsorption behavior13. The BET model assumes uniform adsorption and a homogenous surface. These assumptions may not hold true for materials with heterogeneous surfaces or very small pores, and thus, the application of the BET model must be evaluated for each given material.
The results of the nitrogen adsorption experiment and analysis indicate the successful formation of a UiO-66 microporous, crystalline structure with slight defects. The calculated surface area and pore volume fall within the range reported in literature15,19, leading to the conclusion that the BET model can be applied to the MOF UiO-66 and can be translated to other nanoporous materials if the given assumptions and conditions apply.
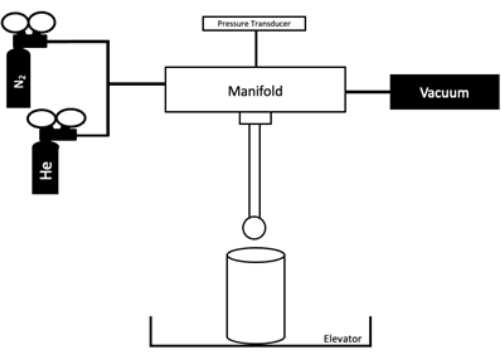
Figure 1: Diagram of adsorption instrument. The sealed sample tube is connected to pressure transducers, a vacuum, and the free space/analysis gas sources. Please click here to view a larger version of this figure.
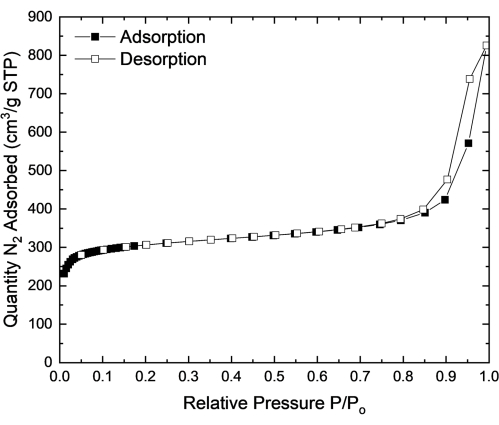
Figure 2: Nitrogen adsorption and desorption isotherm for UiO-66 at 77 K. The nitrogen isotherm of the MOF UiO-66 at 77 K where the BET surface area was measured to be 1211 m2/g and the pore volume was measured to be 0.86 cm3/g. Please click here to view a larger version of this figure.
| BET Area | 1211 m2/g |
| Slope | 0.0035 g/cm3 STP |
| Y-Intercept | 0.000008 g/cm3 STP |
| C | 457 |
| Monolayer Capacity | 278 cm3/g STP |
| Molecular Cross-Sectional Area | 0.1620 nm2 |
| Correlation Coefficient | 0.9999 |
Table 1: Table indicating the values obtained from the BET analysis of UiO-66 at 77 K. The table includes a summary of the key values obtained from the BET analysis in the range of P/P0 of 0.01-0.05 for the MOF UiO-66. The positive C and y-intercept, along with a correlation coefficient of 0.9999 indicates that an acceptable linear region was selected for BET analysis.
Discussion
Applicability and limitations
The BET method requires a few key assumptions: (1) the surface is planar and uniform, (2) the surface is homogenous, and all adsorption sites are energetically identical (3) adsorbates form a monolayer. Because of this, BET may not be suitable for non-porous materials, materials with complex surface structures (different types of surface sites, irregular surface morphology, sites with large energetic differences), or those that do not exhibit monolayer adsorption behavior. Large deviations from assumption conditions may affect the accuracy of specific surface area calculations. Like BET, the BJH method also assumes uniform adsorption and a homogenous surface, along with the assumption of rigid, cylindrical pores. As such, it also may not be suitable for materials with complex surfaces, or breathable structures20. Additionally, since porosimetry requires access to pore space, calculated values will not account for closed pore volume.
