Remote Neuronal Activation Coupled with Automated Blood Sampling to Induce and Measure Circulating Luteinizing Hormone in Mice
In This Article
Summary
Luteinizing hormone (LH) pulsatility is a hallmark of reproductive function. We describe a protocol for remote activation of specific neuronal populations linked to serial automated blood collection. This technique allows timed hormonal modulation, multiplexing, and minimizing manipulation effects on LH levels in conscious freely moving, and undisturbed animals.
Abstract
Circulating luteinizing hormone (LH) levels are an essential index of the functioning of the hypothalamic-pituitary control of reproduction. The role of numerous inputs and neuronal populations in the modulation of LH release is still unknown. Measuring changes in LH levels in mice is often a challenge since they are easily disrupted by environmental stress. Current techniques to measure LH release and pulsatility require long-term training for mice to adapt to manipulation stress, certain restraint, the presence of the investigator, and working on individual animals, reducing its usefulness for many research questions.
This paper presents a technique to remotely activate specific neuronal populations using Designer Receptor Exclusively Activated by Designer Drugs (DREADDs) technology coupled with automated sequential blood sampling in conscious, freely moving, and undisturbed mice. We first describe the stereotaxic surgery protocol to deliver adeno-associated virus (AAV) vectors expressing DREADDs to specific neuronal populations. Next, we describe the protocol for carotid artery and jugular vein cannulation and postsurgical connection to the CULEX automated blood sampling system. Finally, we describe the protocol for clozapine-N-oxide intravenous injection for remote neuronal activation and automated blood collection. This technique allows for programmed automated sampling every 5 min or longer for a given period, coupled with intravenous substance injection at a desired time point or duration. Overall, we found this technique to be a powerful approach for research on neuroendocrine control.
Introduction
The hypothalamo-pituitary-gonadal (HPG) axis is centrally regulated by the pulsatile release of gonadotropin-releasing hormone (GnRH) into the pituitary portal system. In the pituitary gland, GnRH controls the pulsatile release of gonadotropins, luteinizing hormone (LH), and follicle-stimulating hormone (FSH) to the circulatory system. LH pulsatile release serves as a hallmark for central HPG axis functioning1,2,3,4. For example, it displays the effects of genetic alterations or changes in hormonal or environmental factors on the neural part of the axis5,6,7. Until recently, measuring the LH pulsatile pattern was limited to large mammals8 and rats9, given the high frequency of sampling and large blood volumes necessary to identify the pulses.
Detecting LH pulses in mice is desirable since this species has broad genetic models available and can be readily manipulated using genomic engineering technologies to further study specific genes and cell populations. In the last decade, a large advance in the analysis of LH concentrations in mice using a sandwich LH enzyme-linked immunosorbent assay (ELISA) has allowed for detecting LH in a minute amount of blood10. The development of the frequent tail-tip blood sampling technique has made possible the necessary frequent sampling for detecting the frequency and amplitude of LH pulses in mice10,11. However, tail-tip blood sampling is limited to its use in conscious awake animals; it demands a long training period for mice to adapt to the handling and the presence of a designated investigator during sampling. Its success is highly susceptible to environmental stressors and may not be suitable for use in mouse strains showing high levels of anxiety. Intra-atrial cannulation has also been used for frequent blood sampling in freely-moving conscious mice12. However, that setup still requires repeated manual blood sampling and restricts animal moving space, while atrial cannulation can lead to dynamic changes in cardiac function. It is therefore desirable to establish a method for blood collection under stress-free conditions in conscious, freely moving, and undisturbed mice without the need for prior training or human handling or presence.
Automated blood or dialysate sampling has been used before for measuring different hormone levels (e.g., melatonin13,14) and their pulsatile secretion (e.g., growth hormone)15 in unrestrained rodents. We herein present a protocol for automated long-term frequent blood sampling in conscious and unrestrained animals, coupled with a timely remote activation of specific neuronal populations using chemogenetic technologies: the designer receptors exclusively activated by designer drugs (DREADDs). We will describe the stereotaxic delivery of an adeno-associated virus (AAV) vector and the remote activation by an automated intravenous (IV) delivery of clozapine-N-oxide (CNO)16,17. This protocol allows for the sequential detection of basal levels and induced changes in LH pulsatility in multiple animals at the same time. Both the blood sampling and the IV delivery of the compound are conducted in a time-controlled manner via a computer program eliminating the physical presence of the investigator or the requirement for prior mouse training. This method overcomes the main limitations of manual blood sampling. It allows for blood sampling in a stress-free condition, and simultaneous IV compound delivery coupled with remote neuronal activity control. We show representative results of the use of automated blood sampling alone or combined with remote neuronal activation and discuss its advantages, limitations, and additional uses.
Protocol
All animal procedures are performed in agreement with the National Research Council Guide for the Care and Use of Laboratory Animals18 as well as federal, state, and local laws. Adult female mice (3-6 months old) were used for this protocol demonstration, including four C57BL/6J females and four Kiss1-Cre;ChR2-eYFP (Kiss1-eYFP) females. Mice were held under a 12:12 light:dark cycle, temperature-controlled at 22 °C, and fed ad libitum on a low-phytoestrogen diet. Procedures and protocols were approved by the University of Michigan Institutional Animal Care and Use Committee (IACUC, Animal Protocols: PRO00010420 and PRO00010138).
