Donor Posterior Atrial Flap Rotation for Left Atrial Cuff Reconstruction in Lung Transplantation
In This Article
Summary
This protocol describes a new method for effectively reconstructing donor atrial cuff anterior wall defects during lung transplantation through the rotation of the donor posterior atrial flap.
Abstract
We describe in detail a novel surgical technique for repairing donor anterior left atrial wall defect in vivo by rotating the posterior atrial flap during lung transplantation. This method can safely and effectively address the most common type of donor left atrial cuff defect: the anterior wall defect, with the intact posterior wall, typically retained when the donor heart is also used. During atrial cuff anastomosis in lung transplantation, the excess donor posterior atrial wall is trimmed into an atrial flap. After the posterior wall anastomosis is completed, the atrial flap is rotated 180° and used as a patch for anterior wall reconstruction anastomosis.
After restoring blood flow, the flow rate of the pulmonary vein is normal and smooth as confirmed by transesophageal echocardiography. Compared with the traditional patch reconstruction method, the new method effectively reduces the atrial cuff anastomosis time. Our results showed no significant difference in pulmonary vein obstruction after reconstruction. Pulmonary artery systolic blood pressure was significantly lower and pulmonary function improved postoperatively in all groups, with no significant differences among the groups. The new technique provides a feasible strategy for the reconstruction of left atrial cuff defects and can improve the effective utilization rate of donor lungs.
Introduction
Lung transplantation, the only effective treatment for most end-stage lung diseases, is limited by a shortage of donor organs. Therefore, it is necessary to optimize the transplant procedure to effectively utilize the potential donor organs1. The surgical technique for donor lung extraction is an important aspect of this procedure. Currently, it is essential to preserve the intact left atrial cuff, which is routinely used for anastomosis in preference to the pulmonary veins2,3. Left atrial cuff defect is the most common donor lung injury, occurring in 2.7% of cases upon donor heart and lung separation. Retention of the posterior left atrial wall in heart transplant recipients means that the donor posterior atrial wall tissue is typically fully retained by the donor lung (Figure 1A), making defects of the left atrial cuff anterior wall the most common type of atrial cuff injury (Figure 1B), accounting for approximately 71% of cases4. Due to the interatrial groove, anterior atrial wall defect more commonly occurs on the right donor lung5,6.
This paper introduces a new method of atrial cuff reconstruction that can safely and effectively solve the problem of left atrial cuff defect caused during donor lung acquisition, thereby improving the utilization rate of donor lungs. The key aspect of this new method is the retention of the intact posterior atrial wall of the donor lung. By pruning the excess posterior atrial wall, it forms an atrial flap, and the rotation of the atrial flap provides a patch for the repair of the defective anterior wall.
In cases of atrial cuff defects, extra time is required to shape and reconstruct the atrial cuff to smooth the venous outflow channel and prevent stenosis5. The traditional type of patch reconstruction is performed in vitro before the donor lung is placed in the chest, increasing the cold ischemia time. Cuff reconstruction requires the availability of patch material; excess donor pericardial tissue is the most commonly used, although excess arterial tissue can also be utilized4,5,6. Regardless of the type of patch material used, cuff reconstruction is associated with an increased operation time and an increased risk of surgical complications such as venous outflow tract obstruction caused by non-homologous vascular anastomosis.
The new method can repair the anterior wall defect in vivo by using the excess left atrial tissue of the donor. The operation is more convenient, and the anastomosis time is effectively reduced to reduce the cold ischemia time. This method, which we have named posterior atrial flap rotating left atrial cuff reconstruction, offers a novel and feasible strategy for dealing with anterior atrial wall defect which is the most common type of donor lung atrial cuff defects in lung transplantation.
Protocol
This study was approved by the hospital's ethics review board (approval number ChiCTR2400085507), and written informed consent was obtained from all subjects.
1. Preoperative preparation
- Confirm the matching information of the donor and recipient.
NOTE: In our case, confirmation was done by checking the files from the National Organ Transplant Response System. - Remove hair from the surgical area and ensure that they have fasted for more than 12 h.
- Transfer the recipient from the ward to the operating room to await surgery.
- Ensure that all instruments and materials required for the protocol are available (see Table of Materials).
- Confirm that donor lungs are available and suitable for use.
NOTE: All indexes of donor lung, including airway, texture, and laboratory indexes, were up to standard, except for the defect of the anterior atrial sleeve that needed to be repaired. - Initiate anesthesia and extracorporeal membrane oxygenation (ECMO) procedures.
2. Anesthesia and ECMO
- Establish vascular access.
- Administer intravenous anesthesia. Induce anesthesia by administering 0.05-0.1 mg/kg of Midazolam Injection, 0.1-0.3 mg/kg of Etomidate Injectable Emulsion, 0.8 mg/kg of Rocuronium Bromide Injection, and 0.5-1 µg/kg Remifentanil Hydrochloride Injection intravenously. Maintain anesthesia by administering 5-10 mg∙kg-1∙h-1 of Diprivan Injection and 0.5-2 µg∙kg-1∙h-1 of Remifentanil Hydrochloride Injection intravenously.
- Insert double-cavity tracheal intubation and adjust the positions of the left and right openings of the tracheal tube.
- Insert a SwanGanz catheter through the internal jugular vein.
- Insert ECMO via the femoral vein and the internal jugular vein.
NOTE: Depending on the patient's pulmonary artery pressure and heart condition, the surgeon may choose other ECMO modes.
