Measuring Constipation in a Drosophila Model of Parkinson's Disease
In This Article
Summary
This protocol presents an assay for modeling constipation in an alpha-synuclein based Drosophila model of Parkinson's disease.
Abstract
Non-motor symptoms in Parkinson's disease (PD) are common, difficult to treat, and significantly impair quality of life. One prevalent non-motor symptom is constipation, which can precede the diagnosis of PD by years or even decades. Constipation has been underexplored in animal models of PD and lacks specific therapies. This assay utilizes a Drosophila model of PD in which human alpha-synuclein is expressed under a pan-neuronal driver. Flies expressing alpha-synuclein develop the hallmark features of PD: the loss of dopaminergic neurons, motor impairment, and alpha-synuclein inclusions.
This protocol outlines a method for studying constipation in these flies. Flies are placed on fly food with a blue color additive overnight and then transferred to standard food the following day. They are subsequently moved to new vials with standard fly food every hour for 8 h. Before each transfer, the percentage of blue-colored fecal spots compared to the total fecal spots on the vial wall is calculated. Control flies that lack alpha-synuclein expel all the blue dye hours before flies expressing alpha-synuclein. Additionally, the passage of blue-colored food from the gut can be monitored with simple photography. The simplicity of this assay enables its use in forward genetic or chemical screens to identify modifiers of constipation in Drosophila.
Introduction
Parkinson's disease (PD) is a progressive neurodegenerative disorder characterized clinically by the presence of motor symptoms such as bradykinesia, rigidity, and tremor, resulting in significant morbidity1. Pathologically, PD is defined by the loss of dopaminergic neurons in the substantia nigra and the misfolding of alpha-synuclein, leading to the formation of Lewy bodies and Lewy neurites. The pathogenesis of PD remains poorly understood, likely arising from a complex interplay of genetic and environmental factors2,3. Currently, disease-modifying therapies are unavailable, possibly due in part to the advanced stage of pathology present at the time of diagnosis. Studies have shown that more than 60% of dopaminergic neurons in the substantia nigra have already been lost by the onset of motor symptoms, underscoring the need to explore potential biomarkers for early disease detection4. One such clinical biomarker is constipation, which is common in PD patients5,6 and may precede motor symptom onset by years or even decades.
Despite PD's clinical definition based on motor symptoms, constipation is one of several non-motor symptoms that are more challenging to treat symptomatically and cause significant impairment in patients' quality of life7. Alterations in the gut-brain axis, which represents bidirectional communication between the brain and the enteric nervous system, have been implicated in PD pathogenesis. Alpha-synuclein aggregates have been found in tissue samples from the gastrointestinal tracts of PD patients8, and animal models suggest that alpha-synuclein aggregates in the enteric nervous system spread in a prion-like manner to the central nervous system9. Additionally, PD patients exhibit abnormalities in the gut microbiome10 and may experience excessive gut inflammation11. Constipation in PD has been understudied, with few reported models of Parkinson's-associated constipation in flies12,13or rodents14,15.
This assay employs a Drosophila model of PD in which flies express the human alpha-synuclein gene under the control of a pan-neuronal driver, n-synaptobrevin. These flies exhibit all the hallmark features of PD, including alpha-synuclein aggregation, motor dysfunction, and age-related neurodegeneration, resulting in the loss of dopaminergic neurons16,17. Previous studies have introduced the measurement of fecal output in flies to assess gut dysfunction, quantifying fly fecal matter and comparing excreta amounts across various genetic lines to reveal functional differences in the digestive system18,19,20. Here, we demonstrate the constipation assay using flies expressing human alpha-synuclein. This simple yet valuable tool enables the study of an important non-motor symptom of PD.
Protocol
Flies used in this assay: control: nSyb-QF2, nSyb-Gal4/+; alpha-synuclein flies: nSyb-QF2, nSyb-Gal4, QUAS-alpha-synuclein/+; 1- and 10-days post eclosion; male and female (mated and unmated) flies (see Table of Materials).
