Assessment of The Effect of Antidiarrheal Drugs and Plant Extracts on Drosophila melanogaster
In This Article
Summary
Here, a method is described to feed Drosophila melanogaster with drugs and plant extracts and assess their effect on the gastrointestinal tract by analyzing the fruit flies fecal deposits. The drug-treated flies can be used as a model for further research.
Abstract
To study human gastrointestinal physiology, biomedical scientists have relied on the use of model organisms. Although many researchers have used mice as a model to study intestinal function, only a few reports have focused on Drosophila melanogaster (D. melanogaster). Compared to mice, fruit flies present many advantages, such as a short life cycle, cost-effective and simple maintenance, and no ethical issues. Furthermore, the mammalian gastrointestinal physiology, anatomy, and signaling pathways are highly conserved in D. melanogaster. Plant extracts have been used traditionally to treat diarrhea and constipation. For example, Psidium guajava (P. guajava) is one of the most known antidiarrheal agents in the tropics. However, no studies have evaluated the effect of antidiarrheal and laxative drugs and plant extracts in D. melanogaster, and it remains unknown if similar effects (e.g., smaller, more concentrated, and less abundant fecal deposits in the case of antidiarrheal drugs) can occur in the fruit flies compared to mammals. In this study, an antidiarrheal effect induced by P. guajava is demonstrated in a D. melanogaster strain that presents a diarrheic phenotype. Fecal sampling produced by flies is monitored using a dye-supplemented food. This protocol outlines the method used for preparing food with drugs, evaluating the fecal deposits of flies fed on these food preparations, and interpreting the data obtained.
Introduction
The gastrointestinal (GI) tract, also called the digestive tract, is responsible for the digestion and absorption of nutrients and excretion of undigested products1. The GI tract is vulnerable to a range of disorders that can cause discomfort, pain, and disruption to daily life. Gastrointestinal disorders include abdominal pain and discomfort, bloating, heartburn, indigestion or dyspepsia, nausea, vomiting, diarrhea, and constipation2. Diarrhea is the most common symptom of GI disorder3, and it is defined as a disease with at least three loose and watery stool during a 24 h period4. Diarrhea is caused by a wide range of pathogens, including bacteria, viruses, parasites, fungi, and can also be caused by drugs5,6. Worldwide, diarrhea continues to be the second leading cause of mortality among children under 5 years7. Although diarrhea can resolve itself, it can also indicate a more severe underlying condition if it lasts for more than a few days.
To study the intestinal tract, researchers turn to animal models such as mice, rats, and pigs8,9. However, the use of these animals can be expensive and time-consuming because they require specialized facilities and ethical considerations. Recent studies have shown that D. melanogaster can be used as a model to study the GI tract and investigate some mechanisms such as the maintenance of regenerative homeostasis, the development of immune senescence, the loss of epithelial barrier function, and the decline in metabolic homeostasis10,11. D. melanogaster, known as the fruit fly, shares a high degree of genetic homology with humans; approximately 75% of human disease genes are believed to have a functional homolog in fly12. They also have a simple digestive system consisting of a foregut, a midgut, and a hindgut13. D. melanogaster is easy to culture in the laboratory and can be genetically modified in different ways14. Therefore, using D. melanogaster for in vivo testing is a powerful tool that allows researchers to study complex biological processes in a controlled setting.
According to the World Health Organization (WHO), about 80% of people living in developing countries use traditional medicine for their primary health needs15. The high use of medicinal plants can be explained by the fact that they are easily available, inexpensive, and have few side effects16. The main plant parts used in herbal therapy include leaves, bark, roots, seeds17 while the main methods of preparation are infusion, decoction, and maceration18. These herbal remedies contain phytochemical substances such as alkaloids, terpenoids, flavonoids, steroids, tannins and carbohydrates19, which have therapeutic effects on the human body. People use a variety of medicinal plants to treat GI disorders such as diarrhea, stomachache, and dysentery20. For example, Psidium guajava is one of the most commonly used plants to treat diarrhea in the world. Various pharmacological and clinical tests have already showed its safety, which make it a good antidiarrheal candidate to study21,22. However, the major limitations of herbal medicines are the lack of efficiency and safety assessment, as well as a lack of definite and complete information about the composition of plant extracts used23. To validate the efficiency and the safety of herbal medicines, a systematic approach involving experimental and clinical validation is required and the approach should be supported by enough data from in vivo and in vitro studies.
