Bu içeriği görüntülemek için JoVE aboneliği gereklidir. Oturum açın veya ücretsiz deneme sürümünü başlatın.
CRC Organoid Cell Labeling: A Method to Generate GFP Lentivirus-transduced Colorectal Cancer Organoid Cells
Overview
This video describes the technique of generating CRC organoids transduced by GFP lentiviral particles for enabling fluorescent imaging. These GFP labeled organoids, when placed in animal models, help to study tumor invasion and metastasis.
Protokol
1. Generation of the CRC Organoids Cultured on Artificial Extracellular Matrix
Experimental procedures for the CRC organoid culture of the colon PDXs are outlined in Figure 1B (step 4).
- Establish a culture medium suitable for the PDX-derived CRC organoids (see Table of Materials, "the CRC organoid culture medium") employing media previously described for human colon organoids.
- Apply 150 µL of artificial extracellular matrix (see Table of Materials) per well on a 12-well plate on ice and then incubate it for 30-60 min in a 37 °C, 5% CO2 incubator to solidify the gels.
- Suspend the cell pellet of CRC cells obtained from the PDX mouse model with the CRC organoid culture medium (from step 1.1) with 5% FCS to adjust to 3 x 105 cells/mL. Then, seed 1 mL of the medium onto the artificial extracellular matrix-coated plate prepared in step 1.2 and incubate overnight at 37 °C under 5% CO2. Use a hemocytometer for counting the number of tumor cells, including single cells and multicellular clusters (a group of adherent cells) in the cell suspension.
NOTE: 5% FCS is used to quench the residual enzymatic activity of collagenase in this study. - Carefully collect the culture medium, including floating cells detached from the artificial extracellular matrix-coated plate, into a 1.5 mL centrifuge tube on the following day and centrifuge it at 1,400 x g for 5 min. Then, remove the supernatant and resuspend the pellet in 70 µL of artificial extracellular matrix on ice.
- To increase CRC cell viability, overlay the tumor cell-containing artificial extracellular matrix onto the tumor organoid cells attached to the artificial extracellular matrix-coated plate and incubate for 30 min in a 37 °C, 5% CO2 incubator solidify the artificial extracellular matrix coating. Then, incubate it with 1 mL of the CRC organoid cell culture medium with 1% FCS at 37 °C under 5% CO2.
- Change the culture medium every second day. As the CRC organoids fill the medium, it may become necessary to change the medium daily.
2. Generation and Enrichment of GFP Lentiviral Particles
Experimental procedures for the generation and enrichment of GFP lentiviral particles are outlined in Figure 1C (step 5).
- Culture HEK293T cells with Roswell Park Memorial Institute (RPMI) 1640 medium containing 10% FCS and prepare six 10 cm dishes (2 x 106 cells per dish) for the following transfection procedure.
- For transfection per dish, prepare 500 µL of the mixture including 20 µL of the transfection reagent in DMEM with a PRRL-GFP vector (5 µg) and two lentiviral packaging plasmids, such as pCMV-VSV-G (1 µg) and pCMV-dR8.2 dvpr (5 µg), in a sterile 1.5 mL microtube. Keep the mixture at room temperature for 20 min and then overlay it onto each of the 10-cm dishes and incubate them for 18 h.
- Remove the medium, add 5 mL of fresh 10% FCS-RPMI 1640 medium to each dish, and leave standing for 24 h.
- Collect the conditioned medium from each dish into a 50 mL centrifuge tube and store a total of 30 mL of the medium at 4 °C overnight. Add 5 mL of the fresh RPMI 1640 onto each dish and leave standing for 24 h.
- Collect the conditioned medium from each dish into a 50 mL centrifuge tube and keep, in total, 30 mL of the medium on ice. Then, discard the cells.
- Filter 60 mL of the medium (from steps 2.4-2.5) through a 0.45 µm filter to remove the cells and divide this quantity into twelve 5 mL polypropylene centrifuge tubes for ultracentrifugation.
- To concentrate viruses, centrifuge them using a swinging bucket rotor at 85,327 x g for 1.5 h at 4 °C.
- Immediately remove the medium. To resolve the concentrated virus pellet, place 250 µL of Nutrient Ham's Mixture F-12 (F12)/DMEM medium without FCS in each of the twelve tubes and maintain it at 4 °C overnight. Note that the virus pellet is often invisible.