1. Preparing the dyed fly food
- Mix blue soft gel paste food coloring (see Table of Materials) with distilled water in a 1:1 ratio (v/v).
NOTE: Use commercial food coloring that contains only non-absorbable dyes, as some blue dyes have been shown to have neuroprotective effects21. - Microwave the vials of fly food consisting of standard cornmeal-agar (see Table of Materials) in 10-15 s intervals until the food has melted into a liquid or slurry. Do not allow food to boil over.
- Add the dye mixture to each vial of food to obtain a uniform coloring between vials. Mix in the dye mixture until the food is homogenously blue (Figure 1). Add an amount of the blue dye mixture such that the color of the food is saturated, and there is no variation in color between the vials.
NOTE: This step must be done soon after microwaving, as the food solidifies quickly. If the food solidifies, microwave the vial again. - Leave the vials of dyed food to air dry until it solidifies. Place a thin paper towel over vial openings while drying to prevent any stray flies from landing in the vial.
- Once the food has cooled and solidified, transfer the flies that will be used for testing to the blue food.
NOTE: For narrow vials (25 mm), it is recommended that 9-14 flies are added to each vial. For wide vials (28.5 mm), it is recommended that 10-20 flies are added to each vial. Flies are not separated by sex or mating experience and include a mixed population of male and female flies, with both mated and unmated females. - Incubate the vials with flies overnight at 25 °C in an incubator.
2. Imaging the flies
NOTE: This step is optional (Figure 2).
- The following morning (time 0), anesthetize the representative flies with carbon dioxide for 60 s. Place the fly so that the ventral side is facing upwards.
- Using a camera (see Table of Materials), capture images of the flies at each hour to visualize the amount of blue food left in the digestive system.
NOTE: These flies should not be used for the quantitative part of the assay (step 3), as anesthesia may affect the results. - At every hour, anesthetize and image new flies.
3. Performing the constipation assay
- Transfer the remaining (non-anesthetized) flies to vials with standard Drosophila food. Number each vial.
- Leave the flies in the incubator at 25 °C for 60 min.
- After 60 min, transfer the flies to new vials with standard Drosophila food. Begin data recording from the first set of vials.
- Draw a dotted line down the length of the vial to mark the point from which counting will start.
- Manually count the number of small, round dots on the wall of each vial. Do not count dots found on the food or vial plug.
- Begin by counting the number of blue dots on the wall of each vial.
- Count the number of opaque, colorless dots on the wall of each vial.
NOTE: Each dot is fly excrement, which is a combination of urine and fecal matter22. On occasion, a fly may walk over the excrement and leave a trail, in which case, count only the original dot, not each individual mark in the trail. - Record the ratio of blue dots to the total number of dots for each vial.
NOTE: The total number of dots is the number of blue dots on the wall of a vial added to the number of colorless dots on the wall of the same vial. This will be used to calculate the percent of blue fecal matter per hour. - Repeat steps 3.2-3.8 seven more times, collecting data every hour across a total of 8 h.
4. Data analysis
- Graph the percent blue fecal matter per hour for each condition.
- Compare the data between conditions.
NOTE: Statistical significance can be determined in 3 ways: by comparing individual timepoints between conditions, by measuring the slope of the line over time, or by comparing the area under the curve.
Representative Results
Because the Drosophila abdomen is transparent, blue food can be visualized inside the gut in living anesthetized flies. Qualitative differences in gut transit can be assessed by taking images of the flies at various time points. In control flies, the blue food is quickly expelled, whereas in alpha-synuclein flies, the blue food remains present in the gut for as long as 8 hours (Figure 2).
The alpha-synuclein flies display age-related neurodegeneration, with a robust phenotype developing by 10 days post-eclosion10. At this timepoint, differences in gut transit time when compared to control flies are seen by comparing individual timepoints between conditions, by measuring the slope of the line over time, or by comparing the area under the curve (Figure 3). No differences are observed in gut transit between control and alpha-synuclein flies at an earlier timepoint, day 1 post-eclosion, when motor dysfunction and neurodegeneration are also not yet present (Figure 4).