1. Stereotaxic delivery of AAVs to a specific cell population
- Preparation for surgery
- Sterilize all tools. Prepare multiple packages of surgical tools to ensure that each package is used in no more than five animals. Prepare sterile gloves, a minimum of one pair for each animal.
- Pull glass micropipettes for injections using the following settings (see the Table of Materials) for long thin micropipettes that inject slowly and steadily and do not easily clog: Heat 1: 915, Heat 2: 630, Pull: 630. Optimize these settings for each puller.
- Perform surgeries in a designated surgical space. Disinfect surgical surfaces with 70% ethanol.
- Use sterile drapes to maintain the sterility of the surgical field. Wear a clean lab coat or disposable gown and a mask.
- Prepare inhalation anesthesia system. Open oxygen supply and regulate flow rate to 0.8 L/min.
- Surgery
- Place the mouse in an anesthesia box and open the isoflurane to 2.5%. Reduce isoflurane flow to 2% after initial induction; maintain flow throughout the procedure. For alternative methods of anesthesia, refer to animal protocol and local ethical committee guidelines.
- Inject preemptive analgesic (carprofen 5 mg/kg s.c.) following animal use committee recommendation and local regulations.
- Shave the mouse head using clippers.
- Install the animal on a stereotaxic table, place ear bars, and make sure that the head is correctly fixed and stable. Fix the mouse's mouth to the mouthpiece, making sure to place the tongue to the side outside of the mouth to avoid suffocation.
- Verify that the animal is deeply anesthetized with a toe pinch before starting the surgery and monitor the mouse's breath and color throughout the procedure.
- Place elevation support under the mouse to maintain the body and head leveled in a horizontal position. Keep the animal warm with a warm pad covered in paper. Apply eye ointment to both eyes to avoid drying.
- Keep the surgical area as clean as possible. Wear sterile gloves. Disinfect the mouse's head with iodine and alcohol prior to opening the skin. With a scalpel, cut the skin on the head along the midline, approximately from behind the eyes until behind the lambda suture. Keep the skull exposed and clean it with a cotton swab embedded in sterile 0.9% NaCl.
- Find the rostral rhinal vein (RRV) and mark it with a sterile pencil. Use the stereoscope for the rest of the procedure.
NOTE: We obtain better results using the RRV as an anteroposterior reference, but it is standard to use bregma as a reference. - Using a sterile needle as a reference, make sure that the brain orientation is correct before proceeding to stereotaxic measurements. Ensure also that the heights of the skull surface at the RRV and lambda, as well as the lateral inclination, are the same (± 0.02 mm).
NOTE: The lateral inclination becomes more relevant for injection into more lateral brain structures. - Load a sterile glass pipette with the viral solution to be injected. Bring it to the RRV reference for reference 0 anteroposterior (AP). Advance along the sagittal suture to the AP coordinate of choice. Mark this position with a sterile pencil, lift the needle, and proceed to craniotomy.
- Carefully drill a small circle around the marked position to avoid breaking the superior sagittal sinus. Position the drill in a tilted rather than a perpendicular position to reduce pressure while drilling. Remove the piece of the skull with small forceps.
- Once the vasculature is exposed, use the middle of the superior sagittal sinus to use as the mediolateral (ML) reference (point 0); this is more precise than using the sagittal suture. Move to the ML position of choice. Lower the pipette to touch the dura-mater as the dorsoventral (DV) reference (point 0). Slightly break the dura-mater and descend the pipette to the DV position of choice.
- Inject the desired amount of AAV (50-200 nL) and leave the cannula in place for 3 min to allow adequate liquid dispersion. Remove the pipette carefully out from the brain.
- Release the mouse from the ear bars. Close the skin using surgical clips or any method of choice. Put the animal in a separate warmed cage for recovery. Monitor recovery, reactivity, and activity after the mouse is awake. When fully recovered, move the mouse back to its original cage.
- Wait for a minimum of 3-4 weeks for viral expression before proceeding with the second part of the procedure.
2. Jugular vein and carotid artery canulation
- Preparation for surgery
- Perform surgeries in a designated surgical space. Disinfect surgical surfaces with 70% ethanol before initiating the procedures. Prepare multiple packages of surgical tools to ensure that each package is used in no more than five animals.
- Autoclave all surgical instruments. Then, clean with sterile water or saline and disinfect the tools with a hot bead sterilizer for at least 15 s (according to the manufacturer's instructions) between surgeries.
- Use sterile drapes to maintain the sterility of the surgical field. Wear a clean lab coat or disposable gown and a mask.
- Prepare micro-catheters for cannulations in the carotid artery and jugular vein. Construct the artery catheter by joining a small segment of microrenathane tubing (stretched from 0.025 inches outer diameter [OD] x 0.012 inches inner diameter [ID]) with silastic tubing (0.025 inches OD x 0.012 inches ID). Construct the venous catheter only using silastic tubing (0.025 inches OD x 0.012 inches ID). Soak all catheters in 70% ethanol overnight prior to surgery.
- Bevel the cut tips of both catheters at 45° to preestimated lengths based on the animal's body weight and length.
- Surgical procedures
- Anesthetize the animals under 2% isoflurane with a tabletop low-flow isoflurane system to precisely control the anesthesia stage and plane during surgery.
- Inject the preemptive dose of analgesic (carprofen 5 mg/kg s.c.) and apply ophthalmic ointment to prevent desiccation and corneal injuries.