3. Removal of the recipient's diseased lung
- Position the patient horizontally, with the back slightly elevated and hands spread horizontally in a "cross" position and securely fixed.
- Disinfect the skin of the surgical area. Use iodophor disinfectant 3x from the inside out. Sterilize up to the neck, down to the umbilicus, and laterally to the posterior axillary line.
- Ensure that the contralateral lung is ventilated independently, and make a 15-20 cm incision from the fourth intercostal space of the anterior chest wall into the chest without transecting the sternum.
NOTE: For a double-lung transplant, based on the preoperative assessment, the lung with the more severe condition should be transplanted first. If the severity is similar on both sides, the right lung should be transplanted first. - Expose the incision area. Probe the diseased lung and release thoracic adhesions.
- Release the lower lung ligaments, and free the pulmonary artery and pulmonary vein.
- Block the proximal end of the right pulmonary artery using pulmonary artery blocking forceps. Sever the upper and lower pulmonary veins using a vascular cutting and closure device. Clamp the distal end of the pulmonary artery and sever the artery.
- Cut the right main bronchus and surrounding connective tissue at the level of the second branch.
- Remove the diseased lung and fully control bleeding within the chest.
4. Treatment of recipient's vascular and trachea residuals
- Cut 1-2 cartilage rings to expose the right main bronchus and trim them flat to ensure complete hemostasis.
- Prune the recipient's pulmonary artery stump at a distance of 8-10 mm from the blocking clamp.
- Free the upper and lower pulmonary veins within the pericardium. Ensure that the left atrium at the junction of the upper and lower pulmonary veins can be clamped with atrial forceps.
5. Separation and trimming of donor lung
- Divide the posterior atrial wall, ensuring that the excess posterior atrial wall is included on the side with the anterior wall defect.
NOTE: Owing to the position of the interatrial groove, anterior wall defects typically occur on the right side. The excess posterior atrial wall obtained from the right side can provide a source for repairing the short anterior wall (Figure 1B). - Sever the left and right pulmonary arteries at the junction of the left and right pulmonary arteries.
- Free the left main bronchus and sever the bronchus. Separate the left and right donor lungs.
- Place the left donor lung in an organ preservation solution at 4 °C. Trim the right donor lung. Ensure that the main bronchus has 2-3 cartilage rings. Free the pulmonary artery and atrial cuff fully.
6. Anastomosis of the primary bronchus and pulmonary artery
- Place the repaired right donor lung into the right chest.
- Suture the bronchial membrane with a 4-0 PDS line and the bronchial cartilage with a 4-0 poly line. Use a bronchoscope to confirm that the airway is clear and open after bronchial anastomosis is completed.
- Suture the soft tissue around the bronchial anastomosis with 4-0 poly line and ensure the airway anastomosis is covered.
- Anastomose the pulmonary artery with a 5-0 poly line continuous suture.
7. Reconstruction and anastomosis of the left atrial cuff
- Clamp the recipient's left atrium at the proximal end of the intersection of the upper and lower pulmonary veins, ensuring that the junction has 1-2 cm of space for anastomosis.
- Cut the excess posterior wall of the donor atrial cuff from the bottom upward by comparing the distance between the posterior atrial edge of the donor and recipient. Do not let excess posterior wall break off completely. Trim the excess posterior wall to form an atrial flap, connected to the upper edge of the donor atrial cuff by the pedicle (Figure 2A and Figure 3A).
- Anastomose the posterior edges of the atrial cuffs from the bottom to the top using a continuous 4-0 Prolene suture (Figure 2B and Figure 3B). Continue the suture (with 4-0 Prolene) upon reaching the posterior atrial flap to anastomose the edge of the atrial flap nearest the lung with the anterior atrial edge of the recipient. Ensure that the suture ends meet at the lower edge of the anastomosis and tie them off (Figure 2C and Figure 3C).
- Anastomose the other edge of the atrial flap and the anterior atrial edge of the donor from top to bottom with a 4-0 Prolene line continuous suture, and leave the last two needles untightened to serve as exhaust holes (Figure 2D and Figure 3D).
8. Blood flow opening
- Ventilate and dilate the transplanted lung to fully open the alveoli. Temporarily release the pulmonary artery-blocking forceps, allowing the blood to flow out from the exhaust hole of the atrial anastomosis. Clamp the pulmonary artery with blocking forceps, loosen and remove the atrial forceps, and then tighten the Prolene suture of the atrial sleeve anastomosis after fully exhausting the air.
- Release and remove the pulmonary artery-blocking forceps to allow full blood flow. Verify that the anterior wall of the anastomosis is wide and well-filled, with no bleeding manifestations.
9. Chest closure
- Recheck all anastomoses and the chest cavity for bleeding and exudation. Confirm sufficient hemostasis in the chest.
- Flush the chest cavity, place the drainage tube, ventilate the transplanted lung, and close the chest layer by layer using the chest closure sutures.
- Excise the contralateral diseased lung and anastomose the donor lung.
NOTE: The process of excision and anastomosis of the contralateral lung is the same as that for the right, and contralateral left atrial anastomosis is routine due to the intact donor atrial cuff. - After bilateral chest closure, monitor and record the flow rate of bilateral pulmonary veins by esophageal ultrasound.
10. Data logging and leaving the OR
- Adopt a protective lung ventilation strategy to make the transplanted lung fully ventilated, for example, with positive end-expiratory pressure (PEEP) levels of 5-7 cmH2O and tidal values (VT) of 5-7 mL/kg, while airway pressure peaks < 30 cmH2O were monitored.