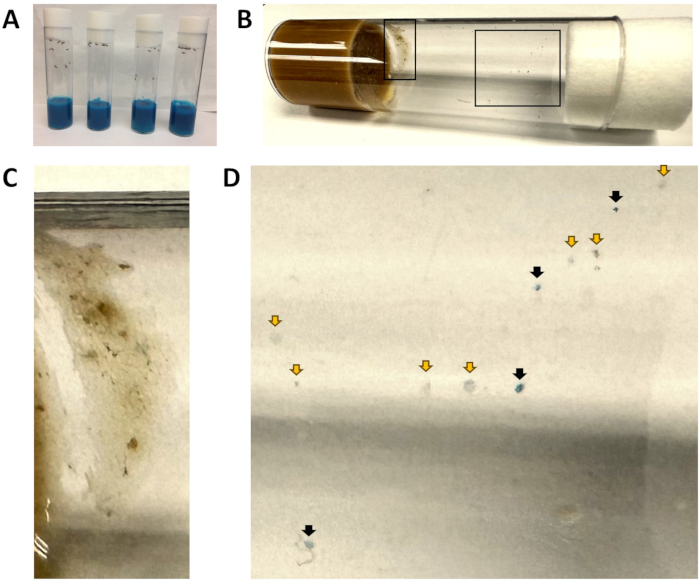
Figure 1: Blue food vials and fecal spots. (A) All vials contain blue-colored food. At this point in time, flies have just been introduced to the blue food, and no blue fecal matter is visible. (B) A vial after w1118 flies have been transferred to fresh food for 1 h, then removed. The left inset is enlarged in (C), and the right inset is enlarged in (D). (C) An area excluded from the analysis due to the presence of fly food. (D) Enlarged view of fecal spots, indicating blue (black arrows) and non-blue spots (yellow arrows). Please click here to view a larger version of this figure.
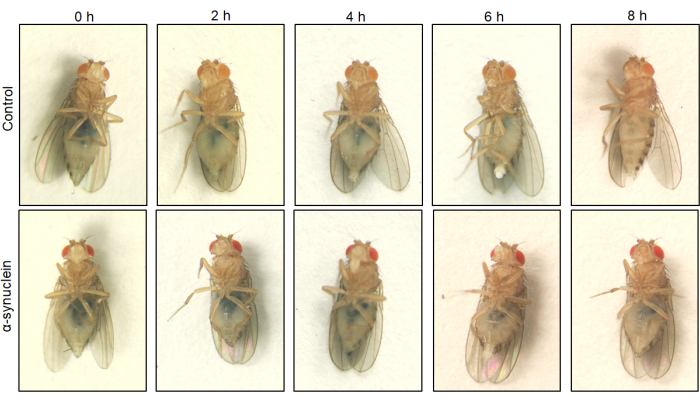
Figure 2: Visualization of gut transit of the dyed food over time. Control flies and flies expressing human alpha-synuclein in neurons were left on blue-dyed food overnight and then transferred to standard media the next day. Flies were serially transferred to new standard fly food every hour. Representative images at time zero and 2, 4, 6, and 8 h after transfer are shown. Control and alpha-synuclein flies show similar patches of blue food in the gut at time zero. At subsequent timepoints, control flies have less blue food than alpha-synuclein flies, and all blue food is gone at the 8 h timepoint in control flies. The genotypes for each fly line are as follows: control (nSyb-QF2, nSyb-Gal4/+); alpha-synuclein flies (nSyb-QF2, nSyb-Gal4, QUAS-alpha-synuclein/+). Female flies are used for imaging due to their easier ability to visualize the abdomen because of their larger size. Please click here to view a larger version of this figure.