- Shave the ventral and back areas of the neck and clean the skin with three iodine scrubs alternated with 70% ethanol. Cut a vertical skin incision (12 mm) between the shoulder blades and cover it with surgical gauze for later use. Place the animal in a supine position with the head towards the surgeon.
- Use a stereo dissecting scope for most parts of the procedures described below.
- Make a small vertical incision (~10 mm) on the right side of the neck, superior to the clavicle to expose the right carotid artery and jugular vein. Cut the skin with scissors and perform a blunt dissection to separate subcutaneous tissues using non-toothed micro forceps with fine tips, exposing the right external jugular vein and the right common carotid artery.
- Tie the distal end of the jugular vein to stop blood flow and cut a small hole in the collapsed vein using micro forceps and scissors. Insert the venous catheter bevel down and proximally using a pair of micro forceps to advance it through the superior vena cava to reach the level of the right atrium. The inserted length is ~10-12 mm for a 30 g lean mouse. Tie the catheter to fix with the vessel using 7-0 silk sutures.
NOTE: With the catheter in the right place, blood can be easily withdrawn; otherwise, the catheter may be misplaced into the lateral thoracic vein and needs to be reinserted. - Carefully dissect connective tissues to expose the right common carotid artery located in a triangle area surrounded by the sternohyoid muscle, sternomastoid muscle, and digastric muscle. Use a wire retractor to separate these muscles if needed. Tie the artery at the level of the bifurcation of internal and external carotid arteries using 7-0 silk sutures. Preplace two untied suture loops proximally.
- Temporarily stop the blood flow by pulling a preplaced suture loop and cut or punch a small hole on the vessel wall using micro scissors or a 27 G needle. Insert the arterial catheter bevel down and proximally to a preestimated length reaching the aorta arch but without touching the aortic valve. The inserted length is ~9-10 mm for a 30 g lean mouse. Tie the catheter to fix with the vessel using the two preplaced sutures.
- Tunnel all catheters subcutaneously and exteriorize them at the back of the neck via the precut incision and join them to the venous or arterial ports of a silicone-coated tubing connector made from 25 G needle tubing: modified MASA19 using two 25 G needle tubing and two 4.0 cm of PE-20 tubing, sleeved with 2.0 cm of 0.062 inch ID silastic tubing attached with a small metal wire-made ring. The total lumen volume of each catheter including the connector is 6-8 µL (Figure 1A).
- Close the ventral incision and fix the connector subcutaneously upon the closure of the back skin with sutures. Fill both catheters with heparinized saline (200 U/mL) and plug them tightly at the end with stainless steel surgical wires.
- Mice recover from isoflurane anesthesia within minutes and fully recover from surgery within 5 days. Place the animals in the automated blood collection housing chamber 24 h after surgery and connect them to the system by joining the tether hook of the system to the metal ring attached to the tubing connector implanted on the back of the neck. Connect the arterial and venous catheters to the injection and sampling lines, respectively, 24 h before the sampling begins (Figure 1B) . The total length of the sampling line is 55 cm in length or 40 µL by lumen volume.
NOTE: The setups of injection and sampling lines, the programming of the collection method, and the cage balance mechanisms of the automated blood sampler system have been described in detail20,21. The system keeps the catheter open by automatically delivering 10 µL of heparinized saline every 20 min. - Set the preinjection sampling, injection, and postinjection sampling time and frequency via the computer program of the system. See step 3.1 below for the current settings.
- The system allows the animal to move freely without tangling the sampling and/or infusion lines by sensing the mouse movement while rotating the housing chamber in an opposite direction to the mouse movement21 (Figure 1).
- Refill the infusion or injection line with the compound and reconnect to the venous catheter at least 2 h before the infusion or injection begins.
NOTE: Surgery complications might occur, and animals need to be monitored closely for recovery and on the days following the surgery. Refer to the animal protocol for correct monitoring, termination requirements, and procedures.
3. Automated blood collection and intravenous injection
- Automated blood collection
- To follow this protocol, use a sampling volume of 20.0 µL and set an interval (i.e., 7.0 min) between each sampling to have a sampling frequency of every 10.0 min per sample. The maximum rate is to sample continuously at 3.0 min/sample; the minimum possible sampling volume is 5.0 µL. An equal amount of saline is automatically given back by the system to replace the sampled blood and maintain body fluid balance.
- Set the total sampling time as 30 (t = -30 - 0) min before and 30 (t = 0 - 30) min after the intravenous injection of CNO (0.5 mg/kg, Figure 2).
- Each collected blood sample (20.0 µL) is automatically diluted in 50 µL of saline (containing heparin at 10 U/mL) and stored individually in a microtube held in a refrigerated sample carousel by the system.
- Automated intravenous injection
- Disconnect the venous line from the venous catheter to refill CNO (individually calculated dose by volume of ~50-60 µL at 0.5 mg/kg) at least 2 h before blood sampling begins.
- Manually withdraw the solution (slightly more than the calculated volume) from the line retrogradely with the injection syringe, leaving a small air bubble between the solution and the existing saline in the line.
- Reconnect the injection line to the venous catheter and set the injection rate at 500 µL/min and the injection starting time at 2 min after t = 0 sampling finishes. The total injection time for each animal is 5-6 s.
NOTE: If required, the compound can also be delivered by a manual intraperitoneal injection (IP), but this will require disturbing the animal during or before the blood collection protocol. We also show this alternative in the results.