To evaluate traditional remedies for their efficacy in the treatment of diarrhea, the use of mice and rats have been predominant in recent decades24,25. Due to the main advantages mentioned previously, i.e., ease of use, affordable, replicable, conserved absorptive and digestive functions between flies and mammals, we propose to use D. melanogaster as a model to evaluate the antidiarrheal activity of plants. The diarrheic phenotype in D. melanogaster can be characterized by several features, including increased abundance of fecal deposits, larger deposit sizes, a lighter coloration (less concentrated), and higher fecal material26. This phenotype can be quantified using various parameters: number of fecal deposits, total area of deposits, mean lightness, and total integrated optical density (IOD). Total IOD is defined as the total dye content of the deposit, meaning the total fecal material excreted27. Previously, an assay has been developed to analyze fecal deposits of D. melanogaster27,28. In this assay, the ultimate reader of dung (T.U.R.D.) was used as a fecal analysis tool, which allows to check for the number, size and lightness of fecal deposits and thus to monitor the intestinal physiology of the fruit flies. However, this method was never applied to evaluate the diarrheic phenotype in flies. The Ion Transport Peptide (ITP) gene is an important endocrine regulator of thirst and excretion and combines water homeostasis with feeding in D. melanogaster. In a recent study, it was shown that the speed of food transit throughout the GI tract and the frequency of defecation events were decreased by ITP over-expression and increased by ITP knockdown. The latter phenotype was described as diarrheic by the authors of this study29.
In this protocol, a modified version of the fecal deposit assay is employed to assess the effect of an antidiarrheal agent (i.e., guava leaf extract) on the gastrointestinal tract of D. melanogaster by using the ITPi strain as a diarrheic model. The overall goal of this method is: 1) to provide an easy and reliable method to evaluate the antidiarrheal effect of drugs and plant extracts, and 2) to allow the discovery of bioactive compounds responsible for the antidiarrheal effect in plant extracts by applying a bioactivity-guided approach.
Protocol
1. Preparing plant extract
- Collect Psidium guajava L. leaves30 from an adult tree and process as follows: dry the leaves in an oven at 40 °C for 6 days, then air-dry for 6 days, then dry again in the oven at 40 °C for 4 days, and finally prepare leaf powder by grinding the dry leaves in a grinding mill or a coffee grinder.
- Macerate 100 g of the dried powder in 1 L of 96% ethanol for 24 h, with continuous stirring using a shaker. Subject the plant residue to the same process once more and evaporate the resulting filtrates to dryness using a vacuum rotary evaporator under reduced pressure (175 mbar) at 40 °C.
- Dissolve the plant extract in ethanol to obtain the desired concentration. Determine the optimal concentration (using the protocol described here) by testing a series of concentrations.
- For plant extracts, test a concentration range of 100 µg/mL, 1 mg/mL, 10 mg/mL, 100 mg/mL and use those that do not affect the survival rate of fly. For pure compounds, test the following range of concentrations: 0.05, 0.5, 5 and 50 mM12.
2. Preparing food medium
- Measure 100 mL of distilled water and pour into the beaker with 4 g of sugar and 0.8 g of agar (see Table of Materials). Heat to 100 °C (while stirring) and hold for 10 min.
- Lower the temperature to 80 °C, add 7.4 g of flour and 2.8 g of yeast while stirring. Heat for at least 20 min, still stirring and controlling the temperature which should be around 80 °C.
- Add the moldex-propionic acid solution (1 mL of moldex and 0.3 g propionic acid mix well). Wait for the temperature to drop to around 50 °C, add the plant extract solution (1 mg/mL) and 0.5 g of bromophenol blue powder.
NOTE: Please refer to section 1.3.1 for more details on the other concentrations to be tested. - Pour the food into the Petri dishes and stop when the Petri dish is full (Figure 1A). Allow the Petri dishes to cool to room temperature (about 3 h), then close the lid, and store in the refrigerator at 4 °C.
NOTE: Petri dishes should be stored in the fridge for no more than 2 weeks to avoid water evaporation.
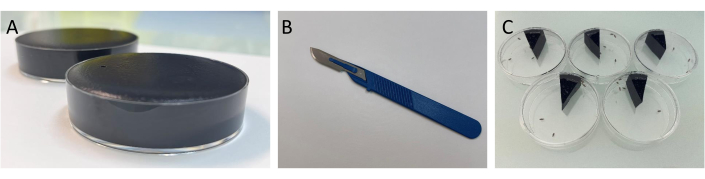
Figure 1: Demonstration of the experimental process for the fecal deposit test. (A) Image showing Petri dishes full of food medium. Make sure to have enough food in the Petri dish, so that no gaps will trap the flies and prevent them from moving. However, do not overload the Petri dish with food so that the surface can be covered evenly. (B) Image of the spatula as described in the protocol. (C) Image of the fecal deposit test as described in the protocol. Please click here to view a larger version of this figure.
3. Preparing flies
- Prepare the CO2 tanks, CO2 blow gun with needles, fly pad, paintbrush, and microscope. The fly station supplies CO2 to both the fly pad and the blow gun. The fly pad is employed for sorting flies, while the CO2 blow gun is utilized to anesthetize flies in vials, bottles, and Petri dishes.
