Adoptive Cell Transfer: Introducing Donor Mouse Splenocytes to a Host Mouse and Assessing Success via FACS
Source: Meunier Sylvain1,2,3, Perchet Thibaut1,2,3, Sophie Novault4, Rachel Golub1,2,3
1 Unit for Lymphopoiesis, Department of Immunology, Pasteur Institute, Paris, France
2 INSERM U1223, Paris, France
3 Université Paris Diderot, Sorbonne Paris Cité, Cellule Pasteur, Paris, France
4 Flow Cytometry Platfrom, Cytometry and Biomarkers UtechS, Center for Translational Science, Pasteur Institute, Paris, France
Adoptive cell transfer is a method for introducing cells into a patient or study organism in order to treat a disease or study a biological process, such as haematopoiesis. Aims of adoptive transfer are various; it can be used in fundamental biology as well as in medical sciences (1, 2). In mouse models, migration and distribution of transferred cells can be studied and followed by a tracking system (cell surface marker, staining by CFSE, etc.). In cancer studies on mouse models, transfer of specific cell populations can be used as experimental treatment against tumors. Another example for this technique is creation of chimeric mice by transfer of bone marrow cells to irradiated mice or mice with a severe immunodeficiency phenotype. This mouse model can be used to assess the impact of gene deletion on a specific cell population for instance. Transfer of bone borrow cells is also used in human medical treatment. When patients are irradiated in case of cancer therapy, adoptive transfer of bone marrow allows immune system reconstitution.
The first step in this technique is to obtain the cell population of interest. The technique chosen to isolate this population depends on the level of specificity of the targeted population. The largest level of selection is the whole organ, in which all cell populations present in the organ are taken. A more precise method is selection of a target cell population, often selected by one cell surface marker. The ideal method to sort cells in this case is by magnetic sorting. Finally, the most stringent level is the selection of cells by several cell surface markers to sort very specific cell populations. Flow cytometry sorting is the most popular method for this level of selection. Once population of interest is obtained, it can be transferred to the host. Before adoptive transfer it is essential to ensure compatibility between host and donor. Indeed, regardless of transfer goal, compatibility is crucial to assure cells adoption by the host without cells rejection.
In this lab exercise, we demonstrate the adoptive cell transfer technique by transferring splenocytes from a CD45.2 mouse into a CD45.1 Ragγc mouse (lacking lymphocytes) and four days later confirm the splenocytes transfer using flow cytometry (see Figure 1).
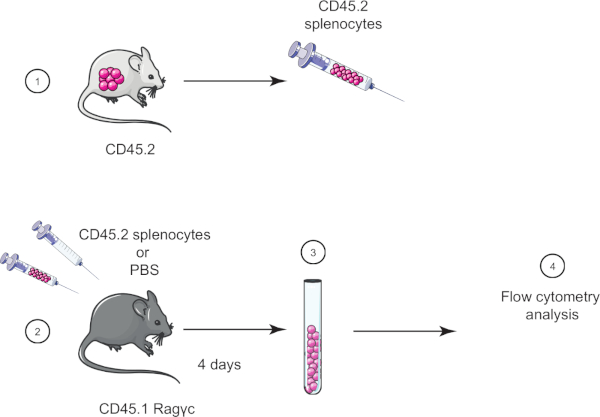
Figure 1: Schematic representation of adoptive transfer. (1) Splenocytes are isolated from CD45.2 mice and (2) transferred in CD45.1 Ragγc mouse, control mouse is injected with PBS only. (3) 4 days after adoptive transfer, splenocytes are recovered from mice and (4) analyzed by flow cytometry. Please click here to view a larger version of this figure.
1. Preparation
- Before beginning, put on laboratory gloves and the appropriate protective clothing.
- Sterilize all the dissection tools, first with a detergent and then with 70% ethanol and then dry thoroughly.
- Prepare 50 mL of Hank's balanced salt solution (HBSS) containing 2% fetal calf serum (FCS).
2. Dissection
- Using a carbon dioxide delivery system, euthanize the mouse by hypoxia. Secure the euthanized
Ragγc mice have an altered immune system composition, mainly lacking lymphocytes. Adoptive transfer of splenocytes allows introduction of lacking population such as T and B cells. Our staining included cell surface markers CD45.1 and CD45.2 to distinguish host and donor cells respectively (Figure 2A). It also included other cell surface marker to highlight cell populations absent in Ragγc mice, such as CD4 T cells ( Log in or to access full content. Learn more about your institution’s access to JoVE content here
Adoptive transfer is a translational technique in different fields of science, with applications in medicine. This technique can be used to study cell migration and tropism or incidence of protein deficiency in specific cell populations. In the last case, different technologies can be used, especially GMO mice where specific cell populations are intrinsically deficient. However, genetic construction to obtain GMO mice can be a very complex and long process. In this case, adoptive transfer of deficient cell population is
- Restifo, N. P., Dudley, M. E. and Rosenberg., S. A. Adoptive immunotherapy for cancer: harnessing the T cell response. Nature reviews. Immunology, 12 (4): 269-281, (2012).
- Bonini, C., and Mondino, A. Adoptive T-cell therapy for cancer: The era of engineered T cells. European journal of immunology, 45 (9): 2457-69, (2015).
Skip to...
ABOUT JoVE
Copyright © 2024 MyJoVE Corporation. All rights reserved