4. Animal perfusion and brain collection (OPTIONAL)
NOTE: This procedure is to be followed only if the brain is required to analyze the brain site of neuronal activation or downstream responses.
- Disconnect the mouse from the blood collection system at the end of the collection protocol. Two hours after the intravenous injection, proceed to perfuse the animal with 10% neutral buffered formalin (NBF), using the preferred method or as described elsewhere22.
- Dissect the brain and conserve it in 20% sucrose in 10%-NBF for 3 h to continue fixation.
- After 3 h, transfer the fixed brain to 20% sucrose in PBS and conserve at 4 °C until ready for sectioning.
NOTE: Avoid overfixation as this will mask the cFOS antigens. In the 20% sucrose solution, the brain should sink to the bottom of the container. - Make sections using a freezing microtome or cryostat and use the preferred method to look at neuronal activation (e.g., cFOS immunohistochemistry is shown).
NOTE: The antibodies used for the current results are described in the Table of Materials.
5. Sample processing and analysis
- Remove blood samples from the automatic sampler immediately after the end of the experiment and put them on ice.
- Spin the samples at 14,000 × g for 30 s.
- Collect the plasma. Store it at -80 °C until analysis.
- Assay the blood samples by LH ELISA as described before10,23.
Representative Results
Kisspeptin-expressing neurons (Kiss1 gene) located in the arcuate nucleus of the hypothalamus are a potent stimulator of GnRH, and thus, of LH release from the pituitary gland24,25. In this protocol demonstration, we have used kisspeptin-induced LH secretion to illustrate the functioning of the automated blood sampling technique. Figure 2 shows representative LH patterns in adult Kiss1-eYFP females that previously received a unilateral stereotaxic injection of AAV-hM3Dq-mCherry in the arcuate nucleus (AP: -4.95, ML: -0.35, DV: -5.7). The ChR2-eYFP was used as a fluorescent reporter for Kiss1 cells. One month after the stereotaxic surgery, the mice underwent carotid artery and jugular vein cannulation and were connected to the automatic blood sampler 4 days post-surgery. Blood collections for determining basal LH levels were started on the next day at 10 min sampling frequency (7 min interval between samples and 3 min/sampling) followed by an automated IV CNO injection and continued blood sampling every 10 min for 30 min. Diestrous LH levels are generally low (Figure 2A), but variations are usually observed because of its pulsatile release (Figure 2B). Following the CNO injection (and activation of kisspeptin neurons), the rise in LH was sharp (within 10 min). The level of increase and the duration of the peak depends on numerous factors, including the site of the injection, the number of neurons activated, or the population targeted.
Figure 3 shows the brain site of injection in the arcuate nucleus of the female mouse represented in Figure 2A. Kiss1-eYFP neurons are labeled in green, while the mCherry immunoreactivity shows the site of the AAV injection and activation following CNO. Activated neurons were detected using cFOS immunoreactivity, labeled with DAB. Most mCherry neurons colocalized with Kiss1-eYFP, and many showed cFOS immunoreactivity, demonstrating that the viral and neuronal activation was specific to the targeted population.
A representative LH pulsatile pattern of release in diestrous wildtype (C57BL/6J) mice followed by the response to an IP injection of kisspeptin-10 is shown in Figure 4. The mouse underwent carotid artery cannulation and was connected to the automatic blood sampling system 4 days after the surgery. The next morning, the estrous cycles were checked and the blood collection and kisspeptin-10 injection were performed on the diestrous day26. Blood samples were collected every 7 min for 2 h (4 min interval, plus 3 min/sampling for 120 min before the injection) to determine the baseline levels and LH pulsatility, followed by an IP injection of kisspeptin-10 (65 µg/kg) and continued blood collection every 7 min for an additional 30 min. Clear LH pulses typical for a female in diestrous were observed, showing low basal LH levels, pulse frequency of ~2 pulses/h, and pulse amplitude ~1 ng/mL27. An immediate and robust increase in LH was detected in response to kisspeptin administration28. The LH secretion patterns and changes after stimulation are in agreement with other studies using manual blood collection10,27,29,30. These results demonstrate that the automated blood sampling method captures typical and stimulated LH secretion under a stress-free condition.
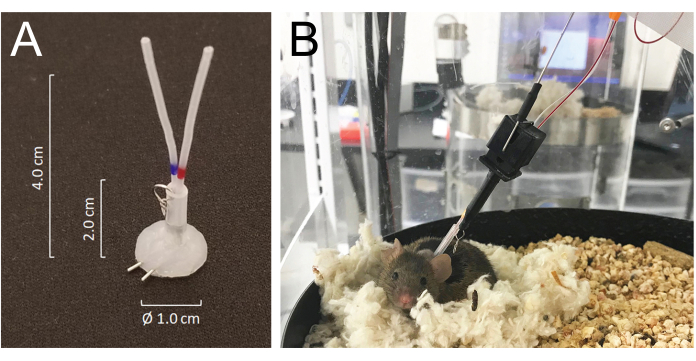
Figure 1: Details of the connector system and mouse connections to the infusion and sampling system tubing. (A) A silicone-coated tubing connector (MASA) constructed using two 25G needle tubing and two PE-20 tubing, sleeved in a silastic tubing attached with a small metal ring. (B) A mouse connected to the infusion and sampling tubing in the sampling cage, resting in its nest during the blood sampling. Please click here to view a larger version of this figure.