- Standardize the age of the flies by selecting vials that contain pupae (at least 10) and discard the adults flies from the tube using the following method. One by one, turn the vials upside down and insert the needle between the cotton plug and the sidewall of the vial. Anesthetize adult flies with the CO2 blow gun until all flies are asleep on the cotton plug (a few seconds is needed to anesthetize them, and the anesthetic action will last for a few seconds after releasing the blow gun). Open the vial above a glass bottle containing 70% ethanol and drop the flies into it. Close the vial with the cotton plug and keep it in an incubator at 25 °C with 60% humidity. Set the light cycle of the incubator to 12 h light/12 h dark.
- After incubation (maximum 8 h), sort them into virgin females and males under the microscope and fly pad by turning them on their back and looking at their genitalia.
- Female genitalia are pale compared to male genitalia, which are of reddish color. Males can also be identified by the presence of dark bristles, called sex combs, on their front pair of legs. Divide the flies in two fresh tubes (one for males and one for females) and incubate them for 6-8 days at 25 °C.
NOTE: At 25 °C, the females remain virgin for approximately 8 h after hatching.
- Female genitalia are pale compared to male genitalia, which are of reddish color. Males can also be identified by the presence of dark bristles, called sex combs, on their front pair of legs. Divide the flies in two fresh tubes (one for males and one for females) and incubate them for 6-8 days at 25 °C.
4. Fecal deposit test
- Label the Petri dishes with the corresponding strain, sex, and drug to avoid confusion between the Petri dishes. Stack the Petri dishes on top of each other.
- Get the Petri dishes containing the dyed food and reverse them on the blotting paper to absorb the extra liquid. Using a spatula (Figure 1B), cut the food into 12 equal parts and then use the spatula to put one slice into an empty Petri dish.
NOTE: Depending on the number of replicates, the number of slices to be cut can be increased up to 20 per Petri dish. Each slice should be of the same size. - Anesthetize the flies with CO2 until all the flies are asleep on the cotton plug. Transfer six healthy flies in each Petri dish (Figure 1C), and close the lid immediately, then place them in the incubator (25°C, 60% humidity, 12 h light/ 12 h dark).
- To ensure that the flies do not escape from the Petri dish during the experiment, attach the top and bottom covers of the Petri dish with a tape. For each test group, prepare six replicate Petri dishes (at least).
- After allowing the flies to rear for 24 h, use CO2 to anesthetize them, transfer the flies to a container filled with 70% ethanol, and dispose of any remaining food.
- Keep the Petri dishes and proceed to step 5.
5. Quantification of Petri dishes
- Set a folder on the computer and rename it, by including the experiment strain name, the sex of the flies, and the type of drugs used. Within this folder, make subfolders named Original, Cut, and Analysis.
- Scan the Petri dishes by using a high-resolution scanner with a 6,400 pixel-per-inch (ppi) optical resolution. Scan the top and bottom covers of each Petri dish separately by placing them individually in the middle of the scanner field.
- Open the application installed on the computer. A welcome window will open on the computer screen with all the general settings (Figure 2A).
NOTE: Do not modify the advanced settings. This step is valid only for users who have the same scanner as proposed in the Table of Materials. Please refer to the guidelines if using another software.- Name the Petri dish in the application, by including sequence number, top or bottom cover, sex, and type of food used. Select the subfolder original set in step 5.1.
- Preview the Petri dish. Click on Preview at the bottom of the window, wait a few seconds for the scanner to pre-scan, then a window appears on the computer screen. Move the square displayed on the screen to surround the Petri dish.
- Click on Scan at the bottom right of the window on the computer screen, the scan is automatically saved as an image in the folder of choice.
- Crop the image using an application (e.g., the open-source Fiji application), so that no artefacts and food residue are considered as deposits.
NOTE: The following steps are valid only for users who have the Fiji application. Please refer to the guidelines if using another software.- Open the Fiji application, wait a few seconds until a toolbar appears on the screen.
- Drag the image to be cropped onto the toolbar. Select the 3rd icon Polygon selections in the toolbar, crop the unwanted part of the photo (the colored pie chart) by clicking on the screen to mark an angle around the pie chart (Figures 2B-1,2).
NOTE: When delineating the cropping region within an image, it is imperative that the chosen frames are seamlessly connected end-to-end. - Click on Edit at the top left of the bar then on Fill/Clear outside (Figure 2B-3).
- To save the photo, click on File at the top left of the bar, then on Save As, and finally on Tiff. Choose the subfolder Cut set in step 5.1.
NOTE: When saving the cropped image, make sure that no special characters (e.g., !, &, $, #, _,-,…) or too many characters are present in the file name .
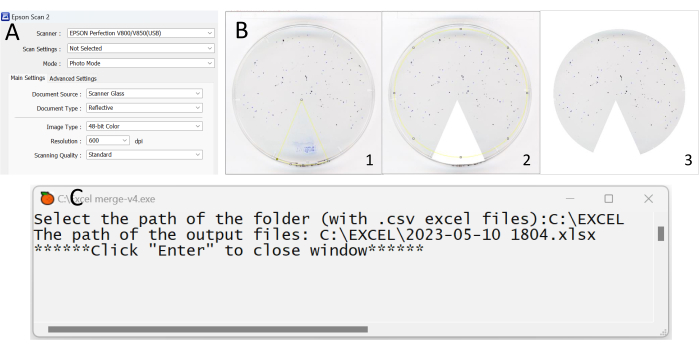
Figure 2: Key steps in the process of analyzing the data from the fecal deposit test. (A) Screenshot showing the setting information of the scan application. (B) Images cropped using the Fiji application. Make sure that no artefacts and food residue are considered deposits. (C) Screenshot showing what it looks like when opening the Excel_merge-v4 application. Please click here to view a larger version of this figure.