- Record the pulmonary artery pressure.
- Insert the esophageal ultrasound probe through the mouth. Monitor and record the flow rate of the bilateral pulmonary veins.
- Switch to single-cavity tracheal intubation and transfer the patient to ICU for further treatment.
11. Follow-up
- Measure cardiac and pulmonary artery blood pressures by external cardiac ultrasound 30 days after transplantation, and analyze arterial blood gas routinely. Review lung function and other routine biochemical test results.
12. Statistical analysis
- Perform statistical analyses and express data as mean ± standard deviation.
- Compare groups using analysis of variance, the Kruskal-Wallis test, the Mann-Whitney U test, or the Wilcoxon matched-pairs test. Set statistical significance at P < 0.05.
Results
Patients were selected by reviewing the records of 931 lung transplants performed at the Lung Transplantation Center of the Second Affiliated Hospital of Zhejiang University School of Medicine or the Lung Transplantation Center of Wuxi People's Hospital from 2021 to 2023. Both centers had the same surgery director leading the operating team, which comprised senior doctors and nurses with extensive experience. All donor organs were allocated fairly from the COPO system according to the principles of organ allocation and scoring.
Primary diseases of the recipients included idiopathic pulmonary fibrosis, connective tissue disease-associated interstitial lung disease, bronchiolitis obliterans syndrome, and chronic obstructive pulmonary disease. A total of 71 patients required donor lung atrial cuff reconstruction, of whom 13 received a single-lung transplant and 58 received a double-lung transplant, reflecting an incidence rate of 6.2% for atrial cuff reconstruction in double-lung transplantation. Among the 58 double-lung transplant recipients included in this study, 23 underwent posterior wall anterior rotation atrial cuff reconstruction (the preferred technique, group 1: posterior atrial flap group) and 35 underwent traditional patch reconstruction. In cases of patch reconstruction; the surgeon determined the most suitable patch material according to the condition of the donor lung. The donor aorta was used in 20 patients (group 2: aortic patch group) and the donor pericardium was used in the remaining 15 patients (group 3: pericardial patch group) Baseline characteristics of the donors and recipients are summarized in Table 1; no significant differences were observed among the groups.
Case of posterior atrial flap rotating left atrial cuff reconstruction
In October 2023, a 17-year-old male (height, 165 cm; weight, 50 kg) was admitted to the hospital because of a cough with sputum persisting for 6 months and aggravated by asthma for 3 months. Six years prior to admission, the patient developed a cough with large amounts of yellow pus sputum, which was diagnosed as bronchiectasis with infection; however, anti-infection treatment was not effective. Genetic testing revealed pulmonary cystic fibrosis. Three months prior to admission, the patient caught a cold and developed asthma, requiring continuous oxygen therapy. After treatment and discharge, but with continuous oxygen therapy at home, the activity endurance of the patient decreased significantly, and a comprehensive assessment identified indications for lung transplantation; the patient was therefore added to the waiting list. After 46 days on the waiting list, a suitable donor was identified.
The donor was a 20-year-old male (height, 170 cm; weight, 60 kg) admitted to the hospital in November 2023 with a traumatic brain injury due to a car accident. The ratio of partial pressure of oxygen in arterial blood to the fraction of inspiratory oxygen concentration was 450 and chest imaging findings were normal. The patient was pronounced brain dead on the fifth day after admission. Family members agreed to organ donation in accordance with the patient's wishes and the lungs were assessed for suitability. After cardiopulmonary intubation perfusion, the entire cardiopulmonary system was isolated, and the heart and lungs were separated in vitro. During the separation of the left atrium, the allowance of sufficient anterior wall tissue to the donor heart resulted in a defect in the atrial cuff anterior wall in the right donor lung (Figure 1B).
The double-lung transplant procedure was performed on November 23, 2023. Single-lung ventilation of the recipient resulted in stable oxygen circulation, and lung transplantation was therefore performed without extracorporeal membrane oxygenation support. During surgery, the posterior wall of the right pulmonary atrial cuff was reconstructed using the posterior atrial flap rotating left cuff reconstruction technique. The left pulmonary vein atrial cuff anastomosis was normal. The anastomosis time for the right and left pulmonary atrial cuffs was 24 min and 16 min, respectively. After all the anastomoses were completed, blood flow was restored and normal double-lung ventilation was performed. Transesophageal ultrasonography indicated a normal bilateral atrial cuff anastomotic flow rate (<140 cm/s) (Figure 4). No primary graft dysfunction (PGD) occurred and the recipient was discharged from the hospital 20 days after the lung transplantation. To date, the recipient has recovered well, with a significantly improved quality of life following the surgery.
Comparison of atrial cuff anastomosis time for 56 patients
Among the 56 patients who underwent right atrial cuff reconstruction, 23 underwent posterior atrial flap rotating left atrial cuff reconstruction and 33 underwent patch reconstruction, 20 with an aortic patch and 13 with a pericardial patch. The right atrial cuff anastomosis time was 20.5 ± 1.99 min, 22.8 ± 5.2 min, and 23.3 ± 5.3 min in the posterior atrial flap, aortic patch, and pericardial patch groups, respectively. Analysis with the Kruskal-Wallis test revealed that the anastomosis time of the posterior atrial flap was significantly shorter than that of the aortic and pericardial patches (P < 0.01); however, there was no significant difference between aortic and pericardial patching.