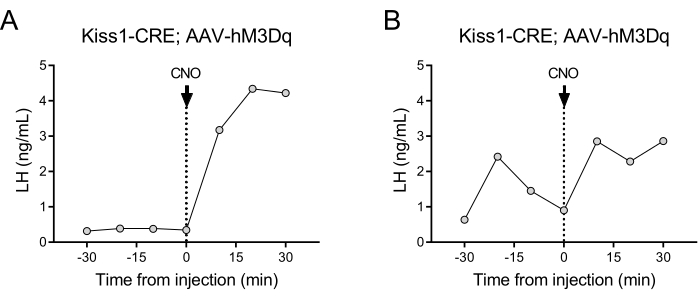
Figure 2: Representative results for LH pulses in Kiss1-Cre female mice injected with AAV-hM3Dq in the arcuate nucleus and remotely activated with CNO. Basal LH levels were measured every 10 min for half an hour. At time 0 following the blood collection, the female received an intravenous injection of clozapine-N-oxide (0.5 mg/kg) and blood continued to be collected from the carotid artery every 10 min for an additional half an hour. (A) Shows a female with low basal LH levels. (B) Shows a female displaying an LH pulse preinjection. Abbreviations: Kiss1 = Kisspeptin; AAV = adeno-associated virus; CNO = clozapine-N-oxide; LH = luteinizing hormone. Please click here to view a larger version of this figure.
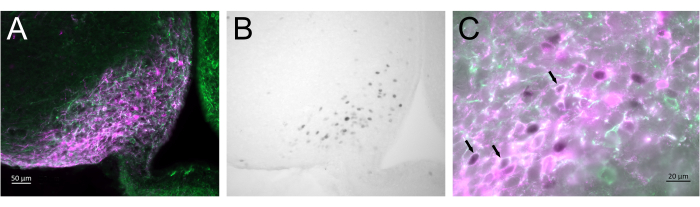
Figure 3: Brain activation in the arcuate nucleus of the Kiss1-Cre;Chr2-eYFP (Kiss1-eYFP) female represented in Figure 2A. ChR2-eYFP was only used as a reporter gene to label kiss1 neurons. (A) Low-magnification fluorescence image showing the site of the AAV injection in the arcuate nucleus. Green: eYFP immunoreactivity, Magenta: mCherry immunoreactivity. (B) Low-magnification brightfield image of the area corresponding to Figure 3A, showing cFOS immunoreactivity (black) in the site of the AAV injection in the Arcuate nucleus. (C) High-magnification fluorescence and brightfield image combined showing a closer look of the neurons in Figure 3A,B. Kiss1-eYFP neurons coexpressing AAV-mCherry that have been activated are those with white cytoplasm and black nucleus (arrows). Scale bars = 50 µm (A,B), 20 µm (C). Abbreviations: Kiss1 = Kisspeptin; AAV = adeno-associated virus; eYFP = enhanced yellow fluorescent protein. Please click here to view a larger version of this figure.
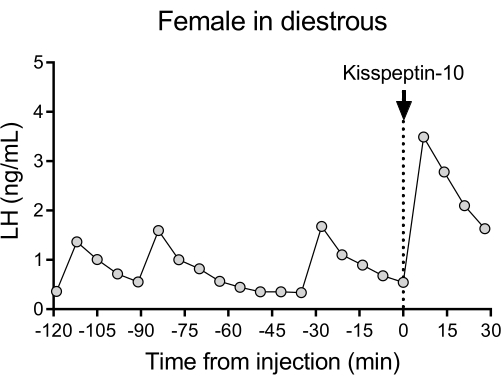
Figure 4: Basal LH pulsatility in a diestrous wildtype female measured every 7 min for 2 h. The female then received an intraperitoneal injection of kisspeptin-10 (65 µg/kg) at time 0, and blood samples were continuously collected every 7 min for half an hour. Abbreviation: LH = luteinizing hormone. Please click here to view a larger version of this figure.
Discussion
Using this protocol, we were able to show basal LH pulsatility and LH secretion after stimulation of a neuronal population. The great advantages of the system are the stress-free environment in which sampling takes place, with no human presence or handling during the blood sampling. Additionally, no prior laborious animal training and adaptation to human presence or handling during the experiment were required. Previous experiments using manual blood sampling required a large amount of time and effort to minimize stressors7,31,32. However, cutting the tail alone is a stressor33. Implementing a non-stressful environment and training paradigm in shared animal facilities, where interruptions are unpredictable, may also be a constraint. In some laboratories, animals often need to be transported to alternative procedure rooms for blood sampling. These limitations may make the manual method inappropriate for the detection of subtle changes in LH levels, and therefore, a hands-off approach can be helpful in these situations. Automated sampling is set in a quiet room where the mice are placed several days in advance to acclimate to the new environment. Our previous experience with this protocol provided precise detection of corticosterone and pulsatile growth hormone secretion patterns in mice, showing no elevated corticosterone levels during the automated sampling15. In the current experiments, all animals were well adapted to the sampling system showing nest-building in the sampling chamber after ~24 h and bright hair color, indicating a lack of stress and overall good health state (Figure 1).
The main difficulty leading to negative results is probably the inappropriate targeting of the AAV to the required neuronal population. Precision in the stereotaxic injections is essential and training should be done in advance to verify the coordinates and injection volumes. Training can be done by injecting a small amount of 0.5-1% Evans Blue to the desired location in non-recovery surgery and then taking a slice of the freshly dissected brain using a mouse brain matrix (e.g., Ted Pella) to check the site and size of the injection using a stereoscope.