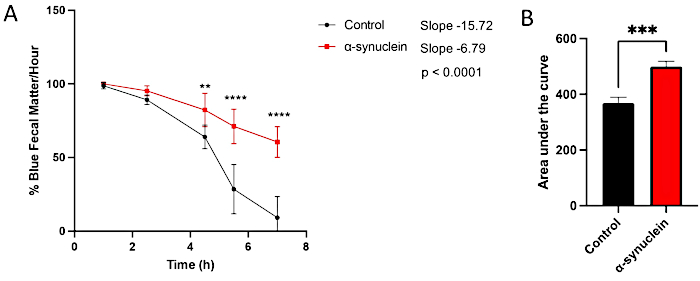
Figure 3: Constipation assay in day 10 post-eclosion flies. Control flies and flies expressing human alpha-synuclein in neurons were left on blue-dyed food overnight and then transferred to standard fly food the next day. Flies were serially transferred to new standard media every hour. (A) Percent blue fecal matter per hour. Error bars represent standard deviation. N = 6 vials per genotype; each vial contained 9-14 flies. All statistical analyses were performed in Graphpad Prism (see Table of Materials). Statistical significance at each timepoint was calculated using two-way ANOVA. The slope of the line and the difference between slopes were calculated using the linear regression analysis. (B) The area under the curve for each genotype was calculated. Error bars represent standard deviation. The genotypes for each fly line are as follows: control (nSyb-QF2, nSyb-Gal4/+); alpha-synuclein flies (nSyb-QF2, nSyb-Gal4, QUAS-alpha-synuclein/+). Both male and female flies are included in roughly equal numbers. Please click here to view a larger version of this figure.
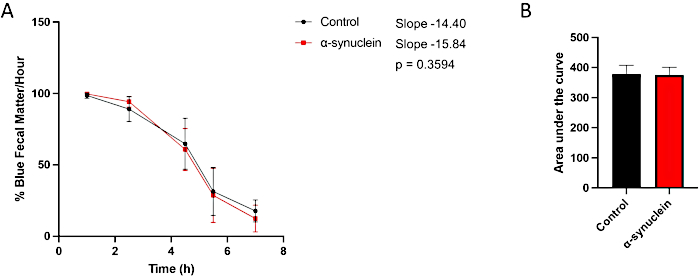
Figure 4: Constipation assay in day 1 post-eclosion flies. Control flies and flies expressing human alpha-synuclein in neurons were left on blue-dyed food overnight and then transferred to standard media the next day. Flies were serially transferred to new standard fly food every hour. (A) Percent blue fecal matter per hour. Error bars represent standard deviation. N = minimum 5 vials per genotype; each vial contained 9-14 flies. All statistical analyses were performed in Graphpad Prism. Statistical significance at each timepoint was calculated using two-way ANOVA. The slope of the line and the difference between slopes were calculated using the linear regression analysis. (B) The area under the curve for each genotype was calculated. Error bars represent standard deviation. The genotypes for each fly line are as follows: control (nSyb-QF2, nSyb-Gal4/+); alpha-synuclein flies (nSyb-QF2, nSyb-Gal4, QUAS-alpha-synuclein/+). Both male and female flies are included in roughly equal numbers. Please click here to view a larger version of this figure.
Discussion
There are several steps in this protocol that will aid in the successful completion of the assay. Firstly, it is important to ensure that the time intervals between each round for each vial are consistent throughout the experiment. Labeling the vials with numbers will help to avoid the need for lengthy genotype descriptions, saving time. Additionally, it is crucial that the method for counting fecal matter22 remains consistent throughout the experiment. While blue fecal matter is visible on the food and vial plug, colorless fecal matter is not. Therefore, do not count the blue dots on the food or vial plug.
With behavioral assays, there is always a possibility of inconsistencies in results due to fluctuations in fly behavior or unknown variables affecting the assay. We recommend using the same Drosophila media, the same food dye, and the same brand of vials for all experiments. Interestingly, in several trials, it was observed that flies tend to defecate less frequently in the early afternoon, possibly due to the flies' circadian rhythm23. However, this behavior is consistent in both control flies and flies expressing alpha-synuclein, so it should not raise concerns.
