JoVE Science Education
Biomedical Engineering
A subscription to JoVE is required to view this content.
Visualization of Knee Joint Degeneration after Non-invasive ACL Injury in Rats
Source: Lindsey K. Lepley1,2, Steven M. Davi1, Timothy A. Butterfield3,4 and Sina Shahbazmohamadi5,
1Department of Kinesiology, University of Connecticut, Storrs, CT; 2Department of Orthopaedic Surgery, University of Connecticut Health Center, Farmington, CT; 3Department of Rehabilitation Sciences, University of Kentucky, Lexington, KY; 4Center for Muscle Biology, Department of Physiology, University of Kentucky, Lexington, KY; 5Biomedical Engineering Department, University of Connecticut, Storrs, CT
Anterior cruciate ligament (ACL) injury to the knee dramatically increases the risk of post-traumatic osteoarthritis (PTOA), as approximately one-third of individuals will demonstrate radiographic PTOA within the first decade following ACL injury. Though ACL reconstruction (ACLR) successfully restores knee joint stability, ACLR and current rehabilitation techniques do not prevent the onset of PTOA. Therefore, ACL injury represents the ideal model to study the development of PTOA after traumatic joint injury.
Rat models have been used extensively to study the onset and effect of ACL injury on PTOA. The most widely used model of ACL injury is ACL transection, which is an acute model that surgically destabilizes the joint. Though practical, this model does not faithfully mimic human ACL injury due to the invasive and non-physiological injury procedures that mask the native biological response to injury. To improve the clinical translation of our results, we have recently developed a novel non-invasive model of ACL injury where the ACL is ruptured through a single load of tibial compression. This injury closely replicates injury conditions relevant to humans and is highly reproducible.
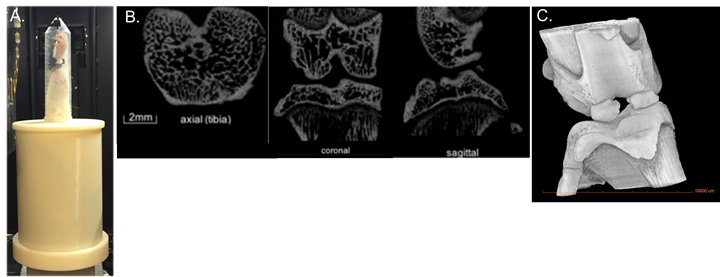
Visualization of joint degeneration through micro-computed tomography (µCT) provides several major advancements over traditional OA staining techniques, including rapid, high-resolution, non-destructive 3D imaging of whole joint degeneration. The goal of this demonstration is to introduce the state of the art non-invasive ACL injury in a rodent model and use µCT to quantify knee joint degeneration.
Non-invasive ACL Injury
- Wear proper personal protection equipment. You may use a respiratory mask, but it is not mandatory for this protocol.
- Anesthetize the rats using an induction chamber with 5% isoflurane and 1 L/min oxygen. Maintain the flow of anesthesia using via a nose cone with 1 - 3% isoflurane and 500 mL/min of oxygen. If the apparatus is not set up on a backdraft or downdraft table, ensure that waste gas is scavenged using a tabletop system and charcoal filters.
- Perform a toe-p
Smaller trabecular number, reduced trabecular thickness and greater trabecular spacing, all hallmark characteristics of PTOA onset, were evident 4 weeks after the non-invasive ACL tear (Table 1 and Figure 3). An image of a dissected ACL of healthy limb versus an acute injured limb is shown in Figure 5. The novel non-invasive model of ACL injury, where the ACL is ruptured through a single load of tibial compressio
This video demonstrates how a linear actuator can be used to produce an isolated non-invasive ACL rupture in rats. This injury closely replicates injury conditions relevant to humans and is highly reproducible. To overcome several of the major limitations of traditional OA staining techniques, this method uses µCT to quantify whole joint degeneration and trabecular structure.
Evidence-based interventions to improve musculoskeletal rehabilitative outcomes is a highly significant area that
- Maerz T, Kurdziel MD, Davidson AA, Baker KC, Anderson K, Matthew HW. Biomechanical Characterization of a Model of Noninvasive, Traumatic Anterior Cruciate Ligament Injury in the Rat. Ann Biomed Eng. 2015;43(10):2467-2476.
- Christiansen BA, Anderson MJ, Lee CA, Williams JC, Yik JH, Haudenschild DR. Musculoskeletal changes following non-invasive knee injury using a novel mouse model of post-traumatic osteoarthritis. Osteoarthritis Cartilage. 2012;20(7):773-782.
- Lockwood KA, Chu BT, Anderson MJ, Haudenschild DR, Christiansen BA. Comparison of loading rate-dependent injury modes in a murine model of post-traumatic osteoarthritis. J Orthop Res. 2014;32(1):79-88.
- Blair-Levy JM, Watts CE, Fiorentino NM, Dimitriadis EK, Marini JC, Lipsky PE. A type I collagen defect leads to rapidly progressive osteoarthritis in a mouse model. Arthritis Rheum. 2008;58(4):1096-1106.
- Mohan G, Perilli E, Kuliwaba JS, Humphries JM, Parkinson IH, Fazzalari NL. Application of in vivo micro-computed tomography in the temporal characterisation of subchondral bone architecture in a rat model of low-dose monosodium iodoacetate-induced osteoarthritis. Arthritis Res Ther. 2011;13(6):R210.
- Jones MD, Tran CW, Li G, Maksymowych WP, Zernicke RF, Doschak MR. In vivo microfocal computed tomography and micro-magnetic resonance imaging evaluation of antiresorptive and antiinflammatory drugs as preventive treatments of osteoarthritis in the rat. Arthritis Rheum. 2010;62(9):2726-2735.
Skip to...
Videos from this collection:

Now Playing
Visualization of Knee Joint Degeneration after Non-invasive ACL Injury in Rats
Biomedical Engineering
8.2K Views

Imaging Biological Samples with Optical and Confocal Microscopy
Biomedical Engineering
35.6K Views

SEM Imaging of Biological Samples
Biomedical Engineering
23.5K Views

Biodistribution of Nano-drug Carriers: Applications of SEM
Biomedical Engineering
9.3K Views

High-frequency Ultrasound Imaging of the Abdominal Aorta
Biomedical Engineering
14.4K Views

Quantitative Strain Mapping of an Abdominal Aortic Aneurysm
Biomedical Engineering
4.6K Views

Photoacoustic Tomography to Image Blood and Lipids in the Infrarenal Aorta
Biomedical Engineering
5.7K Views

Cardiac Magnetic Resonance Imaging
Biomedical Engineering
14.7K Views

Computational Fluid Dynamics Simulations of Blood Flow in a Cerebral Aneurysm
Biomedical Engineering
11.7K Views

Near-infrared Fluorescence Imaging of Abdominal Aortic Aneurysms
Biomedical Engineering
8.2K Views

Noninvasive Blood Pressure Measurement Techniques
Biomedical Engineering
11.8K Views

Acquisition and Analysis of an ECG (electrocardiography) Signal
Biomedical Engineering
104.3K Views

Tensile Strength of Resorbable Biomaterials
Biomedical Engineering
7.5K Views

Micro-CT Imaging of a Mouse Spinal Cord
Biomedical Engineering
7.9K Views

Combined SPECT and CT Imaging to Visualize Cardiac Functionality
Biomedical Engineering
11.0K Views
ISSN 2640-0499
Copyright © 2025 MyJoVE Corporation. All rights reserved
We use cookies to enhance your experience on our website.
By continuing to use our website or clicking “Continue”, you are agreeing to accept our cookies.