It is also important to take into account that blood and plasma collected from the automated blood sampling system will be diluted in heparinized saline (e.g., 20 µL of blood in 50 µL of saline in our results)20, and the dilution ratio may need to be adjusted for the sensitivity of the selected analytical method. We tested LH levels in whole blood diluted in BSA-PBS (as recommended for Ultra-Sensitive LH ELISA)10 or saline and found no differences in LH values. Tween cannot be used in the diluent since this will circulate into the blood system to extract the samples by replacing the sample fluids20. In our experience, dilutions less than 1:10 gave good LH results but slightly underestimated LH levels as compared to 1:3.5. This indicates that the dilution may be further adjusted to reduce the amount of blood collected, if necessary.
An alternative to automated compound delivery is to do manual injections via the venous catheter. In this case, the investigator is briefly present in the room to deliver the injection. However, there is no direct contact with the animals or their housing and surroundings and, unlike intraperitoneal or subcutaneous injections, the entire procedure is often unnoticed by the animal. The advantages of a manual injection are that the compound dilution does not need to be set up in advance, which may be critical for compounds that are too expensive to use in larger volumes or are sensitive to degradation over time; since the operating volume is smaller than in automated delivery, where the infusion line and catheter need to be prefilled with more compound solution.
Automatic blood sampling provides a unique opportunity to study LH variations during sleep for instance. We have regularly observed animals sleeping in their nests during sampling time. It is possible to link this sampling with EEG recordings to generate a more detailed analysis of the relationship between neural activity and LH pattern34. As shown here, the possibilities for using automated blood sampling are many: from basal LH sampling to testing the LH response to endogenous or exogenous compounds, or to activation or suppression of neuronal populations. The neuronal manipulations can be implemented acutely with chemogenetics or optogenetics, or permanently using transgenic mouse models and apoptotic or neuronal silencing tools. Automated blood sampling also allows for the measurement of other hormones with highly pulsatile secretory patterns (e.g., growth hormone15). In female mice, if a specific phase of the estrous cycle is required, vaginal smears can be carefully collected hours before starting the protocol26 without disrupting the infusion and sampling lines. Animals can be connected to the sampling system for 7-10 days with the risk of clotting the arterial line increasing with time.
This technique, however, is limited to use in single-housed animals, and therefore, it may not be suitable for studying social interactions. It is also invasive and requires technically challenging surgery, so it may not be possible to implement with juvenile animals or certain disease models. Finally, because the cost to purchase the system may be too high for a single research laboratory, it would be advisable to set it up in a core laboratory providing the mentioned experimental protocol as services.
In conclusion, this protocol shows how to perform stereotaxic delivery of AAV combined with automated blood sampling. The precise spatial and temporal control achieved with this technique, together with its flexibility of application to different models, measurement protocols, and hormones, makes it a powerful method for the study of hormonal regulation in rodents. Most importantly, the method provides a stress-free environment by eliminating human presence and handling during injection and/or sampling, and prior animal training. These advantages, together with the possibility of multiplexing, make this method a unique tool for studying the neural control of hormonal changes in conscious, freely moving, and undisturbed mice.
Acknowledgements
We thank Dr. Daniel Haisenleder for his help in testing different blood dilution methods. Serum hormone assays were performed at the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core supported by the Eunice Kennedy Shriver NICHD Grant R24 HD102061. The Michigan Mouse Metabolic Phenotyping Center-Live is supported by NIH Center Grant U2C DK135066. JF and NQ are supported by DK020572 (MDRC) and DK089503 (MNORC) grants. CFE and CSM are supported by NICHD grant R21 HD109485 and R01 HD096324.
Materials
| Name | Company | Catalog Number | Comments |
| AAV8-hSyn-hM3D(Gq)-mCherry | Addgene | 44361 | Not necessarily this virus but this was the one used for representative results |
| Alcohol | Disinfection | ||
| Anesthesia Induction box | Vetequip | ||
| Anesthesia induction machine | Kent Scientific Equipment | SomnoSuite | |
| Anesthesia masks for mice | Kent Scientific Equipment | SOMNO-0801 | |