Among the 54 patients with right-side reconstruction and no left-side reconstruction, 21 underwent posterior atrial flap rotating left atrial cuff reconstruction, and 33 underwent aortic patch reconstruction-20 with an aortic patch and 13 with a pericardial patch. The unrepaired left atrial cuff anastomosis time was 17.5 ± 2.8 min, 17.7 ± 3.8 min, and 17.6 ± 4.3 min in the posterior atrial flap, aortic patch, and pericardial patch groups, respectively. The Kruskal-Wallis test revealed no statistically significant differences among the groups (Figure 5).
Comparison of the incidence of re-reconstruction after blood flow restoration
Anastomotic hemorrhage requiring re-reconstruction after blood flow restoration occurred in two patients (8.6%) who underwent posterior atrial flap rotating left atrial cuff reconstruction, three (15.0%) who underwent aortic patch reconstruction, and three (20.0%) who underwent pericardial patch reconstruction. Despite the lower incidence of re-reconstruction following posterior atrial flap rotating left atrial cuff reconstruction, analysis using the chi-squared test revealed no significant difference among the groups (χ2 = 0.808, P = 0.667).
Comparison of outflow velocity
Outflow velocity, as measured by esophageal ultrasound (Figure 6), revealed that patients in all three groups had normal pulmonary vein outflow channels, with no significant differences in flow rate among the groups either for the right (posterior atrial flap rotating left atrial cuff reconstruction, 53.94 ± 16.3 cm/s; aortic patch reconstruction, 60.0 ± 21.5 cm/s; pericardial patch reconstruction, 56.4 ± 17.9 cm/s; P > 0.05) or left (posterior atrial flap rotating left atrial cuff reconstruction, 64.8 ± 18.1 cm/s; aortic patch reconstruction, 68.8 ± 19.8 cm/s; pericardial patch reconstruction, 59.5 ± 16.8 cm/s; P > 0.05) pulmonary vein (Figure 7).
Comparison of cardiac ultrasonography 30 days postoperatively
Preoperative and postoperative cardiac color Doppler ultrasonography results showed no statistically significant differences in preoperative or postoperative pulmonary systolic blood pressure among the three groups (P > 0.05) (Figure 8). However, postoperative pulmonary artery systolic blood pressure was significantly lower than preoperative pulmonary artery systolic blood pressure in all three groups (posterior atrial flap rotating left atrial cuff reconstruction, 24.5 ± 7.5 vs 41.4 ± 16.6 mmHg, P < 0.01; aortic patch reconstruction, 27.6 ± 8.6 vs 35.2 ± 11.1 mmHg, P < 0.05; pericardial patch reconstruction 27.7 ± 7.5 vs 35.9 ± 14.6 mmHg, P < 0.05).
Comparison of postoperative PGD and perioperative survival
A higher incidence of PGD was observed in the posterior atrial flap rotating left atrial cuff reconstruction group (8.6%) than in the aortic (5.0%) or pericardial (13.3%) patch reconstruction groups; however, this difference was not statistically significant (χ2 = 0.431, P = 0.806). Four patients died in the perioperative period, one of whom underwent posterior atrial flap rotating left atrial cuff reconstruction, while three underwent aortic patch reconstruction (χ2 = 2.515, P = 0.284).
Comparison of clinical data 90 days postoperatively
At 90 days postoperatively, there were no significant differences in the relevant clinical characteristics among the groups (Table 2). Pulmonary function, as measured by forced expiratory volume/forced vital capacity and arterial blood gases, was significantly improved 90 days postoperatively compared to preoperatively; however, there were no significant differences in this improvement among the three groups (Table 1 and Table2).
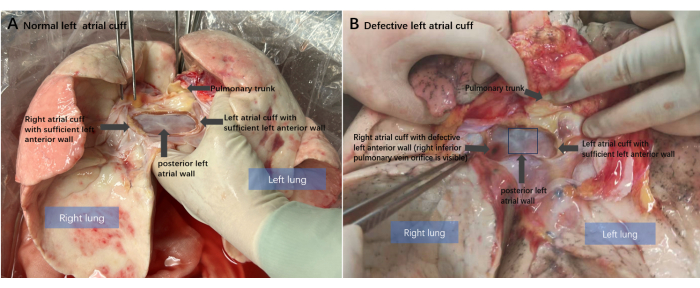
Figure 1: Anatomical diagram of the donor lung. (A) Normal left atrial cuff condition of the donor lung after heart-lung separation. The intact posterior left atrial posterior wall and sufficient anterior wall preserved. (B) The classical donor left atrial cuff anterior wall defect: the anterior atrial wall of the right donor lung is missing. Please click here to view a larger version of this figure.