6. Fecal deposits identification using the ultimate reader of dung open source software
NOTE: The introduction and usage of the ultimate reader of dung software can be found in Supplementary File 1.
- First, open the T.U.R.D. software (Supplementary Figure 1). Create a new experiment and give a name to the document, then save it in the subfolder Analysis set in step 5.1.
- Click on Plates and then on Add plate. Select the Petri dish to be processed. A new window appears with the names of the selected plates and new parameters. Set the block size, offset, min size, and max size (Supplementary Figure 2).
- To verify that the fecal deposits detected are the right ones, click on Plates, then Inspect Selected Plates, then Graphics, and View annotated images (Supplementary Figure 3). Zoom in and look at the counts. If there are only a few deposits that should not be included in the analysis, deselect the deposits to be excluded (Supplementary Figure 4).
- For each new image to process, restart from step 6.3.
- After analyzing the plates with the T.U.R.D. software, modify the number of flies by clicking on No. Flies (Supplementary Figure 5).
- Modify the group name by clicking on Plates > Edit groups > Add, and then on Group column, choose the group name.
- Export the different replicate data separately by clicking on Analyze > Descriptive Statistics > Select Group (Supplementary Figure 6). Keep all spreadsheet files (.csv) in the same folder.
- In order to gather all files (obtained in step 6.7) in a unique spreadsheet, open the application Excel_merge-v4 (Supplementary Coding File 1), wait until the following sentence appear Select the path of the folder (with .csv files):, and then paste the above folder address. For example, the path could be C:\Experiment\Fecal deposit test\, then click Enter twice on keyboard (Figure 2C). After that, a new spreadsheet file is created in the same folder. The new spreadsheet file includes all of the exported files in different sheets.
- In the previous spreadsheet file, add another sheet to gather the mean of each parameter of all replicates (use the VLOOKUP function for processing the data). An example is given in the Supplementary Table 1.
- Analyze the p-value.
Representative Results
The study presented here shows that the measurement of diarrhea in D. melanogaster can be achieved through the use of the fecal deposit assay. Significant differences between the phenotypes (diarrheic or not) can be determined by analyzing various parameters, including the number of fecal deposits, the total area of deposits, the mean area of deposits, the mean lightness, and the total integrated optical density (IOD), which is a measure of the total amount of dye present in the deposit and represents the total fecal material content excreted27.
The ITP gene knockdown in flies can induce a diarrheic phenotype, characterized by increased frequency of defecation, making them a suitable model for studying diarrhea29. In the context of this experiment, the ITPi strain (w1118; daughterless-GeneSwitch, UAS-ITPi /(CyO)) was employed and reared on a standard medium. Psidium guajava leaves extract was selected as the antidiarrheal intervention, given the widespread use of this plant in tropical regions to manage diarrhea. Crofelemer, an antidiarrheal agent, was approved by the US Food and Drug Administration (FDA) to provide symptomatic relief for non-infectious diarrhea in adult patients with HIV/AIDS undergoing antiretroviral therapy31. Crofelemer is an extract from the latex of Croton lechleri Müll.Arg. stem bark32. Loperamide is a synthetic drug used worldwide to treat diarrhea33. Both Crofelemer and Loperamide were used as potential positive controls.
The hypothesis was that feeding flies with P. guajava extract, Crofelemer, and Loperamide would reduce the diarrheic phenotype compared to those fed with normal food. To examine this hypothesis, a measurement of fecal deposits was performed in D. melanogaster by comparing several parameters between flies fed with normal food and those fed with P. guajava extract (1 g/100 mL), Crofelemer (1 g/100 mL), and Loperamide (10 mM). For the experiment setup, 6-7-days-old virgin females or males were used. Each Petri dish contained six flies, and six replicates were carried out. The flies were reared for 24 h, and then each group was analyzed. The student's t-test was used to compare the significant difference between the test group. The results demonstrate that the number of fecal deposits (Figure 3A), the total area of deposits (Figure 3B) and the total IOD (Figure 3C) exhibited significantly higher values in the normal food group compared to the P. guajava extract (1 g/ 100 mL) group, in both virgin females and males. Unfortunately, Loperamide did not show any effect in both genders (but it was already demonstrated that it acts as an antispasmodic agent in D. melanogaster)34 while Crofelemer had an effect on females only.