| Autoclip applier 9 mm | Clay Adams | 427630 | |
| Autoclip remover 9 mm | Clay Adams | 427637 | |
| Autoclips 9 mm | Clay Adams | 427631 | |
| BASi Culex Controller | Culex | SN: 2151, 2152, 2156, 2158 | 4 stations |
| BASi Honey Comb Fraction Collector | Honey Comb | SN: 2105, 2106, 2107, 2108 | 4 stations |
| BASi Ratrun Rotation Control | RATURN 2 | SN: 5680, 5681, 5682, 5683 | 4 stations |
| C57BL/6J mice | JAX # 000664 | ||
| Carprofen | Zoetis | Rimadyl | Analgesic |
| Clippers | Braun | ||
| Clozapine-N-oxide | ENZO | BLM-NS105-0005 | |
| Cotton tipped applicators | |||
| CULEX Automated In Vivo Sampling System | BASi | DS000627 | with CX-4000S Replacement Tubing Sets |
| Curved forceps serrated | FST | 11151-10 | |
| Drill | Dremel | 61100 | |
| Empis control Module | EMPIS CM | SN: 174 | |
| Empis Programmable Infusion System | EMPIS | SN: 2125 , 2126, 2127, 2128 | With CX-7010S 4 BAS-2 Infusion Sets; 4 stations |
| Envigo 2016 diet | low-phytoestrogen diet | ||
| Eye ointment | Dechra | Puralube Vet Ointment | Petrolatum Ophtalmic oinment |
| Glass pipettes | World Precision Instruments | MIB100-6 | |
| Hemostats | Roboz Surgical | RS-7101 | |
| Iodine | Betadine Surgical scrub | ||
| Isoflurane | VetOne | Fluriso | Anesthetic |
| Isoflurane Vaporizer or SomnoSuite Low-Flow Anesthesia System | Surgivet or Kent Scientific Corp | SS-01 | Anesthesia Machine |
| Kiss1-Cre;ChR2-eYFP (Kiss1-eYFP) mice | JAX # 023436 and #024109 | ||
| Kisspeptin-10 | Phoenix Pharmaceuticals | 048-56 | |
| Micro-renathane tubing | Braintree Scientific | MRE025 | Surgical catheterization |
| Micro-Scissors | Roboz Surgical | RS-5606 | |
| Needle Holder | Roboz Surgical | RS-7842 | |
| Picoliter injector | Warner Instruments | PLI-100A | |
| Pipette puller | Sutter Instruments | P30 | |
| Rodent Warmer X2 | Stoelting | 53850 | |
| Scalpel | FST | 10003-12 | |
| Scissors | Roboz Surgical | RS-6808 | |
| Silicon tubing | Liveo Laboratory Tubing | NO.508-001 | 0.012 in I.D x 0.025 in O.D. |
| Stereotaxic table | RWD | E06208 | |
| Sterile 0.9% saline | Baxter | 2F7124 | |
| Sterile towel drapes | Dynarex | 4410 | |
| Surgical blades | SKLAR | 06-3011 | |
| Surgical stereoscope | Zeiss | f-160 | |
| Tweezers | Roboz Surgical | RS-4960 | |
| Tweezers | Roboz Surgical | RS-4972 | |
| Tweezers | Roboz Surgical | RS-5058 | |
| Antibodies | |||
| Anti-cFos | Millipore | ABE457 | Antigen target: N-terminus cFos; Host organism: Rabbit; Dilution used: 1:5,000; RRID: AB_2631318 |
| Anti-GFP | Aves Labs | GFP-1010 | Antigen target: recombinant GFP null; Host organism: Chicken; Dilution used: 1:10,000; RRID: AB_2307313 |
| Biotin-SP-conjugated AffiniPure Donkey Anti-Rabbit IgG | Jackson ImmunoResearch Labs | 711-065-152 | Antigen target: Rabbit IgG (H+L); Host organism: Donkey; Dilution used: 1:1,000; RRID: AB_2340593 |
| Donkey anti-Rat IgG, AlexaFluor 594 | Thermo Fisher Scientific | A-21209 | Antigen target: Rat IgG (H+L); Host organism: Donkey; Dilution used: 1:500; RRID: AB_2535795 |
| Goat anti-Chicken IgY, Alexa Fluor 488 | Thermo Fisher Scientific | A-11039 | Antigen target: Chicken, IgY (H+L); Host organism: Goat; Dilution used: 1:500; RRID: AB_2534096 |
| mCherry monoclonal (16D7) | Thermo Fisher Scientific | M11217 | Antigen target: mCherry tag; Host organism: Rat; Dilution used: 1:5,000; RRID: AB_2536611 |
References
- Kokoris, G. J., Lam, N. Y., Ferin, M., Silverman, A. J., Gibson, M. J. Transplanted gonadotropin-releasing hormone neurons promote pulsatile luteinizing hormone secretion in congenitally hypogonadal (hpg) male mice. Neuroendocrinology. 48 (1), 45-52 (1988).
- Coquelin, A., Desjardins, C. Luteinizing hormone and testosterone in young and old male mice. American Journal of Physiology - Endocrinology and Metabolism. 243 (3), E257-E263 (1982).
- Carmel, P. W., Araki, S., Ferin, M. Pituitary stalk portal blood collection in rhesus monkeys: Evidence for pulsatile release of gonadotropin-releasing hormone (GnRH). Endocrinology. 99 (1), 243-248 (1976).
- Schuiling, G., Gnodde, H. Site of origin of the pulsatile secretion of luteinizing hormone in long-term ovariectomized rats. Journal of Endocrinology. 70 (1), 97-104 (1976).
- Hackwell, E. C. R., Ladyman, S. R., Brown, R. S. E., Grattan, D. R. Mechanisms of lactation-induced infertility in female mice. Endocrinology. 164 (5), 1-12 (2023).
- Bahougne, T., Kretz, M., Angelopoulou, E., Jeandidier, N., Simonneaux, V. Impact of circadian disruption on female mice reproductive function. Endocrinology. 161 (4), (2020).
- Kreisman, M. J., McCosh, R. B., Tian, K., Song, C. I., Breen, K. M. Estradiol Enables Chronic Corticosterone to Inhibit Pulsatile Luteinizing Hormone Secretion and Suppress Kiss1 Neuronal Activation in Female Mice. Neuroendocrinology. 110 (6), 501-516 (2020).
- Moenter, S. M., Evans, N. P. Gonadotropin-releasing hormone GnRH measurements in pituitary portal blood. Journal of Neuroendocrinology. 34 (5), 13065 (2022).