Both the BET and BJH methods should be used cautiously with microporous materials. BJH does not account for fluid-surface interactions or interactions between adsorbate molecules within the pore, both of which become more pronounced in smaller pores. For this reason, BJH is limited to mesopores and small macropores. Since micropores often exhibit pore filling behavior, it can be difficult to locate the linear region of the isotherm that is required to perform BET calculations21.
An additional limitation to both methods is their sensitivity to sample preparation methods. The sample is required to be in a divided form, such as a powder or thin film, which can be challenging to prepare uniformly. This can introduce errors in measurements and make repeatability difficult. The surface area and pore volumes may also be affected by the sample preparation method and conditions, such as material synthesis technique, activation methods/conditions, or drying temperature/time22.
Significance with respect to alternative methods
Nitrogen is the standard adsorbate for BET and BJH data, due to its quadrupole moment - where the orientation of the nitrogen molecule is dependent on the surface chemistry of the adsorbent, allowing for the formation of a monolayer - and its low cost17. However, argon and carbon dioxide23 can also be utilized, particularly for microporous structures. Argon is chemically inert and is a symmetrical, monoatomic molecule; however, 77 K is below its triple point so the bulk reference state is questionable, and the structure of the argon monolayer is heavily dependent on the surface chemistry of the sorbent17.
As both BET and BJH are not universally applicable, other methods of measuring surface area and pore volume should be considered. A Langmuir plot, t-plot, or the Horvath-Kawazoe method can be used to determine micropore surface area, pore volume, and pore size distribution, respectively. Non-local density functional theory (NLDFT) modeling is also an option for pore size distributions and is especially favorable for micropores because it accounts for changes in fluid density with respect to pore size. Mercury porosimetry can be used to determine both porosity and pore volume, but the accessible range for this technique must be considered since it cannot penetrate into micropores. Computational methods can be used to calculate theoretical characterization metrics and provide a point of comparison to experimental results, which can be useful for materials with closed pores. Although BJH produces a pore size distribution, it does not account for non-uniform distribution or fully characterize connectivity between pores. Additional characterization, such as SEM, TEM24, or XRD may be used to gain a more complete understanding of the structure of a porous material. Even when a material cannot be fully represented by BET or BJH, they can still be used as qualitative comparisons between materials. Nitrogen porosimetry can be a very useful tool in combination with other techniques.12
Acknowledgements
This work was supported as part of the Center for Understanding and Control of Acid-Gas Induced Evolution of Materials for Energy (UNCAGE-ME), an Energy Frontier Research Center funded by the U.S. Department of Energy, Office of Science, Basic Energy Sciences under Award #DE-SC0012577. J.S. acknowledges that this material is based upon work supported by the National Science Foundation Graduate Research Fellowship under Grant No. DGE-2039655. Any opinion, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Materials
| Name | Company | Catalog Number | Comments |
| Adsorption Instrument | Micromeritics | TriStar II Plus | |
| Adsorption Software | Micromeritics | TriStar II Plus Version 3.03 | |
| Balance | |||
| Dewar | Liquid N2 Dewar | ||
| Dimethyl Formamide (DMF) | Fisher Scientific | D119-1 | |
| Helium | Airgas | HE UHP300 | Ultra-High Purity |
| Nitrogen | Airgas | NI 230LT22 | Industrial Grade Liquid N2 |
| Nitrogen | Airgas | NI UHP300 | Ultra-High Purity Gaseous N2 |
| Sample Holder | Micromeritics | 302-61001-02 | Glass Sample Holder |
| Sample Preparation System | Micromeritics | 061-00030-00 | VacPrep 061 |
| Terephthalic Acid (H2BDC) | Sigma Aldrich | 185361 | |
| ZrCl4 | Sigma Aldrich | 221880 | Zirconium(IV) chloride, ≥99.5% trace metals basis |
References
- Zhou, H. C., Long, J. R., Yaghi, O. M. Introduction to Metal-Organic Frameworks. Chemical Reviews. 112 (2), 673-674 (2012).