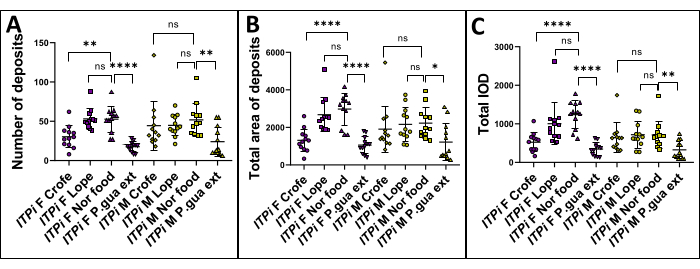
Figure 3: ITPi strain analysis. The ITPi strain was analyzed under four conditions: feeding on normal food, food supplemented with 1 g/100 mL P. guajava extract, 1 g/100 mL Crofelemer, and 10 mM Loperamide. The data are presented as mean ± SD of each condition in both females and males (for six replicates of two sides of a Petri dish). Statistical analysis was performed using a student's t-test comparing two groups. p-values are shown as follows: *: p < 0.05; **: p < 0.01; ***: p < 0.001, ****: p < 0.0001. (A) Number of fecal deposits of ITPi strain was compared in flies fed with food supplemented with 1 g/100 mL Crofelemer, 10 mM Loperamide, 1 g/100 mL P. guajava extract and in flies fed with normal food. Additionally, the difference in the number of fecal deposits between virgin females and males was also analyzed. In both groups, the number of fecal deposits was significantly higher in flies fed with normal food than those fed with 1 g/100 mL P. guajava extract. (B) Total area of fecal deposits of the ITPi strain was compared in flies fed with normal food and in flies fed with food supplemented with 1 g/100 mL P. guajava extract, 1 g/100 mL Crofelemer, and 10 mM Loperamide. In males and females, the total area of fecal deposits was significantly higher in flies fed with normal food than those fed with 1 g/100 mL P. guajava extract. (C) The difference in the total IOD of the ITPi strain was analyzed between flies fed on normal food and flies fed on food supplemented with 1 g/100 mL P. guajava extract, 1 g/100 mL Crofelemer, and 10 mM Loperamide. In males and females, the total IOD was significantly higher in flies fed with normal food than those fed with 1 g/100 mL P. guajava extract. Abbreviations: F = Female; M = Male; Crofe = Crofelemer; Lope = Loperamide; Nor food = Normal food; P. gua ext = Psidium guajava extract. Please click here to view a larger version of this figure.
To demonstrate that the reduced excretion observed in the P. guajava extract group is due to the extract's inhibitory effect and not to a reduced food consumption, we performed the direct intake estimation and tracking of solid food consumption (DIETS) method35. The results showed that there were no significant differences in food consumption between the drug-supplied groups and those without drugs, except for Loperamide in males, which caused flies to consume less food than normal (Figure 4).
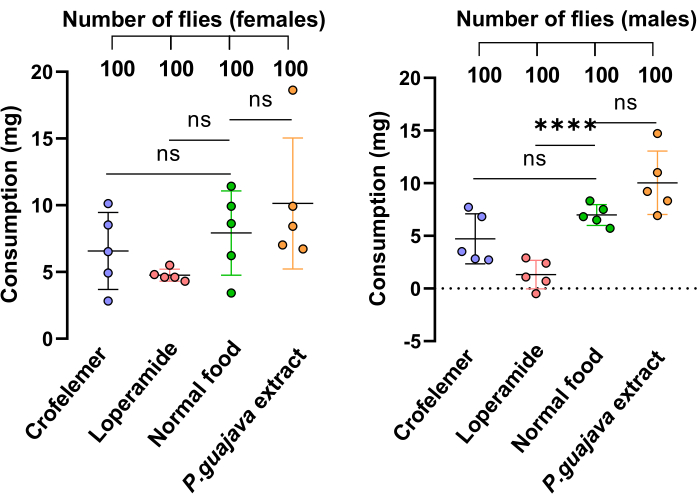
Figure 4: Feeding assay. The feeding assay measured solid food consumption in flies. Flies were fed with four different mediums: 1 g/100 mL P. guajava extract, 1 g/100 mL Crofelemer, 10 mM Loperamide and normal food. Each group consisted of 20 flies with five replicates. The data are presented as mean ± SD of each condition in both females and males. Statistical analysis was performed using a student's t-test comparing two groups. p-values are shown as follows: *: p < 0.05; **: p < 0.01; ***: p < 0.001, ****: p < 0.0001. Please click here to view a larger version of this figure.
The fecal deposits and the feeding assay results showed that P. guajava extract is a reliable medicinal plant to treat diarrhea in fruit flies.
Supplementary Figure 1:T.U.R.D. opening window. Please click here to download this File.
Supplementary Figure 2: T.UR.D. window with settings to be adjusted. Please click here to download this File.
Supplementary Figure 3: T.U.R.D. window with an annotated image. Please click here to download this File.
Supplementary Figure 4: T.U.R.D. window presenting each detected spot from an image already processed. Please click here to download this File.