- Gently pipette the medium to collect 3 mL of the concentrated 5x GFP lentivirus stock, in total, presumably including GFP lentiviral particles with 108 transducing units per mL (TU/mL). Then, store the twelve 1.5 mL microtubes (including 250 µL per tube) under sterile conditions at -80 °C for up to a year. Prepare many small aliquots of the original solution to avoid multiple freeze-thaw cycles.
3. Labelling of CRC Organoid Cells with GFP Lentiviral Particles Cultured on Artificial Extracellular Matrix
Experimental procedures for labeling the CRC organoid cells with GFP lentivirus are outlined in Figure 1C (step 6).
- Allow the CRC organoids prepared in step 1.6 to grow without interference for 7-10 days, harvest them mechanically using a sterile cell scraper, and transfer them into a 1.5 mL microtube.
NOTE: The growth of CRC organoids in culture depends on the nature of the original tumors from patients. Avoid overgrowth of the CRC organoids by maintaining them at 60-70% confluence before transfer at a 1:2 split ratio onto a new artificial extracellular matrix-coated 12-well plate. - Centrifuge the microtube for 3 min at 1,400 x g, remove the culture medium and resolve the cell pellet in 500 µL of PBS by gentle tapping.
- Centrifuge the microtube for 3 min at 1,400 x g and eliminate PBS.
- To dissociate the adherent CRC organoids, add 500 µL of a cell detachment solution of proteolytic and collagenolytic enzymes to the cell pellet in the tube and mix it by gentle tapping. Then, leave the tubes to settle for 10 min at room temperature.
- Very gently pipette the cell suspension with an additional 500 µL of 1% FCS-DMEM several times in a 1.5 mL microtube to roughly dissociate the cells. Generation of single cells from the CRC organoids by harsh pipetting markedly reduces cell viability. Thus, it is essential to leave the mass of cells visually detectable in the cell suspension by gently pipetting in a 1.5 mL microtube.
- Centrifuge the cell suspension for 3 min at 1,400 x g and remove the supernatant.
- Resolve the cell pellet with a mixture of the 100 µL of 5x GFP lentivirus stock (>108 TU/mL) and 400 µL of the CRC organoid culture medium in a 1.5 mL microtube by gentle tapping to adjust to a concentration of 5 x 105 dissociated tumor cells per 500 µL. Use a hemocytometer for counting the number of tumor cells, including single cells and groups of cells in the cell suspension, as described above (step 1.3).
- Prepare an artificial extracellular matrix-coated 12-well plate, as described in step 1.2. Then, place 500 µL of the cell suspension prepared in step 3.7 on the plate and leave it for 18 h at 37 °C under 5% CO2.
- Collect the medium with the floating cells detached from the artificial extracellular matrix-coated plate into a 1.5 mL centrifugation tube. Centrifuge it at 1,400 x g and then remove the supernatant and re-suspend the pellet in 70 µL of artificial extracellular matrix on ice.
- To increase CRC cell viability, overlay the tumor cell-containing artificial extracellular matrix onto the tumor organoids attached to the artificial extracellular matrix-coated 12-well plate, as described in step 1.5. Next, incubate the plate for 30 min in a 37 °C, 5% CO2 incubator to solidify the artificial extracellular matrix coating and then culture it with 1 mL of the CRC organoid culture medium with 1% FCS at 37 °C under 5% CO2.
- Observe the cells at three days after infection under a fluorescence microscope to confirm nearly 100% GFP positivity due to using a high titer of lentiviral particles (see Figure 2A).
- Culture the CRC organoids for 7-10 days to expand cell growth prior to injection into recipient mice.