If the flies do not excrete blue fecal matter at the beginning of the assay, it is possible that the dye used is not pigmented enough. In this case, one can increase the ratio of dye to distilled water accordingly. It is also possible that when there is only a small amount of blue food left in the fly's digestive tract, it may be difficult to determine whether the fecal matter is pale blue or colorless. When this occurs, placing a white sheet of paper behind the vial will help determine the color of the fecal dot. Even if the fecal matter is very light blue, it is best to record it as blue fecal matter rather than colorless fecal matter.
One limitation of this assay is that it requires manual counting of fecal spots. To improve the potential for high-throughput screening, this protocol may be modified in the future to allow for automated quantification of blue fecal spots produced by individual flies in multi-well plates. Another limitation is that, although the alpha-synuclein model has the potential to be developed into a prodromal model of PD, an optimal timepoint at which constipation is present without neurodegeneration has not yet been identified.
In summary, this method offers a simple, straightforward approach to modeling constipation, an understudied non-motor PD symptom, in a Drosophila model of PD.
Acknowledgements
We acknowledge Dr. Mel Feany at Brigham and Women's Hospital and Harvard Medical School for the kind gift of the control and alpha-synuclein expressing Drosophila lines. We acknowledge the following sources of grant support to Dr. Olsen: NINDS K08, American Parkinson Disease Association George C. Cotzias Fellowship, Department of Defense Parkinson's Disease Early Investigator Award.
Materials
| Name | Company | Catalog Number | Comments |
| 1400 g sucrose | MP Biomedicals | 904713 | |
| 1800 g dextrose | MP Biomedicals | 901521 | |
| 2884 g yeast | MP Biomedicals | 903312 | |
| 428 g agar | Fisher Scientific | 10253156 | |
| 4600 mL molasses | Grandma's Molasses | 7971942 | |
| 68 L water | N/A | N/A | |
| 680 mL tegosept mix (1200 g tegosept in 6 L ethanol) | |||
| 6864 g cornmeal | Pearl Milling | 125045 | |
| 800 mL acid mix (83 mL phosphoric acid in 1 L water + 836 mL propionic acid in 1 L water) | |||
| cellSens Standard software | Olympus | N/A | |
| Ethanol | Pharmco-Aper | 111ACS200 | |
| Flugs for wide plastic vials | Genesee Scientific | 49-101 | |
| Flystuff wide Drosophila vials, polystyrene | Genesee Scientific | 32-117 | |
| Graphpad Prism | GraphPad | N/A | Version 9.5.1 |
| Olympus DP23 camera | Olympus | N/A | |
| Olympus SZX12 Stereo Microscope | Olympus | N/A | |
| Phosphoric Acid | Fisher Scientific | S25470A | |
| Propionic Acid | Fisher Scientific | A258 - 500 | |
| Soft gel paste food color, Royal blue | AmeriColor | 202 | |
| Tegosept | Apex | 20-258 | |
| Drosophila Stocks | |||
| nSyb-QF2, nSyb-Gal4 | All lines provided by Dr. Mel Feany | N/A | Lines are available directly from Dr. Feany |
| nSyb-QF2, nSyb-Gal4, QUAS-alpha synuclein | N/A | ||
| w1118 | N/A |
References
- Kalia, L. V., Lang, A. E. Parkinson's disease. Lancet. 386 (9996), 896-912 (2015).
- Bloem, B. R., Okun, M. S., Klein, C. Parkinson's disease. Lancet. 397 (10291), 2284-2303 (2021).
- Ball, N., Teo, W. P., Chandra, S., Chapman, J. Parkinson's disease and the environment. Front Neurol. 10, 218 (2019).
- Zhou, J., Li, J., Papaneri, A. B., Kobzar, N. P., Cui, G. Dopamine neuron challenge test for early detection of Parkinson's disease. NPJ Parkinsons Dis. 7 (1), 116 (2021).