Supplementary Figure 5: T.U.R.D. window presenting each processed image. Please click here to download this File.
Supplementary Figure 6: T.U.R.D. window showing the process to export the data for each group. Please click here to download this File.
Supplementary File 1: Quick guideline for using the T.U.R.D. software. Please click here to download this File.
Supplementary Table 1: Example of the final spreadsheets ready for analysis. Please click here to download this File.
Supplementary Coding File 1: Application for merging spreadsheets. Please click here to download this File.
Discussion
D. melanogaster has been widely accepted as a model for various biological processes due to the similarity in genes between D. melanogaster and humans36. The use of D. melanogaster as a model to study the intestinal tract is prevalent and the application of T.U.R.D. has been used to estimate the number, area, and amount of fecal deposits. However, the phenotypic detection method has not been used to assess the diarrhea in fruit flies. Therefore, this protocol introduces a new method to roughly assess the presence of diarrhea by detecting the fecal deposits.
Fecal deposits are an essential indicator of intestinal tract function and health37. In this context, a method is proposed for rearing D. melanogaster on drug-contained medium to investigate various parameters of fecal deposits. By monitoring the number of deposits, it is possible to determine the frequency of defecation and assess whether a drug has any impact on the intestinal transit. The total area of the deposits can be measured to evaluate the concentration and dilution of fecal matter, which is an important factor in determining the overall health of the intestinal tract. In addition, the total integrated optical density (IOD) can be used to detect the total amount of fecal material present in the deposits. This protocol provides an efficient method to screen and evaluate drugs as well as plant extracts that affect the intestinal tract. When D. melanogaster is used as a model organism, it is possible to assess the efficacy of potential drugs, which can help accelerate the drug discovery process. By applying this method on plant extracts, researchers can help to validate their use as antidiarrheal agents.
There are several critical steps to consider when using this protocol to study fecal deposits in D. melanogaster. Firstly, it is essential to calculate the mass required to achieve the desired concentration of the drug in the medium. Moreover, it is important to ensure good preparation condition when adding the drug to the medium, as high temperatures can degrade the drug and affect its potency. Second, the selection of female flies is important in this protocol. It is important to use virgin female flies to avoid the differences in fecal output between virgin and mated females. For example, the spots produced by virgin females are more circular than mated females, and mated females tend to excrete more fecal material than virgin females27,28. Therefore, it is recommended to collect flies before 8 h of eclosion to ensure that all females collected are virgins. Additionally, the tested flies should be strong and healthy, as their health can influence food intake and fecal output. For example, flies having an anormal shape of wings may have difficulty getting the food. Finally, to use T.U.R.D. successfully, the block size (pixels) and offset settings are crucial. Due to the difference in the light contrast of the images, it may be necessary to try different settings to achieve the best possible identification of fecal deposits.
Although the method presented is effective, there are several limitations. One is the accuracy of the drug concentration in the medium. As the medium is heated during preparation, some water may evaporate, which can affect the concentration of the drug. Another limitation is the scanning of the Petri dishes. Some parts of the Petri dishes (i.e., edges) are not scanned, and this could result in a miscalculation of the total fecal deposits. In addition, the flies do not produce the same amount of fecal deposits on the top and bottom covers of the Petri dishes. Because they tend to produce more deposits on the bottom cover, the standard deviation of the analysis between the top and bottom cover may be high, which may affect the accuracy of the results.
Using this protocol, researchers can study diarrhea in D. melanogaster. By modifying the drug-containing medium, this method can be used to screen antidiarrheal plants, which provides a novel approach to drug discovery. Traditional medicine and natural products have been used for centuries to treat different diseases, including gastrointestinal disorders. By using this protocol to evaluate the efficacy of plant extracts on fecal deposits, potential new treatments for intestinal tract disorders can be identified and a scientific rationale for their use as antidiarrheal agents can be provided. This approach can provide a valuable contribution to the field of drug discovery and ethnopharmacology.
Acknowledgements
We thank Dr. Martina Gáliková for providing us with the Drosophila strains. We are grateful to the Michelle Crozatier-Borde and Marc Haenlin team for giving feedback on our study and helping us improve our model. We would like to thank Napo Pharmaceuticals Company for providing us the drug Crofelemer. The authors are also thankful to the guest editor Dr. Hugues Petitjean for providing us with the opportunity to publish this protocol. This study was funded by the Agence Nationale de la Recherche (ANR) under the project ANR-22-CE03-0001-01.