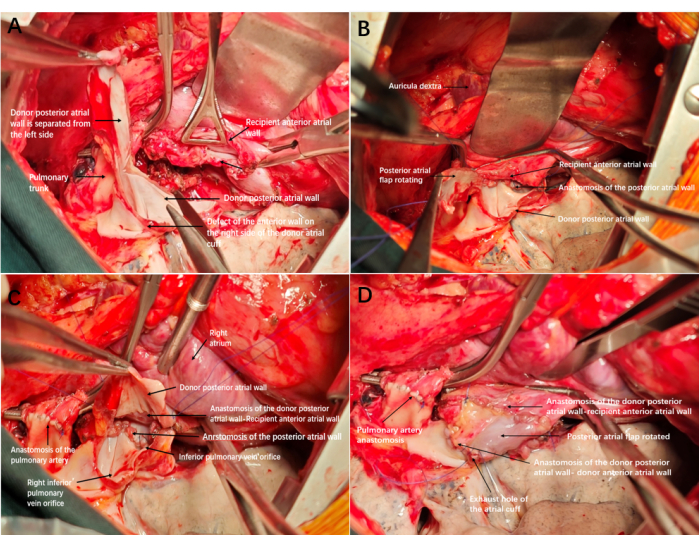
Figure 2: Reconstruction and anastomosis diagram of the left atrial cuff. (A) Defect of the anterior wall on the right side of the donor atrial cuff; after anastomosis of the trachea and artery is completed, the excess posterior wall of the atrium is cut from the bottom up to form an atrial flap. (B) Completing the anastomosis of the posterior atrial wall. (C) The edge of the atrial flap was anastomosed to the anterior wall of the recipient's atrial cuff, with the inner membrane of the atrial flap facing inward. (D) The remaining edge of the atrial cuff was anastomosed to the anterior wall of the recipient. After tightening and opening the exhaust, the cuff expanded well, with no bleeding and no need for re-reconstruction. Please click here to view a larger version of this figure.
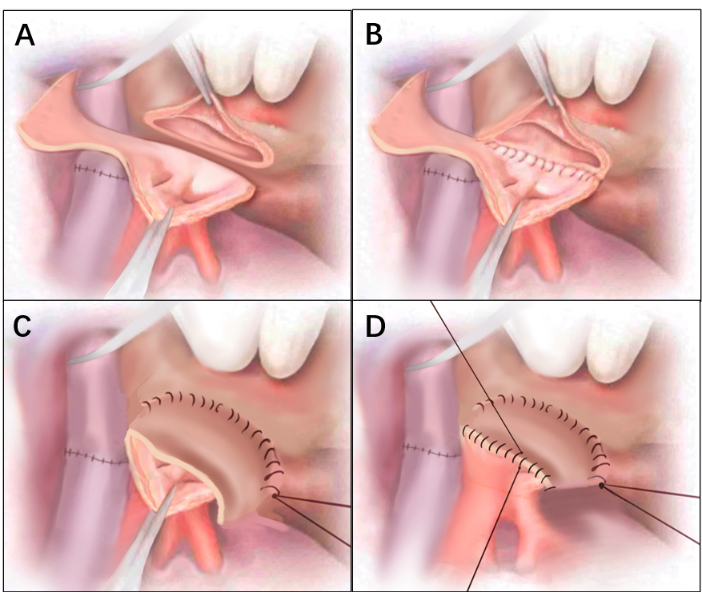
Figure 3: Line drawings of Figure 2. (A) Defect of the anterior wall on the right side of the donor atrial cuff. After anastomosis of the trachea and artery is completed, the excess posterior wall of the atrium is cut from the bottom up to form an atrial flap. (B) Completing the anastomosis of the posterior atrial wall. (C) The edge of the atrial flap was anastomosed to the anterior wall of the recipient's atrial cuff, with the inner membrane of the atrial flap facing inward. (D) The remaining edge of the atrial cuff was anastomosed to the anterior wall of the recipient. Please click here to view a larger version of this figure.
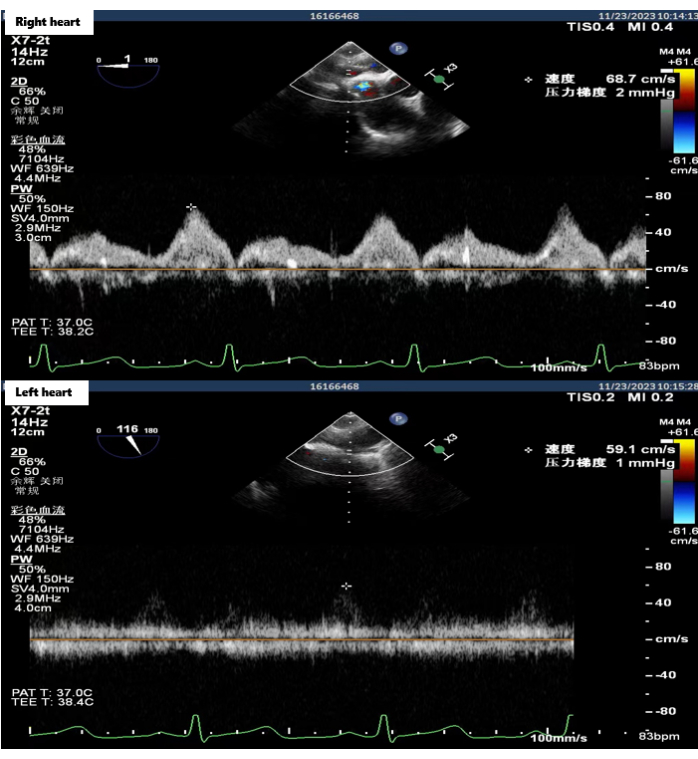
Figure 4: Esophageal ultrasonography of the bilateral atrial anastomotic flow rate. Transesophageal ultrasound images were acquired in the operating room after all anastomosis was completed, showing that bilateral atrial anastomotic flow rate was normal. Please click here to view a larger version of this figure.