- Maeda, K. I., et al. The LHRH pulse generator: A mediobasal hypothalamic location. Neuroscience and Biobehavioral Reviews. 19 (3), 427-437 (1995).
- Steyn, F. J., et al. Development of a methodology for and assessment of pulsatile luteinizing hormone secretion in juvenile and adult male mice. Endocrinology. 154 (12), 4939-4945 (2013).
- Steyn, F. J., et al. Development of a method for the determination of pulsatile growth hormone secretion in mice. Endocrinology. 152 (8), 3165-3171 (2011).
- Minabe, S., Uenoyama, Y., Tsukamura, H., Maeda, K. Analysis of pulsatile and surge-like luteinizing hormone secretion with frequent blood sampling in female mice. Journal of Reproduction and Development. 57 (5), 660-664 (2011).
- Perreau-Lenz, S., Kalsbeek, A., Pévet, P., Buijs, R. M. Glutamatergic clock output stimulates melatonin synthesis at night. European Journal of Neuroscience. 19 (2), 318-324 (2004).
- Herwig, A., Pévet, P., Bothorel, B., Steinlechner, S., Saboureau, M. Trans-pineal microdialysis in the Djungarian hamster (Phodopus sungorus): A tool to study seasonal changes of circadian clock activities. Journal of Pineal Research. 40 (2), 177-183 (2006).
- Adams, J. M., Otero-Corchon, V., Hammond, G. L., Veldhuis, J. D., Qi, N., Low, M. J. Somatostatin is essential for the sexual dimorphism of GH secretion, corticosteroid-binding globulin production, and corticosterone levels in mice. Endocrinology. 156 (3), 1052-1065 (2015).
- Alexander, G. M., et al. Remote control of neuronal activity in transgenic mice expressing evolved G protein-coupled receptors. Neuron. 63 (1), 27-39 (2009).
- Krashes, M. J., et al. reversible activation of AgRP neurons drives feeding behavior in mice. Journal of Clinical Investigation. 121 (4), 1424-1428 (2011).
- National Research Council. Guide for the Care and Use of Laboratory Animals. Eighth edition. National Research Council. , (2011).
- Ayala, J. E., et al. Hyperinsulinemic-euglycemic clamps in conscious, unrestrained mice. Journal of Visualized Experiments. (57), e3188 (2011).
- Peters, S., et al. Culex ABS Part I: Introduction to automated blood sampling. Current Separations. 18 (4), 139-145 (2000).
- Bohs, C., Cregor, M., Gunaratna, G., Kissinger, C. Culex Automated blood sampler part II Managing freely-moving animals and monitoring their activity. Current Separations. 18 (4), 147-151 (2000).
- Gage, G. J., Kipke, D. R., Shain, W. Whole animal perfusion fixation for rodents. Journal of Visualized Experiments. (65), e3564 (2012).
- Kreisman, M. J., Mccosh, R. B., Breen, K. M. A Modified ultra-sensitive ELISA for measurement of LH in mice. Endocrinology. 163 (9), (2022).
- Pielecka-Fortuna, J., Chu, Z., Moenter, S. M. Kisspeptin acts directly and indirectly to increase gonadotropin-releasing hormone neuron activity and its effects are modulated by estradiol. Endocrinology. 149 (4), 1979-1986 (2008).
- Kumar, D., et al. Specialized subpopulations of kisspeptin neurons communicate with GnRH neurons in female mice. Endocrinology. 156 (1), 32-38 (2015).
- Caligioni, C. S. Assessing reproductive status/stages in mice. Current Protocols in Neuroscience. , 1-8 (2009).
- Czieselsky, K., et al. Pulse and surge profiles of luteinizing hormone secretion in the mouse. Endocrinology. 157 (12), 4794-4802 (2016).
- Wang, L., et al. Genetic dissection of the different roles of hypothalamic kisspeptin neurons in regulating female reproduction. eLife. 8, 43999 (2019).
- McCosh, R. B., Kreisman, M. J., Breen, K. M. Frequent tail-tip blood sampling in mice for the assessment of pulsatile luteinizing hormone secretion. Journal of Visualized Experiments. (137), e57894 (2018).
- Vanacker, C., Defazio, R. A., Sykes, C. M., Moenter, S. M. A role for glial fibrillary acidic protein (Gfap)-expressing cells in the regulation of gonadotropin-releasing hormone (GnRH) but not arcuate kisspeptin neuron output in male mice. eLife. 10, e68205 (2021).
- Dulka, E. A., Defazio, R. A., Moenter, S. M. Chemogenetic suppression of GnRH neurons during pubertal development can alter adult GnRH neuron firing rate and reproductive parameters in female mice. eNeuro. 7 (3), 0223 (2020).
- Talbi, R., et al. Characterization of the Action of Tachykinin Signaling on Pulsatile LH Secretion in Male Mice. Endocrinology. 162 (8), 1-9 (2021).
- Tuli, J., Smith, J., Morton, D. Corticosterone, adrenal and spleen weight in mice after tail bleeding, and its effect on nearby animals. Laboratory Animals. 29 (1), 90-95 (1995).
- Lucien, J. N., Ortega, M. T., Shaw, N. D. Sleep and puberty. Current Opinion in Endocrine and Metabolic Research. 17, 1-7 (2021).
This article has been published
Video Coming Soon
ABOUT JoVE
Copyright © 2024 MyJoVE Corporation. All rights reserved