Materials
| Name | Company | Catalog Number | Comments |
| Chemical & Food medium | |||
| Agar | Sigma Aldrich | A7002 | 5 Kg bucket |
| Bromophenol blue | Sigma Aldrich | 34725-61-6 | B5525-25G |
| Corn flour | Nature et Cie | *910007 | 25 Kg bag |
| Crofelemer | Napo pharmaceuticals | - | - |
| Ethanol 96% | - | - | - |
| Loperamide | Sigma Aldrich | L4762 | 5 grams |
| Moldex | VWR | 1.06757.5000 | 5 Kg bag |
| Propionic acid | Dutscher | 409553-CER | 1 Liter bottle |
| Sugar | Pomona EpiSaveurs | 52705 | 1 Kg bag |
| Yeast | Dutscher | 789195 | 10 Kg bag |
| Materials | |||
| Beaker | DWK LIFE SCIENCE | - | 250 mL |
| Centrifugation tube | Eppendorf | 30119401 | Eppendorf tubes 5.0 mL |
| CO2 tank | - | - | - |
| Erlen Meyer flask | - | - | 500 mL (for extraction) |
| Filter paper grade | Whatman | - | 3 mm chr. |
| Flowbuddy socle | Genesis | - | - |
| Flugs Narrow Plastic vials | Genesis | 49-102 | - |
| Flystuff Blow gun | Genesis | - | - |
| Flystuff Ultimate Flypad | Genesis | - | - |
| Flystuff Foot pedal | Genesis | - | - |
| Forceps | Dumostar | 11295-51 | - |
| Graduated cylinder | - | - | 100 mL |
| Inox spatula | - | - | - |
| Micropipette | Eppendorf | 4924000088 | Eppendorf Reference 2 |
| Micropipette tip | Eppendorf | 30000919 | epT.I.P.S. Standard |
| Narrow Drosophila vials | Genesis | 32-120 | - |
| Paintbrush | - | - | - |
| Petri dish | Greiner | 628162 | Size: 60 x 15mm |
| Round-bottom flask | - | - | 500 mL (for evaporation) |
| Thermometer | Avantor | 620-0916 | |
| Whisk | - | - | - |
| Equipments | |||
| Chiller | HUBER | Minichiller | - |
| Heating bath | BÜCHI | B-490 | - |
| Heating plate | BIOBLOCK SCIENTIFIC | - | Magnetic stirrer hot plate |
| Incubator | Memmert | - | HPP110eco |
| Rotary evaporator | BÜCHI | R-200 | - |
| Scanner | Epson | V850 pro | - |
| Shaker | Edmund Bühle | KS 10 | - |
| Stereomicroscope binocular | Zeiss | Stemi 305 | - |
| Vacuum pump | VACUUBRAND | PC500 series | - |
| Vortex mixer | Sigma Aldrich | CLS6776-1EA | Corning LSE vortex mixers |
| Weighing scale | OHAUS Scout | SKX622 | - |
References
- Cheng, L. K., et al. Gastrointestinal system. WIREs Sys Bio Med. 2 (1), 65-79 (2010).
- Greenwood-Van Meerveld, B., Johnson, A. C., Grundy, D. Gastrointestinal physiology and function. Handb Exp Pharmacol. 239, 1-16 (2017).
- Doyle, L. A., et al. A clinicopathologic study of 24 cases of systemic mastocytosis involving the gastrointestinal tract and assessment of mucosal mast cell density in irritable bowel syndrome and asymptomatic patients. Am J Surg Pathol. 38 (6), 832-843 (2014).
- Levine, G. A., Walson, J. L., Atlas, H. E., Lamberti, L. M., Pavlinac, P. B. Defining pediatric diarrhea in low-resource settings. J Pediatric Infect Dis Soc. 6 (3), 289-293 (2017).
- Abraham, B., Sellin, J. H. Drug-induced diarrhea. Curr Gastroenterol Rep. 9 (5), 365-372 (2007).
- Badry, A. H. H., Jameel, A. Y., Mero, W. M. S. Pathogenic microorganisms associated with arrhea in infants and children in Duhok Province, Kurdistan Region / Iraq. Sci J Uni Zakho. 2 (2), 266-275 (2014).
- Manetu, W. M., M'masi, S., Recha, C. W. Diarrhea disease among children under 5 years of age: A global systematic review. Open J Epidemiol. 11 (3), 207-221 (2021).
- Fu, J., et al. Aquatic animals promote antibiotic resistance gene dissemination in water via conjugation: Role of different regions within the zebra fish intestinal tract, and impact on fish intestinal microbiota. Mol Ecol. 26 (19), 5318-5333 (2017).
- Zhang, Q., Widmer, G., Tzipori, S. A pig model of the human gastrointestinal tract. Gut Microbes. 4 (3), 193-200 (2013).
- Cox, C. R., Gilmore, M. S. Native microbial colonization of Drosophila melanogaster and its use as a model of Enterococcus faecalis pathogenesis. Infect Immun. 75 (4), 1565-1576 (2007).
- Jasper, H. Exploring the physiology and pathology of aging in the intestine of Drosophila melanogaster. Invertebr Reprod Dev. 59, 51-58 (2015).
- Pandey, U. B., Nichols, C. D. Human disease models in Drosophila melanogaster and the role of the fly in therapeutic drug discovery. Pharmacol Rev. 63 (2), 411-436 (2011).
- Miguel-Aliaga, I., Jasper, H., Lemaitre, B. Anatomy and physiology of the digestive tract of Drosophila melanogaster. Genetics. 210 (2), 357-396 (2018).