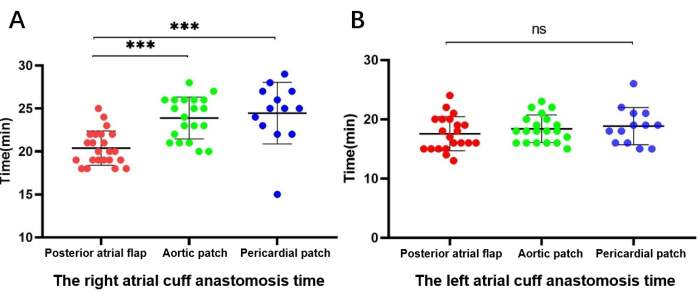
Figure 5: Comparison of the duration of atrial cuff anastomosis. (A) Right-side (reconstruction) atrial cuff anastomosis time. (B) Left-side (unreconstructed) atrial cuff anastomosis time. Please click here to view a larger version of this figure.
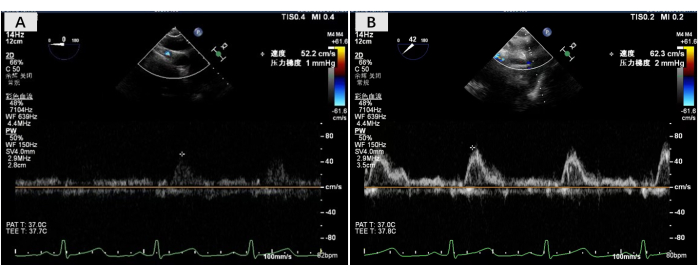
Figure 6: Esophageal ultrasonography of bilateral outflow flow rate. (A) Flow velocity measurement at the right pulmonary atrial anastomosis. (B) Flow velocity measurement at the left pulmonary atrial anastomosis. Please click here to view a larger version of this figure.
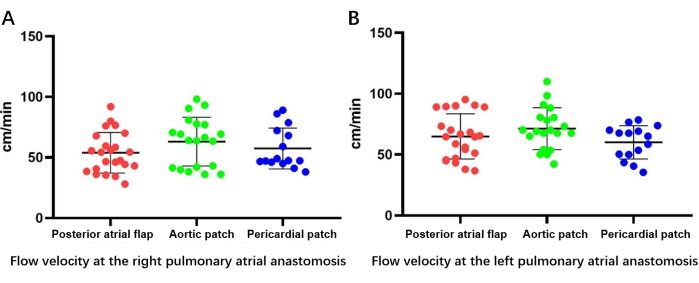
Figure 7: Bilateral outflow velocity as determined by esophageal ultrasonography. (A) Flow velocity measurement at the right pulmonary atrial anastomosis. (B) Flow velocity measurement at the left pulmonary atrial anastomosis. Please click here to view a larger version of this figure.
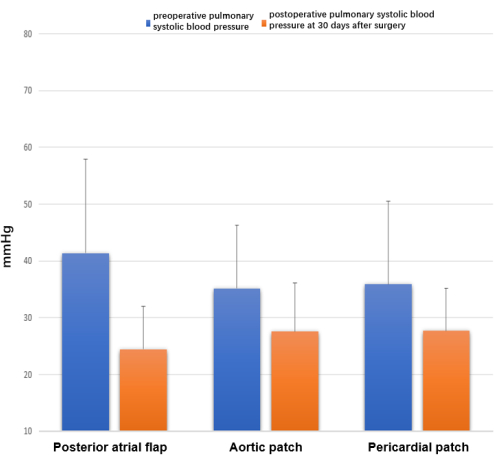
Figure 8: Echocardiography at 30 days after surgery. Differences were observed in preoperative and postoperative pulmonary systolic blood pressure, measured as 4V²TR + RPA mmHg. Please click here to view a larger version of this figure.
| Characteristic | Technique used for cuff reconstruction | P-value | ||
| Posterior atrial flap (N = 23) | Aortic patch (N = 20) | Pericardial patch (N = 15) | ||
| Donor | ||||
| Sex (F/M) | 5/18 | 4/16 | 4/11 | ns |
| Age (years) | 35.0 ± 12.9 | 36.1± 12.1 | 36.7± 10.9 | 0.86 |
| Arterial PO2/FiO2 | 401 ± 102 | 422± 106 | 426 ± 101 | 0.14 |
| Recipient | ||||
| Sex (F/M) | 2/21 | 4/16 | 6/9 | - |
| Age (years) | 47.3 ± 21.0 | 52.3± 16.2 | 49.5 ± 21.4 | ns |
| PO2 (mmHg) | 91.6 ± 21.6 | 106.7±38.3 | 102.2±35.2 | ns |
| PCO2 (mmHg) | 55.2 ± 21.7 | 54.6 ± 17.3 | 47.4 ± 11.2 | ns |
| SatO2 (%) | 96.6 ± 2.1 | 92.9± 20.3 | 91.9 ± 17.3 | ns |
| FEV1/FVC | 49.4 ± 23.9 | 48.7± 30.0 | 63.7 ± 26.0 | ns |
| PAP (cm/s) | 38.8 ± 17.9 | 44.4± 23.0 | 39.1 ± 24.4 | ns |
Table 1: Baseline characteristics of lung transplant donors and recipients. Values are presented as mean ± standard deviation. The Kruskal-Wallis test was used to compare the groups. Abbreviations: F = female; FEV1 = forced expiratory volume; FiO2 = fraction of inspired oxygen; FVC = forced vital capacity; M = male; PAP = pulmonary artery pressure; PCO2 = partial pressure of carbon dioxide; PO2 = partial pressure of oxygen; SatO2 = oxygen saturation.