- Tian, Y., Wu, J. A comprehensive analysis of the BET area for nanoporous materials. AIChE Journal. 64 (1), 286-293 (2017).
- Farha, O. K., et al. Metal-organic framework materials with ultrahigh surface areas: is the sky the limit. Journal of the American Chemical Society. 134 (36), 15016-15021 (2012).
- Li, J. R., Sculley, J., Zhou, H. C. Metal-organic frameworks for separations. Chemical Reviews. 112 (2), 869-932 (2012).
- Yang, D., Gates, B. C. Catalysis by Metal Organic Frameworks: Perspective and Suggestions for Future Research. ACS Catalysis. 9 (3), 1779-1798 (2019).
- Kreno, L. E., et al. Metal-organic framework materials as chemical sensors. Chemical Reviews. 112 (2), 1105-1125 (2012).
- Wang, T. C., et al. Ultrahigh surface area zirconium MOFs and insights into the applicability of the BET theory. Journal of the American Chemical Society. 137 (10), 3585-3591 (2015).
- Ongari, D., et al. Accurate Characterization of the Pore Volume in Microporous Crystalline Materials. Langmuir. 33 (51), 14529-14538 (2017).
- Thommes, M., et al. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure and Applied Chemistry. 87 (9-10), 1051-1069 (2015).
- Brunauer, S., Emmett, P. H., Teller, E. Adsorption of Gases in Multimolecular Layers. Journal of the American Chemical Society. 60 (2), 309-319 (1938).
- Barrett, E. P., Joyner, L. G., Halenda, P. P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. Journal of the American Chemical Society. 73 (1), 373-380 (1951).
- Lowell, S., Shields, J. E., Thomas, M. A., Thommes, M. . Characterization of Porous Solids and Powders: Surface Area, Pore Size and Density. , (2004).
- Osterrieth, J. W. M., et al. How Reproducible are Surface Areas Calculated from the BET Equation. Advanced Materials. 34 (27), 2201502 (2022).
- Cavka, J. H., et al. A New Zirconium Inorganic Building Brick Forming Metal Organic Frameworks with Exceptional Stability. Journal of the American Chemical Society. 130 (42), 13850-13851 (2008).
- Winarta, J., et al. A Decade of UiO-66 Research: A Historic Review of Dynamic Structure, Synthesis Mechanisms, and Characterization Techniques of an Archetypal Metal-Organic Framework. Crystal Growth & Design. 20 (2), 1347-1362 (2020).
- Valenzano, L., et al. Disclosing the Complex Structure of UiO-66 Metal Organic Framework: A Synergic Combination of Experiment and Theory. Chemistry of Materials. 23 (7), 1700-1718 (2011).
- Rouquerol, F., Rouquerol, J., Sing, K. S. W., Maurin, G., Llewellyn, P. . Adsorption by Powders and Porous Solids (Second Edition). , (2014).
- Agrawal, M., Han, R., Herath, D., Sholl, D. S. Does repeat synthesis in materials chemistry obey a power law). Proceedings of the National Academy of Sciences of the United States of America. 117 (2), 877-882 (2020).
- Rouquerol, J., Llewellyn, P., Rouquerol, F. . Studies in Surface Science and Catalysis. , 160 (2007).
- Howarth, A. J., et al. Best Practices for the Synthesis, Activation, and Characterization of Metal-Organic Frameworks. Chemistry of Materials. 29 (1), 26-39 (2017).
- Kim, K. C., Yoon, T. U., Bae, Y. S. Applicability of using CO2 adsorption isotherms to determine BET surface areas of microporous materials. Microporous and Mesoporous Materials. 224, 294-301 (2016).
- Bau, S., Witschger, O., Gensdarmes, F., Rastoix, O., Thomas, D. A TEM-based method as an alternative to the BET method for measuring off-line the specific surface area of nanoaerosols. Powder Technology. 200 (3), 190-201 (2010).
This article has been published
Video Coming Soon
ABOUT JoVE
Copyright © 2024 MyJoVE Corporation. All rights reserved