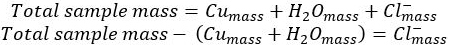Determining the Empirical Formula
Source: Laboratory of Dr. Neal Abrams - SUNY College of Environmental Science and Forestry
Determining the chemical formula of a compound is at the heart of what chemists do in the laboratory every day. Many tools are available to aid in this determination, but one of the simplest (and most accurate) is the determination of the empirical formula. Why is this useful? Because of the law of conservation of mass, any reaction can be followed gravimetrically, or by change in mass. The empirical formula provides the smallest whole-number ratio among elements (or compounds) within a molecular compound. In this experiment, gravimetric analysis will be used to determine the empirical formula of copper chloride hydrate, CuxCly·nH2O.
1. Dehydrating the Hydrate
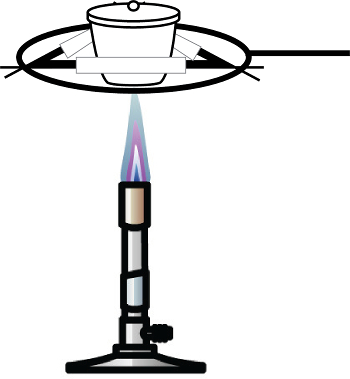
- Accurately weigh a sample of copper chloride hydrate and place it into a pre-dried and tared crucible. It is important that the crucible is dried above 120 °C to drive off any adsorbed moisture. Typically, 1–2 g of compound will suffice.
- Heat the sample using a Bunsen burner or other flame source until it changes color from greenish-blue to a reddish-brown (Figure 1). This color change is indicative of the anhydrous form of copper chloride.
- Experiment
- Heat 1.25 g of copper chloride hydrate in a crucible. After heating and then cooling, the final mass is 0.986 g of copper chloride, CuxCly.
- Dissolve the CuxCly sample in 50 mL of deionized water and add 0.2 g of fine aluminum mesh to the beaker.
- After reacting and dissolving the excess aluminum, 0.198 g of dried copper metal is recovered.
- Subtract the mass of both copper and water from the initial copper chloride hydrate to yield&#
In one example, suppose an unknown biomolecule containing only C, H, and O is found to act well as a new fuel. One way to determine the formula of the fuel would be to combust it in air and analyze the products:
CxHyOz + O2 → mCO2 + nH2O
While O2 is in excess, we would know all the carbon in CO2 originated from the biomolecule and all the hydrogen would be present in H
Skip to...
Videos from this collection:

Now Playing
Determining the Empirical Formula
General Chemistry
178.0K Views

Common Lab Glassware and Uses
General Chemistry
650.4K Views

Solutions and Concentrations
General Chemistry
271.4K Views

Determining the Density of a Solid and Liquid
General Chemistry
552.5K Views

Determining the Mass Percent Composition in an Aqueous Solution
General Chemistry
382.6K Views

Determining the Solubility Rules of Ionic Compounds
General Chemistry
140.8K Views

Using a pH Meter
General Chemistry
343.0K Views

Introduction to Titration
General Chemistry
421.1K Views

Ideal Gas Law
General Chemistry
77.5K Views

Spectrophotometric Determination of an Equilibrium Constant
General Chemistry
157.8K Views

Le Châtelier's Principle
General Chemistry
262.4K Views

Freezing-Point Depression to Determine an Unknown Compound
General Chemistry
159.3K Views

Determining Rate Laws and the Order of Reaction
General Chemistry
195.4K Views

Using Differential Scanning Calorimetry to Measure Changes in Enthalpy
General Chemistry
44.2K Views

Coordination Chemistry Complexes
General Chemistry
90.6K Views
Copyright © 2025 MyJoVE Corporation. All rights reserved