- Ueki, A., Otsuka, M. Life style risks of Parkinson's disease: association between decreased water intake and constipation. J Neurol. 251 (Suppl 7), vII18-vII23 (2004).
- Houser, M. C., Tansey, M. G. The gut-brain axis: is intestinal inflammation a silent driver of Parkinson's disease pathogenesis. NPJ Parkinsons Dis. 3, 3 (2017).
- Pfeiffer, R. F. Non-motor symptoms in Parkinson's disease. Parkinsonism Relat Disord. 22 (Suppl 1), (2016).
- Klann, E. M., et al. The Gut-brain axis and its relation to parkinson's disease: A review. Front Aging Neurosci. 13, 782082 (2021).
- Mukherjee, A., Biswas, A., Das, S. K. Gut dysfunction in Parkinson's disease. World J Gastroenterol. 22 (25), 5742-5752 (2016).
- Yemula, N., Dietrich, C., Dostal, V., Hornberger, M. Parkinson's disease and the gut: symptoms, nutrition, and microbiota. J Parkinsons Dis. 11 (4), 1491-1505 (2021).
- Chen, S. J., Lin, C. H. Gut microenvironmental changes as a potential trigger in Parkinson's disease through the gut-brain axis. J Biomed Sci. 29 (1), 54 (2022).
- Hawrysh, P. J., et al. PRKN/parkin-mediated mitophagy is induced by the probiotics Saccharomyces boulardii and Lactococcus lactis. Autophagy. 19 (7), 2094-2110 (2023).
- Liu, W., Lim, K. L., Tan, E. K. Intestine-derived α-synuclein initiates and aggravates pathogenesis of Parkinson's disease in Drosophila. Transl Neurodegener. 11 (1), 44 (2022).
- Rota, L., et al. Constipation, deficit in colon contractions and alpha-synuclein inclusions within the colon precede motor abnormalities and neurodegeneration in the central nervous system in a mouse model of alpha-synucleinopathy. Transl Neurodegener. 8, 5 (2019).
- Diwakarla, S., et al. ATH434 reverses colorectal dysfunction in the A53T mouse model of Parkinson's disease. J Parkinsons Dis. 11 (4), 1821-1832 (2021).
- Ordonez, D. G., Lee, M. K., Feany, M. B. α-synuclein Induces mitochondrial dysfunction through spectrin and the actin cytoskeleton. Neuron. 97 (1), 108.e6-124.e6 (2018).
- Olsen, A. L., Feany, M. B. Glial α-synuclein promotes neurodegeneration characterized by a distinct transcriptional program in vivo. Glia. 67 (10), 1933-1957 (2019).
- Cognigni, P., Bailey, A. P., Miguel-Aliaga, I. Enteric neurons and systemic signals couple nutritional and reproductive status with intestinal homeostasis. Cell Metab. 13 (1), 92-104 (2011).
- Urquhart-Cronish, M., Sokolowski, M. B. Gene-environment interplay in Drosophila melanogaster: chronic nutritional deprivation in larval life affects adult fecal output. J Insect Physiol. 69, 95-100 (2014).
- Popovic, R., et al. Blocking dPerk in the intestine suppresses neurodegeneration in a Drosophila model of Parkinson's disease. Cell Death Dis. 14 (3), 206 (2023).
- Poteet, E., et al. Neuroprotective actions of methylene blue and its derivatives. PLoS One. 7 (10), e48279 (2012).
- Miguel-Aliaga, I., Jasper, H., Lemaitre, B. Anatomy and physiology of the digestive tract of Drosophila melanogaster. Genetics. 210 (2), 357-396 (2018).
- Schlichting, M., et al. A neural network underlying circadian entrainment and photoperiodic adjustment of sleep and activity in Drosophila. J Neurosci. 36 (35), 9084-9096 (2016).
This article has been published
Video Coming Soon
ABOUT JoVE
Copyright © 2024 MyJoVE Corporation. All rights reserved