| Characteristic | Technique used for cuff reconstruction | P-value | ||
| Posterior atrial flap (N = 23) | Aortic patch (N = 20) | Pericardial patch (N = 15) | ||
| PO2 | 113.2 ± 24.1 | 97.3 ± 27.5 | 98.8 ± 27.0 | ns |
| PCO2 | 40.4 ± 5.7 | 39.2 ± 10.8 | 36.4 ± 7.2 | ns |
| SatO2 | 98.1 ± 0.9 | 93.0 ± 21.1 | 93.7 ± 16.7 | ns |
| FEV1/FVC | 86.1 ± 10.3 | 87.1 ± 19.4 | 81.9 ± 17.6 | ns |
Table 2: Clinical characteristics of lung transplant recipients 90 days postoperatively. Values are presented as mean ± standard deviation. The Kruskal-Wallis test was used to compare the groups. Abbreviations: FEV1 = forced expiratory volume; FVC = forced vital capacity; PCO2 = partial pressure of carbon dioxide; PO2 = partial pressure of oxygen; SatO2 = oxygen saturation.
Discussion
Between 2021 and 2023, the team at the Second Hospital of Zhejiang University/Wuxi Lung Transplant Center performed 931 lung transplants, of which 71 involved donor left atrial cuff reconstruction. To reduce heterogeneity, only 58 double-lung transplants were included in this study. Artificial patches are rarely used in our center, and they were therefore not included in this study.
The anastomosis time of the reconstruction side was significantly shorter in the posterior atrial flap rotating atrial cuff reconstruction group than in the aortic and pericardial patch reconstruction groups, indicating that the new surgical technique effectively shortened the operation time. There was no difference in the anastomosis time of the unrepaired side, which suggests surgical consistency, indicating that the difference in anastomosis time on the repaired side was due to the difference in technique.
Esophageal ultrasonography indicated that there was no significant difference in the velocity of bilateral outlet flow, demonstrating the feasibility of the new approach. Cardiac ultrasonography and patient outcomes also indicated good short-term recovery following the new technique, with no significant differences in short-term complications and adverse reactions compared with the traditional patch reconstruction method. This was further supported by assessments of pulmonary function and arterial blood gases 90 days after transplantation.
To ensure smooth blood flow in the pulmonary vein, the left atrial cuff must be anastomosed during lung transplantation3. During donor lung extraction, ideal cardiopulmonary separation requires preservation of the intact left atrial cuff, including the intact posterior and adequate anterior atrial wall2. Atrial cuff defects are common injuries sustained during donor lung extraction3, when the need to utilize both the donor heart and lung often results in competition in some anatomical areas, particularly the left atrial area, which can lead to an atrial cuff defect in the donor lung7. Whether donor cardiopulmonary separation should be performed in vivo or in vitro remains controversial.
At present, in vivo cardiopulmonary separation is the preferred technique2,8. However, in vitro cardiopulmonary separation can better expose the left atrial area, where cardiopulmonary competition may exist5. An anterior atrial wall defect of the right-sided donor lung is the most common type of donor atrial cuff defect due to the presence of the interatrial groove4. Meticulous dissection and dissociation of the interatrial groove can better expose the left anterior atrial wall on the right side and prevent defects in the atrial cuff of the right donor lung3.
When atrial cuff defects occur, intraoperative anastomosis becomes more difficult, and it is often necessary to shape and reconstruct the atrial cuff to prevent bleeding and related venous outflow channel complications, which increases the cold ischemia time. Studies have shown that although prolonged cold ischemia time due to increased anastomotic duration has no effect on long-term survival, it can affect survival at 90 days postoperatively and increase the risk of airway complications9. Defects in the atrial cuff also cause donors to be downgraded from ideal to marginal10.
When atrial cuff defects occur, forced anastomosis with tension or separate anastomosis of the upper and lower pulmonary veins increases the risk of venous outflow channel obstruction11. Instead, a slightly more compact posterior atrial anastomosis and looser, tension-free anterior anastomosis can open the left atrial outflow channel. Hammond et al.5 introduced the use of pericardial tissue adjacent to the pulmonary vein for reconstruction, resulting in a wide and smooth atrial cuff anastomosis and a bilateral perfusion flow rate matching the recipient. It has been argued that direct anastomosis of the peripheral pericardium without reinforcing the pulmonary vein stump to the pericardium can reduce the risk of blocking or narrowing the vein opening12. The availability of pericardial tissue and its good outcomes make it the most important material for atrial cuff patch reconstruction. Sugimoto et al.6 reported a method of using excess donor pulmonary artery to reconstruct atrial cuffs, with good surgical outcomes. However, there have been no reports on the use of the surplus posterior left atrial wall of the donor lung to reconstruct anterior wall defects.
Here, we introduce this new reconstruction method for the donor atrial cuff, named the posterior atrial flap rotating left atrial cuff reconstruction technique, and compared and analyzed its safety. In terms of surgery, the posterior atrial flap rotating left atrial cuff reconstruction method is more convenient and rapid, as it does not require in vitro atrial cuff formation but can be repaired directly during anastomosis. Moreover, because the reconstructed area is made from the same atrial tissue, tissue compatibility is good, and there is maximal preservation of the blood supply to the repaired area. Regarding prognosis, the posterior atrial flap method can keep the anterior atrial cuff loose after anastomosis and reduce the occurrence of complications related to venous outflow, bleeding, and re-reconstruction compared with patch reconstruction methods, as well as decrease the cold ischemia time through a faster anastomosis. This simple surgical method provides a feasible strategy for the reconstruction of atrial cuff defects in the donor pulmonary vein, with good clinical outcomes.