Sonuçlar
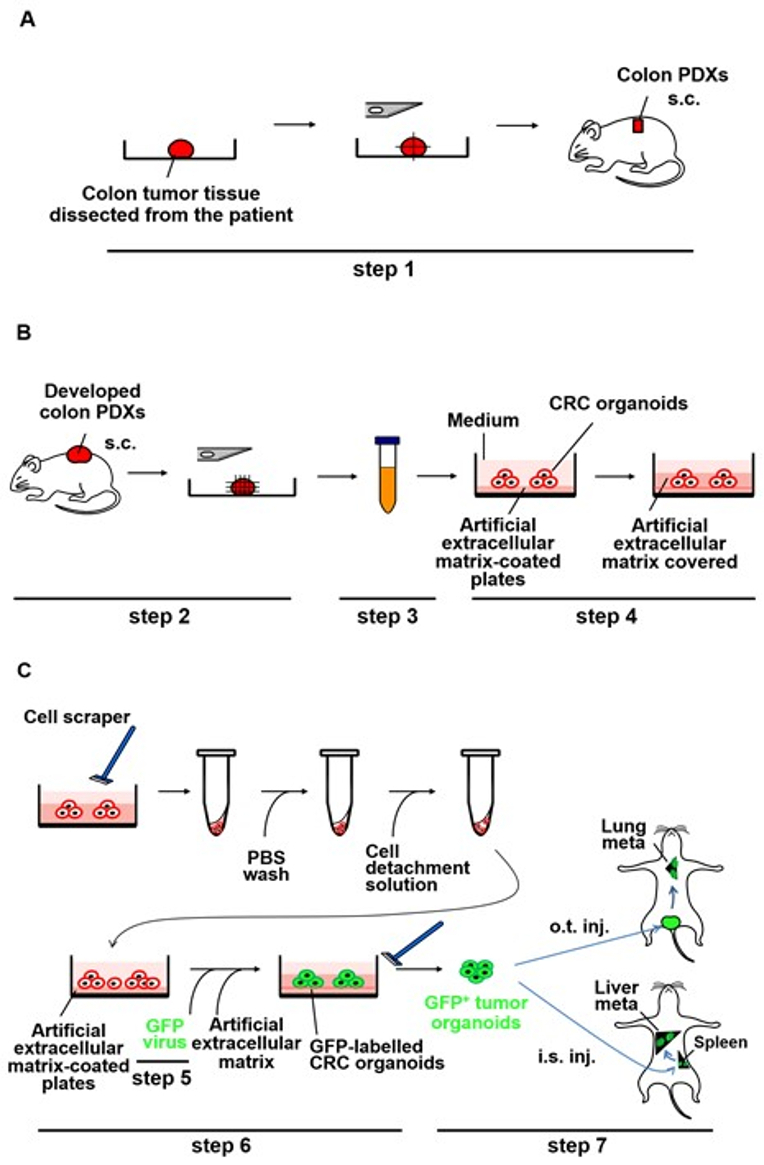
Figure 1: Schematic representation of the generation of metastases by the PDX-derived CRC organoids labeled with GFP lentivirus in NOG mice. (A) Implantation of small pieces of the CRC tissue subcutaneously into NOG mice (step 1). The CRC tissue surgically dissected from the patient was cut into pieces and implanted subcutaneously into NOG mice. s.c.: subcut...
Açıklamalar
Malzemeler
| Name | Company | Catalog Number | Comments |
| 12-well plate | BMBio | #92412 | |
| Microtube | Eppendorf | #0030120086 | Autoclave before use |
| Hemocytometer | Erma | #03-202-1 | |
| Matrigel basement membrane matrix | Corning | #354234 | Store aliquots at -20°C. Place on ice until use |
| Collagenase type 1 | Sigma | #C1030 | 150 mg/ml collagenase type1 in 1×PBS. Store aliquots at -20°C for up to 1 year |
| DMEM/F-12 with GlutaMAX™ | Gibco | #10565018 | |
| Penicillin | Gibco | #15140122 | Store at 4°C. Use within 1 month |
| hEGF | PEPROTECH | #AF-100-15 | Store at -20°C. Add to medium on same day as use |
| Y27632, a ROCK inhibitor | Wako | #253-00591 | Store at -20°C. Add to medium on same day as use |
| Streptomycin | Gibco | #15140122 | Store at 4°C. Use within 1 month |
| CRC organoid culture medium with 1% or 5% FCS | DMEM/F-12 with GlutaMAX™ supplement (Gibco #10565018) supplemented with 1% or 5% FCS, 100 U/ml penicillin, 100 µg/ml streptomycin, 2 ng/ml hEGF and 10 µM Y27632, a ROCK inhibitor. Store at 4°C. Use within 1 month. | ||
| PRRL-GFP vector | Gift from Dr. Robert A. Weinberg | ||
| FuGENE 6 transfection regent | Roche | 11814 443001 | |
| pCMV-VSV-G | Gift from Dr. Robert A. Weinberg | ||
| pCMV-dR8.2 dvpr | Gift from Dr. Robert A. Weinberg | ||
| Zeiss Axioplan 2 stereo fluorescence microscope | Zeiss |
This article has been published
Video Coming Soon
Source: Okazawa Y. et al., High-sensitivity Detection of Micrometastases Generated by GFP Lentivirus-transduced Organoids Cultured from a Patient-derived Colon Tumor. J. Vis. Exp. (2018).
JoVE Hakkında
Telif Hakkı © 2020 MyJove Corporation. Tüm hakları saklıdır