- Jennings, B. H. Drosophila- a versatile model in biology & medicine. Materials Today. 14 (5), 190-195 (2011).
- Kumar, V. S., Navaratnam, V. Neem (Azadirachta indica): Prehistory to contemporary medicinal uses to humankind. Asian Pac J Trop Biomed. 3 (7), 505-514 (2013).
- Shrestha, P., Adhikari, S., Lamichhane, B., Shrestha, B. G. Phytochemical screening of the medicinal plants of Nepal. J Environ Sci Tech Food Tech. 1 (6), 11-17 (2015).
- Perveen, S., Al-Taweel, A. Pharmacognosy: Medicinal Plants. IntechOpen. , (2019).
- Noumi, E., Yomi, A. Medicinal plants used for intestinal diseases in Mbalmayo Region, Central Province, Cameroon. Fitoterapia. 72 (3), 246-254 (2001).
- Njoku, V. O., Obi, C., Onyema, O. M. Phytochemical constituents of some selected medicinal plants. African J Biotechnol. 10 (66), (2011).
- Rokaya, M. B., et al. Traditional uses of medicinal plants in gastrointestinal disorders in. Nepal. J Ethnopharmacol. 158, 221-229 (2014).
- Birdi, T., Krishnan, G. G., Kataria, S., Gholkar, M., Daswani, P. A randomized open label efficacy clinical trial of oral guava leaf decoction in patients with acute infectious diarrhoea). J Ayurveda Integr Med. 11 (2), 163-172 (2020).
- van Vuuren, S. F., Nkwanyana, M. N., de Wet, H. Antimicrobial evaluation of plants used for the treatment of diarrhoea in a rural community in northern Maputaland, KwaZulu-Natal, South Africa. BMC Complement Altern Med. 15, 53 (2015).
- Firenzuoli, F., Gori, L. Herbal medicine today: Clinical and research issues. Evid Based Complement Alternat Med. 4, 37-40 (2007).
- Rawat, P., Singh, P. K., Kumar, V. Evidence based traditional anti-diarrheal medicinal plants and their phytocompounds. Biomed Pharmacother. 96, 1453-1464 (2017).
- Palombo, E. A. Phytochemicals from traditional medicinal plants used in the treatment of diarrhoea: modes of action and effects on intestinal function. Phytother Res. 20 (9), 717-724 (2006).
- Koyama, T., et al. A nutrient-responsive hormonal circuit mediates an inter-tissue program regulating metabolic homeostasis in adult Drosophila. Nat Commun. 12 (1), 5178 (2021).
- Wayland, M. T., et al. Spotting the differences: Probing host/microbiota interactions with a dedicated software tool for the analysis of faecal outputs in Drosophila. J Insect Physiol. 69, 126-135 (2014).
- Cognigni, P., Bailey, A. P., Miguel-Aliaga, I. Enteric neurons and systemic signals couple nutritional and reproductive status with intestinal homeostasis. Cell Metab. 13 (1), 92-104 (2011).
- Gáliková, M., Dircksen, H., Nässel, D. R. The thirsty fly: Ion transport peptide (ITP) is a novel endocrine regulator of water homeostasis in Drosophila. PLoS Genet. 14 (8), 1007618 (2018).
- Chassagne, F., Quave, C. L. Collection, extraction, and in vitro antibacterial evaluation of plants used in traditional medicine. Methods Mol Biol. 2296, 19-41 (2021).
- Patel, T. S., Crutchley, R. D., Tucker, A. M., Cottreau, J., Garey, K. W. Crofelemer for the treatment of chronic diarrhea in patients living with HIV/AIDS. HIVAIDS. 5, 153-162 (2013).
- Cottreau, J., Tucker, A., Crutchley, R., Garey, K. W. Crofelemer for the treatment of secretory diarrhea. Expert Rev Gastroenterol Hepatol. 6 (1), 17-23 (2012).
- Wu, P. E., Juurlink, D. N. Loperamide cardiac toxicity: Pathophysiology, presentation, and management. Can J Cardiol. 38 (9), 1378-1383 (2022).
- Benguettat, O., et al. The DH31/CGRP enteroendocrine peptide triggers intestinal contractions favoring the elimination of opportunistic bacteria. PLoS Pathog. 14 (9), 1007279 (2018).
- Thakare, M. R., et al. Direct intake estimation and longitudinal tracking of solid-food consumption (DIETS) in Drosophila. bioRxiv. , 543033 (2023).
- Miller, J., et al. Drosophila melanogaster as an emerging translational model of human nephrolithiasis. J Urol. 190 (5), 1648-1656 (2013).
- Zierer, J., et al. The fecal metabolome as a functional readout of the gut microbiome. Nat Genet. 50 (6), 790-795 (2018).
This article has been published
Video Coming Soon
ABOUT JoVE
Copyright © 2024 MyJoVE Corporation. All rights reserved