This study has certain limitations. For example, the statistical difference in the ratio of male to female recipients among the three groups and the inability to maintain the same quality of donor lungs due to the complexity of lung transplantation may have affected the results of the study. This method is only applicable to the most common type of atrial cuff defect, the anterior atrial wall defect, and requires an intact posterior atrial wall. If the posterior atrial wall is incomplete, patch reconstruction or other formation methods are required.
In conclusion, this study describes a new method of donor lung left atrial cuff reconstruction. This new method can effectively address atrial cuff anterior wall defects during lung transplantation by rotating the posterior atrial flap. Although no significant differences were observed in complication rates and venous obstruction in the short term between the posterior atrial flap and patch reconstruction methods, use of the posterior atrial flap method effectively reduced the atrial cuff anastomosis time. Compared with the traditional patch reconstruction method, it also reduces the difficulty of the operation and has the potential to reduce the risk of long-term complications such as anastomotic ischemia and mural thrombosis. This technique therefore provides a feasible strategy for atrial cuff defect reconstruction and may improve the effective utilization rate of donor lungs.
Disclosures
The authors declare that they have no competing interests.
Acknowledgements
This research was supported by National Key Research and Development Program of China. (Project No. 2023YFC2507100).
Materials
| Name | Company | Catalog Number | Comments |
| ACE+7 SHEARS | ETHICON | HARH23 | |
| Articulating Endoscopic Linear Cutter | ETHICON | PSEE45A | |
| Atraumatic tissue forceps | Delacroix-Chevalier | DC13200-24/DC13200-20 | |
| Atrial blocjing forceps | Delacroix-Chevalier | DC40672-26/DC40673-26 | |
| Blue monofilament non-absortable suture (4-0) | ETHICON | REF8521 | |
| Blue monofilament non-absortable suture (5-0) | ETHICON | HS6856 | |
| Bronchoscope | OLYMPUS | BF-Q170 | |
| Coated vicryl plus antibacterial suture (1-0) | ETHICON | VCP359 | |
| Diagnostic Ultrasound System | PHILIPS | EPIQ 7C | |
| Diprivan Injection | AstraZeneca | 1%w/v | |
| Disposable Skin Stapler | Pride | P-PF-35R | |
| ECMO | Maquet | CARDIOHELP-i | |
| Electrosurgical Pencil | AESCULAP | GN300 | |
| Needle Holder | Delacroix-Chevalier | DC51170-24/G30154 | |
| Negative pressure suction device | SMAF | YX930 | |
| PDS II absorbable suture (4-0) | ETHICON | W9109 | |
| Pulmonary artery blocking forceps | Delacroix-Chevalier | DC13010-23/DC13010-23 | |
| Remifentanil Hydrochloride Injection | CNPIC | H20223422 | |
| Thoratrak MICS Retractor System | Medtronic | 28605 | |
| Tissue scissors | Delacroix-Chevalier | B25925 |
References
- Young, K. A., Dilling, D. F. The future of lung transplantation. Chest. 155 (3), 465-473 (2019).
- Copeland, H., et al. Donor heart and lung procurement: A consensus statement. J Heart Lung Transplant. 39 (6), 501-517 (2020).
- Sundaresan, S., Trachiotis, G. D., Aoe, M., Patterson, G. A., Cooper, J. D. Donor lung procurement: assessment and operative technique. Ann Thorac Surg. 56 (6), 1409-1413 (1993).
- Oto, T., et al. Techniques of reconstruction for inadequate donor left atrial cuff in lung transplantation. Ann Thorac Surg. 81 (4), 1199-1204 (2006).
- Hammond, G. L., Franco, K. L., Baldwin, J. C. Method of single-lung transplantation in the absence of a left atrial cuff. Ann Thorac Surg. 54 (2), 379-380 (1992).
- Sugimoto, S., et al. Pulmonary artery patch for an inadequate donor atrial cuff in the absence of donor pericardium in lung transplantation. Surg Today. 47 (3), 399-401 (2017).
- Contreras, F. J., et al. Dual procurement of lung and heart allografts does not negatively affect lung transplant outcomes. J Surg Res. 259, 106-113 (2021).
- Copeland, H., Copeland, J., Hayanga, J. W. A. Cardiac and pulmonary donor procurement. Curr Opin Organ Transplant. 23 (3), 281-285 (2018).
- Mulvihill, M. S., et al. The association of donor age and survival is independent of ischemic time following deceased donor lung transplantation. Clin Transplant. 31 (7), (2017).
- Courtwright, A., Cantu, E. Evaluation and management of the potential lung donor. Clin Chest Med. 38 (4), 751-759 (2017).
- Gamez, P., Alvarez, R., Hernández, H., Córdoba, M., De Pablo, A. Lung transplantation: how to do the venous anastomosis when the pulmonary graft has no auricular cuff. J Heart Lung Transplant. 24 (8), 1123-1125 (2005).
- Yarbrough, W. M., et al. Alternative technique for salvage of donor lungs with insufficient atrial cuffs. Ann Thorac Surg. 88 (4), 1374-1376 (2009).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved